Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas
Abstract
Neuromuscular fatigue is the exercise-dependent decrease in the ability of muscle fibres to generate force. To investigate whether manipulation of brain excitability by transcranial direct current stimulation (tDCS; 1.5 mA, 10 min, 0.026 C/cm2) modulates neuromuscular fatigue, we evaluated the effect of brain polarization over the right motor areas of the cerebral cortex of healthy subjects on the endurance time for a submaximal isometric contraction of left elbow flexors. In 24 healthy volunteers the study protocol comprised an assessment of the maximum voluntary contraction (MVC) for the left elbow flexors and a fatiguing isometric contraction (35% of MVC), before and immediately after brain polarization. One hour elapsed between baseline (T0) and postconditioning (T1) evaluation. After tDCS, MVC remained unchanged from baseline (mean ± SEM; anodal tDCS: T0, 154.4 ± 18.07; T1, 142.8 ± 16.62 N; cathodal tDCS: T0, 156 ± 18.75; T1, 141.86 ± 17.53 N; controls: T0, 148.8 ± 6.64; T1, 137.6 ± 7.36 N; P > 0.1). Conversely, endurance time decreased significantly less after anodal than after cathodal tDCS or no stimulation (−21.1 ± 5.5%, −35.7 ± 3.3% and −39.3 ± 3.3%, respectively; P < 0.05). None of the evaluated electromyographic variables changed after tDCS. Anodal tDCS could improve endurance time by directly modulating motor cortical excitability, modulating premotor areas, decreasing fatigue-related muscle pain, increasing motivation and improving synergist muscle coupling. Our findings, showing that anodal tDCS over the motor areas of the cerebral cortex improves muscle endurance, open the way to increasing muscle endurance and decreasing muscle fatigue in normal (i.e. sports medicine) and pathological conditions.
Introduction
Neuromuscular fatigue is the exercise-dependent decrease in muscle force. It involves both peripheral and central factors (Gandevia, 2001). Whereas peripheral fatigue has been defined as a loss of force related to processes lying at or distal to the neuromuscular junction, central fatigue represents a progressive failure of the nervous system to drive the muscle maximally during exercise (Taylor et al., 2006). ‘Central fatigue’ is relevant for daily motor activities in normal conditions and has a major role in several disorders of the central nervous system. Central fatigue has been related both to changes in the activity of spinal motoneurons due to modulation of afferent inputs or intrinsic cellular properties (spinal fatigue) and to reduced supraspinal drive (supraspinal fatigue). Evidence from studies using transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (TES) suggests that the development of reduced supraspinal drive is accompanied by changes in motor cortex excitability (for a review see Gandevia, 2001).
Transcranial direct current stimulation (tDCS) is a noninvasive technique for modulating brain function (Priori et al., 1998; Nitsche & Paulus, 2000; Priori, 2003; Paulus, 2004; Wassermann & Grafman, 2005). Weak electrical direct current (< 1.5 mA) applied over the scalp induces prolonged changes in brain excitability persisting long after the current has ceased (Nitsche & Paulus, 2001; Nitsche et al., 2003a). In particular, several studies, investigating changes in motor evoked potentials (MEPs) elicited by TMS, have shown that tDCS delivered over the motor cortex modulates corticospinal output (Priori et al., 1998; Nitsche & Paulus, 2000, 2001; Priori, 2003;Paulus, 2004; Ardolino et al., 2005). The after-effects of brain polarization on motor cortical excitability are due to both synaptic and nonsynaptic functional changes (Liebetanz et al., 2002; Ardolino et al., 2005).
In this study we investigated whether brain polarization modulates neuromuscular fatigue. To do so we evaluated the after-effects of anodal and cathodal tDCS over the motor areas of the cerebral cortex in healthy subjects on the endurance time (Et) for a submaximal isometric contraction of elbow flexors. We also assessed a group of control subjects without stimulation. Finally, in an additional experiment to investigate the mechanisms underlying the action of tDCS on fatigue, we delivered anodal tDCS and evaluated the changes in MEPs elicited by TMS.
Materials and methods
Subjects
A group of 24 healthy right-handed volunteers (14 women and 10 men, mean age 24.3 years) participated in the study. None of the subjects were engaged in competitive sport activities specifically involving elbow flexor muscles (i.e body-building). Before enrolment and after local ethical committee approval (Fondazione IRCCS Ospedale Maggiore Policlinico Mangiagalli Eregina Elena), the study protocol was explained to each subject and informed written consent was obtained. The experimental procedures were in accordance with the declaration of Helsinki.
Force measurement
Subjects sat in a chair with the left elbow on a padded support, the elbow joint at a right angle, and the wrist connected to a load cell (BC 302; DS Europe, Milan, Italy; Fig. 1A). The motor task required the subject to exert an upward-directed force with the wrist activating the elbow flexor muscles (biceps brachii, brachialis, coracobrachialis, and brachioradialis). To determine the maximal voluntary contraction (MVC) force, subjects were instructed to increase the force from zero to maximum and to hold it for 3 s. Subjects performed nine MVC trials subdivided in three sessions and rested for 60 s between successive trials. The mean of the best three performances was taken as the MVC force and used as the reference to calculate the target force for the fatiguing contraction.
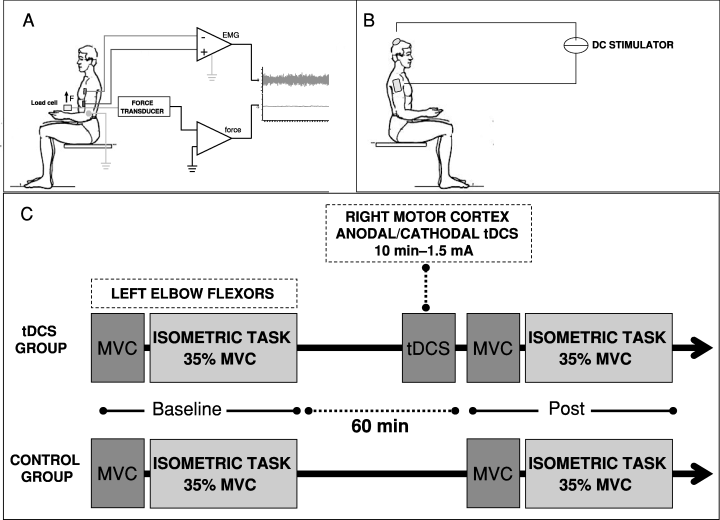
(A) Motor task. Subjects sat in a chair with the left elbow on a padded support, the elbow joint at a right angle and the wrist connected to a load cell. The motor task required the subject to exert an upward-directed force with the wrist using the elbow flexor muscles. (B) Technique used for tDCS. The direct current was applied between two electrodes, one on the scalp over the right motor cortex (4 cm lateral to the vertex) and the other above the right shoulder. tDCS polarity refers to the cephalic electrode. (C) Experimental design. Subjects were randomized into one of two groups: a ‘tDCS’ and a ‘control’ group. The ‘tDCS’ group underwent anodal and cathodal stimulation (1.5 mA, 10 min) over the right motor cortex. The study protocol comprised an assessment of the MVC force for the left elbow flexors and performance of a fatiguing isometric contraction (35% of MVC) before and immediately after brain polarization. One hour elapsed between baseline and postconditioning evaluation. Controls underwent a baseline evaluation of MVC and endurance time followed 1 h later by a second fatiguing contraction, without tDCS.
Muscle endurance was assessed as the time for which subjects could sustain an isometric contraction with the elbow flexor muscles at 35% of the MVC force (‘time to task failure’), previously determined. During the fatiguing task each subject received force feedback on a computer monitor showing a 35% MVC target. The subject was encouraged to keep the force at this level.
The contraction was terminated when the subject deviated from the target force (the subject remained below the force marker on the monitor) for > 3 s despite strong verbal encouragement.
The signal from the load cell (range: 0–60 kg; sensitivity, 2 mV/V; total error, < 0.5%; repeatability, < 0.1%) was preamplified and low-pass filtered (< 1000 Hz) with an analogue amplifier [Signal Conditioner Cambridge 1902; Cambridge Electronic Design (CED), Cambridge, England]. The output signal was digitized (Cambridge Micro 1402; CED) with a sampling rate of 2500 Hz and 12-bit quantization with 5 V range. The digitized signal was stored on a personal computer and displayed by the Spike2 software (version 5.11; CED).
Electromyographic (EMG) signal recording
The EMG, recorded during MVC and fatiguing contraction, was captured with surface electrodes positioned over the left biceps brachii with a belly tendon montage (Fig. 1A). The EMG signal was preamplified, band-pass filtered (2–1000 Hz), and differentially amplified (×1000) with an analogue amplifier (Signal Conditioner Cambridge 1902; CED). The output signal was digitized (Cambridge Micro 1402; CED), with a sampling rate of 2500 Hz and 12-bit quantization with 5 V range. The digitized signal was stored on a personal computer, displayed and analysed offline using Spike2 software (version 5.11; CED).
Transcranial magnetic stimulation and motor evoked potentials
TMS was delivered by a Novametrix Magstim 200 stimulator through a flat coil (outer diameter 13.5 cm) centred over the vertex with currents flowing clockwise (viewed from above). MEPs were recorded by standard nonpolarizable Ag–AgCl surface electrodes (diameter 9 mm; Meditec, San Paolo di Torrile, Parma, Italy) placed over the belly of the biceps brachii muscle and on the skin overlying the biceps' tendon of the left arm. The motor threshold was defined as the lowest intensity able to produce MEPs of 50 µV in five out of 10 consecutive trials of stimulation during a slight contraction of the biceps (5% of MVC). Stimulation intensity was 120% of the baseline MEP threshold. A total of 16 MEPs were recorded in response to 16 stimuli delivered at 0.15 Hz. The peak-to-peak amplitude was measured before and immediately after tDCS. The MEP signals were preamplified, band-passed from 20 Hz to 5 kHz, amplified, acquired and stored by a Nikolet Viking IV P.
Transcranial direct current stimulation
tDCS (1.5 mA, 10 min) was delivered by an electrical stimulator through a constant-current unit and an isolation unit (Priori et al., 1998) connected to a pair of electrodes, one on the scalp over the right motor cortex (4 cm lateral to the vertex) and the other above the right shoulder (Fig. 1B). Stimulating electrodes were thick (0.3 cm) square (35 cm2) pieces of saline-soaked synthetic sponge. To guarantee safety we applied current at a density of 0.043 mA/cm2 and delivered a total charge of 0.026 C/cm2. These criteria, in accordance with Nitsche et al. (2003b), are far below the threshold for tissue damage and have been used by others (Hummel & Cohen, 2005; Boggio et al., 2006; Fregni et al., 2006a). The wide electrode surface avoided the possible harmful effects of high current density. Apart from occasional, transient and short-lasting tingling and burning sensations below the electrodes, direct current stimulation strength remained below the conscious cutaneous sensory threshold throughout the experimental session. tDCS polarity (cathodal or anodal) refers to the electrode over the right motor area.
Experimental procedures
Experiment 1
Subjects were randomly assigned to one of two groups: a ‘brain polarization’ group (9 subjects: five women and four men) and a ‘control’ group (15 subjects: nine women and six men). The brain polarization group underwent anodal and cathodal tDCS. The two conditions were tested in random order and at least 1 week elapsed between sessions. The conditioning stimulus was a cathodal or anodal tDCS at 1.5 mA delivered for 10 min (0.026 C/cm2) over the right motor cortex. The subjects were blinded about conditioning stimulation. The ‘brain polarization’ protocol comprised an assessment of the MVC force (baseline MVC) for the left elbow flexors followed by a fatiguing contraction (35% of baseline MVC) before (baseline evaluation, T0) and immediately after (< 60 s) brain polarization (postconditioning evaluation, T1; Fig. 1C). One hour elapsed between baseline and postconditioning evaluation. Control subjects underwent a baseline evaluation (T0) of MVC (baseline MVC) and Et (35% of baseline MVC) followed, 1 h later, by a second evaluation (MVC and fatiguing contraction at 35% of baseline MVC), without a conditioning stimulus (T1).
Experiment 2
In six subjects (one woman and five men), TMS was delivered at near-threshold intensity (120% of the baseline MEP threshold) during an isometric contraction of the left biceps brachii at 5% of MVC. MEPs from biceps brachii were recorded before (T0) and immediately after (T1) anodal tDCS (1.5 mA, 10 min, 0.026 C/cm2). The force signal was monitored to ensure the same level of voluntary activation before and after tDCS.
Data and statistical analysis
Experiment 1
MVC (N) and Et (s) at T0 and T1 for the polarization group (anodal and cathodal tDCS) and controls are expressed throughout the text as mean ± SEM. For each subject we also calculated the normalized MVC (n-MVC) and normalized endurance time (n-Et) as (T1 − T0)/T0. In a preliminary analysis we used a one-way anova (NCSS Software, Number Cruncher Statistical Systems, Kayseville-Utah, USA) to compare baseline MVC and baseline Et values in anodal, cathodal and control groups. We then evaluated the effect of anodal and cathodal tDCS on MVC and Et. First, we performed a two-way anova on absolute data at T0 and T1 after log transform. The first main factor was time (two levels, T0 and T1; repeated measures) and the second main factor was stimulation (three levels: anodal tDCS, cathodal tDCS and no stimulation; independent measures). Second, a one-way anova was used to compare n-MVC and n-Et obtained with the different stimulation modalities with stimulation as the main factor (three levels: anodal tDCS, cathodal tDCS and no stimulation; independent measures). A Bonferroni-corrected unpaired t-test was used for post hoc comparison (P < 0.016).
EMG root mean square (RMS) amplitude values were calculated with a 0.5-s window during MVC tasks. The maximum RMS value at the peak of the nine MVC contractions was considered the representative RMS for MVC (MVC RMS). EMG RMS values during fatiguing tasks were calculated for consecutive 5-s time windows and were expressed as a percentage of MVC RMS. We estimated a linear fit of these values for each trial (PRISM software, v.3.2; GraphPad Sofware Inc., SA, CA, USA). The slope of the linear fit was used to quantify the increase in the EMG signal during fatiguing tasks whereas the y-intercept represented the muscle activation level at task starting point. Slope and y-intercept values were compared between the polarization group and the control group at T0 and T1. We performed a two-way anova with time as first main factor (two levels: T0 and T1; repeated measures) and stimulation as second main factor (three levels: anodal tDCS, cathodal tDCS and no stimulation; independent measures). Tukey–Kramer test for a two-factor interaction was used for post hoc analysis (P < 0.05).
Experiment 2
Peak-to-peak MEP amplitudes (mV) were calculated for T0 and T1 and are expressed throughout the text as mean ± SEM. The Wilcoxon signed-rank test was used to compare values at baseline and after anodal tDCS (P < 0.05).
Results
None of the subjects complained of adverse reactions to tDCS.
Experiment 1
We found no significant differences in baseline MVC and Et between anodal, cathodal and controls (Et: P = 0.317; cathodal tDCS, 261.88 ± 18.9; anodal tDCS, 258.22 ± 22.76; controls, 226.47 ± 14.91 s; MVC: P = 0.73; cathodal tDCS, 156 ± 18; anodal tDCS, 154.4 ± 18.07; controls, 148.8 ± 6.64 N).
The two-way anova on MVC disclosed a significant effect of the factor time (P < 0.0001), baseline MVC being significantly higher than MVC evaluated after stimulation (T0, 152.29 ± 7.44 vs. T1, 140.18 ± 7.12 N). Neither MVC nor n-MVC were significantly affected by the factor stimulation (MVC: P = 0.9; anodal tDCS: T0, 154.4 ± 18.07; T1, 142.8 ± 16.62 N; cathodal tDCS: T0, 156 ± 18.75; T1, 141.86 ± 17.53 N; controls: T0, 148.8 ± 6.64; T1, 137.6 ± 7.36 N; n-MVC: P = 0.77; Anodal tDCS, −7.11 ± 2.36; cathodal tDCS, −9.44 ± 1.51; controls, −7.86 ± 2.09%) (Fig. 2A).
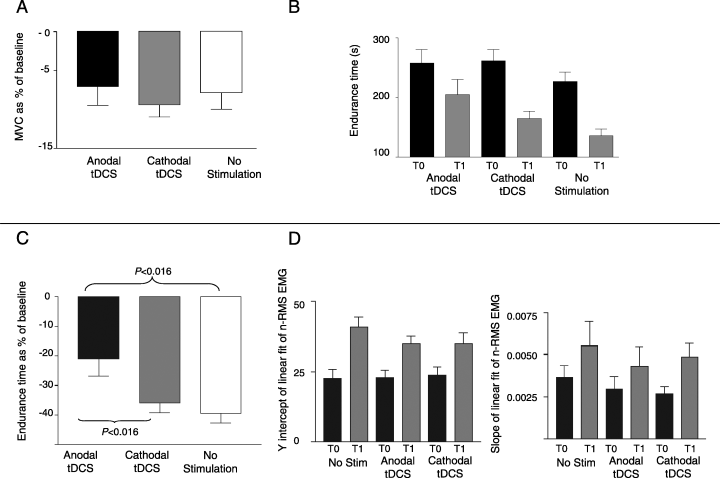
(A) Effect of tDCS on normalized maximal voluntary contraction (n-MVC) represented on the y-axis. n-MVC was not significantly affected by tDCS (P > 0.05). (B) Effect of tDCS on endurance time (Et, y-axis). At postconditioning evaluation Et was significantly reduced from baseline after cathodal tDCS and after no stimulation but not after anodal tDCS. Error bars are SEM. (t-test, P < 0.016) (C) Effect of tDCS on normalized endurance time (n-Et, y-axis). Anodal tDCS induced a significantly lower decrease in Et at 1 h than did cathodal tDCS or no stimulation (t-test, P < 0.016). (D) Effect of tDCS on electromyographic signals (EMG). Y-intercept (left) and slope (right) of liner fit of normalized EMG RMS amplitude values were significantly higher at T1 than at T0 for cathodal tDCS, anodal tDCS and no stimulation (P < 0.05), with no differences due to the factor ‘stimulation’.
The two-way anova disclosed a significant effect of time (P < 0.0001) on Et, Et being significantly higher at T1 than at baseline for all groups (cathodal tDCS: T0, 261.88 ± 18.9; T1, 165.44 ± 12.08 s; anodal tDCS: T0, 258.22 ± 22.76; T1, 205.22 ± 24.92 s; controls: T0, 226.47 ± 14.91; T1, 136.13 ± 14.91 s; 2, 3). Whereas the factor stimulation was not significant (P = 0.071), the stimulation × time interaction was significant (P = 0.019). Although no difference was found between n-Et values at 1 h after cathodal tDCS and no stimulation (−35.77 ± 3.39 vs. −39.33 ± 3.32%; P = 0.53), anodal tDCS induced a significantly smaller decrease in Et at 1 h than did no stimulation (−21.11 ± 5.52%; P = 0.0029) and cathodal tDCS (P = 0.015; Fig. 2C).
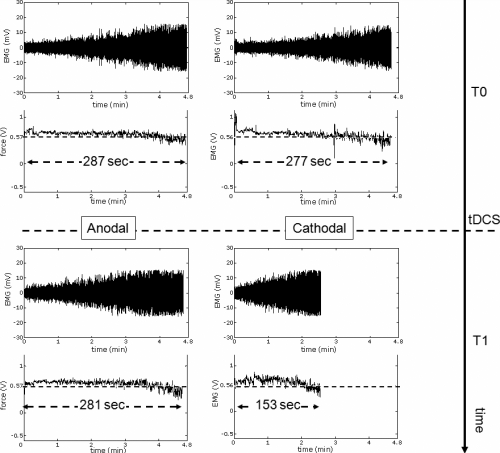
Raw force and EMG signals from a representative subject before and after tDCS. Top panels are (top trace) the EMG and (bottom trace) the force at the baseline evaluation before anodal (left) and cathodal (right) tDCS. The bottom panel on the left is after anodal tDCS and the bottom panel on the right is after cathodal tDCS. The force signal is in V before the conversion into N. Note the relatively prolonged endurance time after anodal tDCS (bottom left trace) than after cathodal tDCS (bottom right trace).
EMG slope and y-intercept were significantly higher at T1 than at T0 for both the polarization and the control group (anova main factor time: slope, P = 0.0002; y-intercept, P < 0.00001). The two-factor anova disclosed no significant differences due to either the factor ‘stimulation’ or the time × stimulation interaction (Fig. 2D).
Experiment 2
After the end of anodal tDCS the amplitude of MEPs during a slight isometric biceps brachii contraction (5% of MVC) increased significantly from baseline (T0, 1.26 ± 0.1; T1, 1.59 ± 0.12 mV; P = 0.0156; Fig. 4).
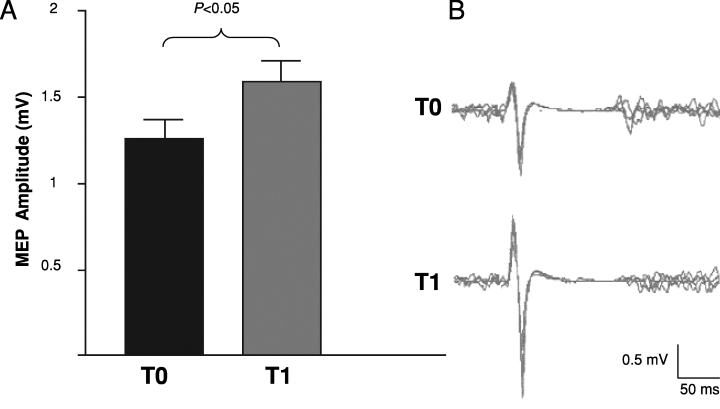
(A) Effect of tDCS on motor evoked potential (MEP) amplitude elicited by transcranial magnetic stimulation (TMS). After the end of anodal tDCS the amplitude of MEPs (peak-to-peak, mV) during a slight isometric biceps brachialis contraction (5% of MVC) increased significantly from baseline (Wilcoxon signed-rank test, P < 0.05). (B) MEP recording from a representative subject showing the increase in MEP amplitude immediately after the end of anodal tDCS. Each trace is the superimposition of eight sweeps. T0, baseline evaluation; T1, 0 min after anodal tDCS.
Discussion
Our findings in healthy subjects show that anodal tDCS delivered over the cortical motor areas induced a relative increase in the flexor muscles' Et for a sustained submaximal isometric contraction: at the postconditioning evaluation (1 h after the baseline fatiguing task), the expected shortening of the Et owing to residual tiredness was reduced by ∼15% after anodal tDCS compared with cathodal tDCS and with no stimulation, showing that brain polarization can modulate neuromuscular fatigue.
Our data are in line with recent data demonstrating that anodal tDCS delivered over the motor cortex improves hand function in healthy subjects (Boggio et al., 2006) and in patients with stroke (Hummel & Cohen, 2005). Benwell et al. (2006) have shown that paired-pulse rTMS can modulate the rate of force loss in a brief MVC task of the hand, suggesting that the rTMS-induced manipulation of cortical excitability improves muscle force.
Studies using TMS and TES have reported exercise-dependent changes in motor cortex excitability. During and immediately after brief or fatiguing exercise, whereas motor responses to TMS increase in amplitude (Brasil-Neto et al., 1993; Samii et al., 1996), those elicited by TES decrease in size (Modugno et al., 1998; Gandevia et al., 1999). These data suggest a motor cortical facilitation related to voluntary contraction which offsets alpha-motoneuron relative inhibition. The increased cortical excitability outlasts the fatiguing effort by a few minutes (postcontraction facilitation) and is followed by a depression of the MEPs (postcontraction depression) not related to spinal motoneuron inhibition (Brasil-Neto et al., 1993). Further support for the existence of focal cortical changes related to central fatigue comes from studies on the cortical silent period. During prolonged MVC or submaximal efforts the silent period which follows the motor response evoked by TMS progressively lengthens (Taylor et al., 1996; Taylor et al., 1999). This phenomenon has been interpreted as an increase in local intracortical inhibition. Because the relationship between these cortical events and the development of supraspinal fatigue and task failure is unclear, some have suggested that changes in cortical excitability are epiphenomena and not causal factors in motor fatigue (Gandevia et al., 1996; Gandevia, 2001). Our findings on the relative Et prolongation induced by anodal tDCS of motor cortex support the hypothesis that manipulation of cortical function by tDCS may play a role in determining task failure during sustained effort.
The Et changes we observed after anodal tDCS could have several explanations. The most obvious is that anodal tDCS could modulate supraspinal fatigue by balancing the aforementioned changes in motor cortex excitability. Anodal and cathodal scalp tDCS induce well-known changes in the MEP size (Nitsche & Paulus, 2000; Priori, 2003) and TMS motor threshold (Ardolino et al., 2005). Because we used a different electrode setup (motor cortex–right deltoid) from previous studies, to evaluate the effect of anodal tDCS on motor cortex excitability we conducted an additional experiment. We found that the amplitude of MEPs evoked by TMS had increased by nearly 30% at the end of anodal stimulation. This result is similar to that observed by others using the ‘motor cortex–contralateral forehead’ setup (Liebetanz et al., 2002; Lang et al., 2005), and confirms an increase in motor cortex excitability related to anodal polarization. Hence, anodal tDCS may increase supraspinal drive by inducing a prolonged facilitation of corticospinal neurons, thereby prolonging Et.
As expected for the presence of a residual tiredness due to the basal fatiguing task, EMG slope and y-intercept were significantly higher at postconditioning evaluation for both the polarization and the control group but statistical analysis did not show any differences between groups related to the factor ‘stimulation’. Why surface EMG variables in biceps brachii remained unchanged despite the Et prolongation remains unclear. First, the evaluated EMG parameters may be too insensitive to detect subtle changes in central recruitment of spinal motorneurons. Second, the effect of anodal tDCS on the force endurance may arise not from a postsynaptic effect on corticomotoneuronal projections but could be related to a presynaptic effect on the motor cortex interneuronal network. Moreover, we cannot rule out possible concomitant mechanisms involving cortical areas neighbouring the active cephalic electrode. In this regard, imaging studies showed that polarization of motor cortex induces widespread activation changes (Baudewig et al., 2001; Lang et al., 2005). The decline in voluntary activation during prolonged effort is thought to be related to ‘upstream’ failure of the motor cortical neuron (Gandevia et al., 1996; Gandevia, 2001; Taylor et al., 2006). Furthermore, brain activation measured by functional magnetic resonance imaging during a sustained maximal contraction shows an initial increase followed by a significant reduction, seen not only in the primary sensorimotor areas but also in the secondary and association cortices (Liu et al., 2002). Hence, t-DCS might act by modulating nonprimary premotor motor areas. A further possibility is that the prolonged Et may be related to the tDCS-induced modulation of the feedback inhibitory systems limiting motor cortical output to ‘protect’ the motor system from overload. tDCS of the motor cortex has been reported to decrease pain (Fregni et al., 2006a) and pain afferent input from muscle tissue is probably involved in muscle fatigue (Gandevia et al., 1996; Gandevia, 2001). The increased Et after anodal tDCS could therefore depend on a reduced pain sensation arising from muscle tissue during sustained and prolonged voluntary contraction. A similar mechanism is involved in the well-known ergogenic effects of caffeine during prolonged efforts (Plaskett & Cafarelli, 2001). Another possible mechanism is increased voluntary activation. Because tDCS delivered over the dorsolateral prefrontal cortex improves depression (Fregni et al., 2006b), although none of our subjects reported consistent mood changes or euphoria after brain polarization, tDCS could increase motivation and the voluntary drive for sustained and prolonged muscle contraction. Finally, because tDCS induces persistent increases in intermuscular coherence (Power et al., 2006), the prolonged Et could also arise from a more optimal coupling of synergistic flexor muscles (biceps brachii, brachialis, coracobrachialis and brachioradialis).
The net effect (increased or reduced excitability) produced by tDCS depends on several technical issues including the relative position of the reference electrode as well as the orientation of excitable tissue with respect to the electrical field (Priori, 2003). In this study, we used an extracephalic reference electrode positioned on the right deltoid (Priori et al., 2007). This experimental setup differs from that used in previous works (Nitsche & Paulus, 2000; Priori, 2003; Ardolino et al., 2005; Hummel & Cohen, 2005; Boggio et al., 2006). Modelling studies suggested that current flow is localized beneath the stimulating electrodes, both the anode and the cathode (Nathan et al., 1993; Miranda et al., 2006). Thus, anodal tDCS of one cortical area is combined with cathodal stimulation of another, and vice versa (Nitsche et al., 2007). Using the conventional ‘motor cortex–contralateral forehead’ setup with a complex motor behaviour such as a fatiguing task would generate confusion regarding the source of the observed effect as it could originate from the active as well as the cephalic reference electrode. In this regard, a single cephalic electrode on the scalp helps to resolve this ambiguity, as the observed aftereffects are unlikely to be related to the extracephalic reference electrode.
Among critical technical points to consider in choosing the extracephalic electrode setup are safety criteria and possible charge flow to brainstem (Nitsche et al., 2003b). Given our stimulating dipole geometry, we cannot a priori exclude brainstem involvement. Nevertheless, this possibility seems unlikely for several reasons. First, in accordance with the results reported by Accornero et al. (2007) our experimental setup left heart rate and body temperature unchanged and none of our subjects showed signs or symptoms attributable to the modulation of brainstem activity. Second, most of the current delivered by transcranial stimulation gets shunted through the scalp and the current density delivered to the cortex by the stimulation decreases rapidly with depth (Nathan et al., 1993). Lippold & Redfearn (1964) reported one case of disturbed breathing, speech arrest and psychosis possibly related to brainstem excitability changes after direct current stimulation using an extracephalic electrode setup (active electrodes above each eyebrow and reference electrode above the right knee). Nonetheless, these authors applied battery-delivered small currents (100–500 µA) for long time periods (up to 5 h), delivering values of total charge a thousand-fold higher than in our experiments. Moreover, previous experiences on humans during surgery demonstrated that for eliciting a motor evoked potential by directly stimulating the human brainstem a current density of ∼ 2–9 mA/cm2 is required (Cedzich et al., 1998); this is far above the current density we delivered.
In conclusion, anodal tDCS applied over the right motor cortical areas prolonged the Et for contralateral elbow flexors in a submaximal isometric task, thus showing that brain polarization can modulate human neuromuscular fatigue. Anodal tDCS could improve the Et through several different mechanisms, for example by increasing motor cortical excitability, acting on premotor areas, decreasing fatigue-related muscle pain, inducing behavioural disinhibition and increasing motivation or improving synergist muscle coupling. Our observations open the possibility of improving muscle performance and decreasing muscle fatigue in normal (i.e. sports medicine) and pathological (i.e. stroke and primary or secondary chronic fatigue syndrome, rehabilitation) conditions.
Acknowledgements
The authors wish to thank Mr Vito Antonio Di Pace for building the experimental apparatus, Lorenzo Rossi, PhD, for setting the experimental instrumentation, and Dr Simona Mrakic-Sposta, Dr Ettore Accolla and Dr Francesca Mameli for their helpful cooperation. S.M. is a PhD student supported by Fondazione IRCCS Ospedale Maggiore, Policlinico, Mangiagalli and Regina Elena di Milano (Italy) at the Dipartimento di Bioingegneria, Politecnico di Milano.
Abbreviations
-
- Et
-
- endurance time
-
- MVC
-
- maximal voluntary contraction
-
- MEP
-
- motor evoked potential
-
- n-Et
-
- normalized endurance time
-
- n-MVC
-
- normalized maximal voluntary contraction
-
- RMS
-
- root mean square
-
- T0
-
- baseline evaluation
-
- T1
-
- postconditioning evaluation
-
- tDCS
-
- transcranial direct current stimulation
-
- TES
-
- transcranial electrical stimulation
-
- TMS
-
- transcranial magnetic stimulation