The effects of fear conditioning on cerebellar LTP and LTD
Abstract
Long-term potentiation (LTP) and depression (LTD) at parallel fibre–Purkinje cell synapses have been described in vitro in the cerebellar cortex, but the physiological roles of these two forms of plasticity have not been well defined. Here we show that, in cerebellar slices taken from rats that had undergone fear conditioning, there was a significant occlusion of electrically induced LTP at parallel fibre–Purkinje cell synapses. This effect was long-lasting and related to associative processes, as LTP was not occluded in unpaired animals. Notably, in conditioned animals the LTP-inducing protocol produced LTD in some cells instead of LTP. Conversely, synaptic depression induced by conjunctive stimulation of parallel fibers and climbing fibres was impaired in tissue taken immediately following aversive stimulation in both paired and unpaired subjects. This effect was not, however, long-lasting as the incidence and extent of LTD returned to normal levels 24 h after behavioural testing. These findings suggest that LTP takes part in the mechanisms underlying aversive associative memories in the cerebellum.
Introduction
Recent studies indicate that the cerebellum plays an important role in aversive memory (see Sacchetti et al., 2005). Changes in heart rate induced by repeated pairing of an acoustic neutral stimulus (conditioned stimulus; CS) with an aversive one (unconditioned stimulus; US) are hampered by a vermal lesion performed either before or after conditioning (Supple & Leaton, 1990; Supple & Kapp, 1993). Similar results are observed in patients with medial cerebellar lesions (Maschke et al., 2002). In humans, the presentation of coloured lights that signal in advance of a painful stimulation correlates with an increased cerebellar activity (Ploghaus et al., 1999, 2000). More recently, it has been shown that fear learning is associated with a long-term potentiation (LTP) at parallel fibre (PF)–Purkinje cell (PC) synapses (Sacchetti et al., 2004). This synaptic locus is involved in emotional learning as, in hotfoot mice, which have a primary deficiency of the PF-PC synapse, fear memories are affected (Sacchetti et al., 2004). The fear-induced potentiation was postsynaptic in nature and related to associative processes, as it was present in subjects that received CS and US in a temporally paired way but not in those receiving the same two stimuli separately. Recently, it has been found that a form of postsynaptic LTP can be induced in vitro at PF-PC synapse (Lev-Ram et al., 2003; Coesmans et al., 2004). Therefore, it may be that fear- and electrically induced LTP share common mechanisms, i.e. the synaptic strengthening that occurs in the cerebellum during fear learning may engage signal transduction pathways that are in common with those activated by electrical stimulation.
In order to examine whether fear- and electrically induced LTP share common mechanisms, we tested their interaction by eliciting LTP electrically in animals previously submitted to behavioural training. By using this approach it has previously been demonstrated that electrically induced LTP is occluded after learned fear in the hippocampus (Sacchetti et al., 2001, 2002b) and amygdala (Tsvetkov et al., 2002).
Since the theoretical papers by Marr and Albus (Marr, 1969; Albus, 1971), it has been proposed that long-term depression (LTD) induced at PF-PCs by paired stimulation of PFs and the climbing fibre (CF) represent the neuronal correlate of motor learning (Marr, 1969; Albus, 1971; Ito, 1984; Kim & Thompson, 1997; Hansel et al., 2001). In the light of a recent study which showed that in vitro PF-PC LTP can be reversed by the electrical induction of LTD and vice versa (Coesmans et al., 2004; Boyden et al., 2004; Jorntell & Hansel, 2006), we investigated whether fear-induced LTP could be reversed by the electrical induction of LTD. Moreover, by means of the presentation of paired CS and US, we studied the relationships between associative learning and electrically induced LTD.
Materials and methods
Behavioural procedures
Behavioural and electrophysiological studies were performed at postnatal days 15–17 on Wistar rats (Harlan, Verona, Italy), in which the cerebellar circuitry has already achieved its final architecture at qualitative but not quantitative levels (Ito, 2001). At this age, postsynaptic LTP and LTD can be electrically induced (Sims & Hartell, 2006) and fear conditioning is already present (Sacchetti et al., 2004). Indeed, as the dendrites develop, voltage-clamp recordings become less reliable because of dendritic filtering and space-clamp error.
As in our previous study (Sacchetti et al., 2004), animals were randomly divided into four behavioural groups. The first two groups underwent Pavlovian fear conditioning training (conditioned animals). The rats were placed in a basic Skinner box module (Coulbourn Instruments, Allentown, PA, USA) and left undisturbed for 1 min and 24 s. A training session consisting of seven presentations (with intervals of 30 s) of tone (7 s, 1000 Hz, 70 dB; CS), coterminating with an electric footshock (2 s, 1 mA; US), was then delivered to the animals. The third group of rats (unpaired animals) received tone (1000 Hz, 70 dB) continuously for 40 s. Immediately afterwards the animals were placed back in their home cages. After at least 45 min, the animals were put in a different apparatus where they immediately received seven presentations of footshock, each of 1 s with < 1 s interval. This procedure was designed to make it difficult for the animals to associate the footshock with the tone and the environment (Sacchetti et al., 2004). The fourth group (naïve animals) were always kept in the home cages before surgery.
Fear retention was evaluated by measuring freezing, the expression of fear behaviour (Sacchetti et al., 1999, 2002a, 2004), during the administration of the CS alone at 10 min and 24 h after the acquisition session or after the same time lapse in the home cage for the naïve animals. Freezing (immobility) was defined as the complete absence of somatic mobility except for respiratory movements. The subjects were placed inside the conditioning apparatus and left there for 2 min and 24 s. Thereafter, a series of seven acoustic stimuli (CS) were administered, identical to those used during the acquisition session. All behavioural procedures were performed between 10.00 and 12.00 h to minimize circadian influence.
Slice preparation
Cerebellar slices were prepared 10 min or 24 h after the final acquisition session from the conditioned, unpaired and naïve subject groups. The rats were anaesthetized by inhalation of isoflurane (Rhodia Organique Fine Ltd, Bristol, UK) and decapitated. The cerebellar vermis was immediately removed and chilled rapidly in 95% O2- and 5% CO2-saturated ice-cold artificial cerebrospinal fluid (ACSF; in mm: NaCl, 120; KCl, 2.7; NaH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; NaHCO3, 25; and glucose, 20). Parasagittal slices (200 µm thick) were cut on a vibratome (Vibroslice 752; Campden Instruments, Loughborough, UK) and left to recover for 1 h in 95% O2- and 5% CO2-bubbled ACSF at 25 °C. One slice at a time was transferred to a recording chamber and constantly superfused with 95% O2- and 5% CO2-saturated ACSF at a rate of 2 mL/min at room temperature.
Electrophysiological recordings
Whole-cell patch-clamp recording was performed from PC soma as previously described (Zhu et al., 2006) on lobules V-VI (Fig. 1A), where we previously demonstrated fear-induced LTP at PF-PC synapses (Sacchetti et al., 2004). The experimental setup consisted of an upright microscope (Axioskope, Zeiss, Germany) with a 40× water-immersion objective and an EPC9 patch-clamp amplifier (HEKA Elektronik, Lambrecht, Germany). Cells were held in voltage-clamp mode at a holding potential of −70 mV. Signals were low-pass-filtered at 3 kHz and sampled at 10 kHz. PFs were stimulated with 100-µs pulses delivered by an isolated pulse stimulator (A-M Systems, Washington, USA) through a glass pipette filled with ACSF placed in the external half of the molecular layer (Fig. 1B). The baseline stimulation rate was 0.1 Hz. Excitatory postsynaptic currents (EPSCs) evoked by PF stimuli were recorded. Bicuculline (20 µm) was applied in the superfusate to inhibit GABAergic activity. To establish the pre- or postsynaptic origin of changes in synaptic strength, pairs of pulses separated by 100 ms were delivered and the paired-pulse ratio (PPR) of the second to the first EPSC amplitude was calculated.
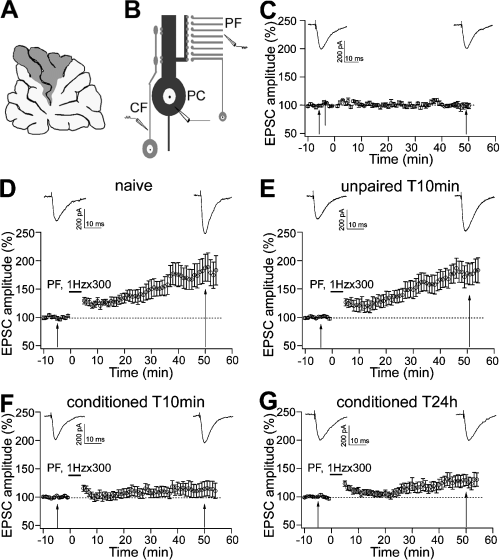
Electrically induced LTP was occluded after learned fear. (A) Electrophysiological recordings were performed on lobules V and VI (grey area) of cerebellar vermis. (B) PC with two excitatory inputs, PF and CF, and electrophysiological experimental scheme. (C) Basal stimuli delivered at 0.1 Hz gave rise to constant amplitude PF EPSCs. (D and E) LTP was induced by 300 PF stimuli at 1 Hz (horizontal bar) in naïve and unpaired T10min groups. (F and G) LTP induced by the same protocol is reduced in conditioned T10min and conditioned T24h groups. Representative traces in C–G were taken 5 min before and 50 min after the repetitive stimulation. Data are expressed as mean ± SEM.
For LTP experiments, patch pipettes (3–4 MΩ) pulled from borosilicate capillary were filled with an intracellular solution containing (in mm): K-gluconate, 110; NaCl, 8; MgCl2, 2; 1,2-bis- (2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA), 10; HEPES, 30; Na2ATP, 4; and Na3GTP, 0.3; pH was adjusted to 7.3 using KOH. When a 10-min stable baseline of PF EPSCs was achieved, an LTP induction protocol consisting of 1 Hz stimulation for 5 min (Lev-Ram et al., 2002) was delivered to PFs. Stimulation was resumed at the baseline frequency of 0.1 Hz for a further 50 min.
For LTD experiments, the intracellular solution consisted of (in mm): K-gluconate, 130; NaCl, 8; MgCl2, 2; EGTA, 0.5; HEPES, 30; Na2ATP, 4; and Na3GTP, 0.3; pH was adjusted to 7.3 with KOH. An additional ACSF-filled pipette was placed in the granular layer, positioned close to the PC soma to recruit the CF input (Fig. 1B). A stimulus isolator (A.M.P.I, Jerusalem, Israel) was used to activate the CF. Once a 10-min period of stable EPSC baseline was achieved, cells were switched from voltage-clamp to current-clamp mode and held at −70 mV. CF and PF inputs were activated conjunctively at 1 Hz for 5 min (Karachot et al., 1994). Following the induction protocol, PCs were switched back to voltage-clamp mode and PFs were activated at baseline rate for the remainder of the recording (50 min).
An 80-ms, −4 mV test hyperpolarizing pulse preceding each PF stimulus was delivered to monitor the series and input resistances of the PC throughout experiments. Series resistance and input resistance were evaluated by measuring the negative peak amplitude and the steady-state amplitude, respectively, from the response to the preceding pulse. Recordings were discarded from the analysis if the leak current exceeded −500 pA or if the input resistance changed significantly or if the series resistance changed by > 20%. Normalized EPSC amplitudes were calculated every 1 min by averaging six successive EPSC amplitudes and expressed as a percentage of the mean baseline response. Normalized PPRs were calculated in the same way.
Data were acquired using Pulse software (HEKA Elektronik, Lambrecht, Germany) and analysed offline with the program Igor Pro (Wavemetrics, Lake Oswego, USA). Reported values are mean ± SEM. Statistical tests for data group comparisons were one-way and two-way repeated-measure anova.
The experimental plan was designed according to the European Community Council Directive of 24 November 1986 (86/609/EEC) for care and use of experimental animals and approved by the Bioethical Committee of the University of Turin.
Results
Electrically induced LTP was occluded in fear-conditioned animals
In order to examine whether the involvement of the cerebellum in fear learning shares common mechanisms with those of electrically induced LTP, we employed the same learning paradigm that, in our previous study, induced LTP at PF-PC synapses (Sacchetti et al., 2004). As in that study, the subjects were divided in three groups: naïve animals that received no training, conditioned animals that received a pairing of CS and US, and unpaired subjects that received CS and US separated from each other.
As in our previous studies (Sacchetti et al., 2004; Zhu et al., 2006), fear retention was evaluated by measuring freezing response 10 min or 24 h after the acquisition trial. At both time intervals, in the fear-conditioned rats the periods of immobility, expressed as percentage of the total time, were increased relative to the unpaired and naïve groups (P < 0.005 in all instances; one-way anova and Newman–Keuls post-test; see Table 1).
| Group | Freezing responses (%) | n |
|---|---|---|
| Naïve | 34.8 ± 6.4 | 16 |
| UnpT10min | 43.0 ± 7.5 | 17 |
| CondT10min | 85.0 ± 4.7 | 15 |
| CondT24h | 80.1 ± 5.2 | 16 |
- Data are presented as means ± SEM. n = number of animals in the group.
In other naïve, unpaired and conditioned animals, we prepared parasagittal slices from the vermis of the cerebellum at 10 min and 24 h after the behavioural procedures. In order to electrically induce LTP, we employed an LTP induction protocol (300 PF stimuli at 1 Hz) which has been shown to produce in vitro LTP of postsynaptic origin (Lev-Ram et al., 2002, 2003; Coesmans et al., 2004; Kakegawa & Yuzaki, 2005; Sims & Hartell, 2006). In accordance with these studies, we used a K+-based solution containing BAPTA in order to chelate intracellular Ca2+. BAPTA was added to the internal solution to maximize the probability of LTP induction (Lev-Ram et al., 2002). PF stimulus intensities were limited to obtain PC EPSCs with amplitudes in the range between 100 and 250 pA in order to reduce the likelihood of postsynaptic Ca2+ entry. Previous studies have shown that raised intensities of PF stimulation at rate of 1 Hz result in LTD instead of LTP, due to the large entry of Ca2+ (Hartell, 1996). Prior to testing the effects of the LTP protocol, responses to stimulation at the baseline rate of 0.1 Hz were examined in a separate group of five cells. At this frequency, no change in the EPSC amplitude was observed over a period of 60 min (Fig. 1C). Therefore, neither the baseline stimulus parameters nor the patch-clamp conditions significantly affected PF-PC responses over this time period.
Figure 1D–G shows the effects of 1 Hz stimulation on PF EPSCs in naïve, unpaired and conditioned animals. One-way anova showed no differences among the absolute EPSC amplitudes of the tested groups measured 5 min before the repetitive stimulation (F3,67 = 2.406, P = 0.075; see Table 3). Therefore, by adjusting PF stimulus intensities we were able to obtain EPSC responses with similar amplitudes in the different behavioural groups. (It should be pointed out that the experimental procedure we employed in the present study is quite different from that used in our previous work: Sacchetti et al., 2004). In that study, by using identical stimulus intensities among the different behavioural groups we were able to observe an increase in the amplitude of the PF response in the conditioned subjects (Sacchetti et al., 2004) Then, EPSC amplitudes expressed as a percentage of baseline responses measured 50 min after the 1-Hz stimulation were compared among groups. Repeated-measures two-way anova (4 × 2), with four behavioural groups as between-subjects variable and two time intervals as within-subject variable, revealed significant difference in EPSC amplitude among the four behavioural groups (F3,67 = 4.453, P = 0.006) and between the two time intervals (F1,67 = 38.89, P < 0.001). There were also significant interactions between groups and time intervals (F3,67 = 4.984, P = 0.004). The Newman–Keuls test showed no significant difference between naïve and unpaired T10min groups (P > 0.05; Fig. 1D and E), and between conditioned T10min and conditioned T24h groups (P > 0.05; Fig. 1F and G). However, in the latter two conditioned groups EPSC amplitude was smaller than that in the former two groups (P < 0.05; see Table 3). These results indicate that fear learning affects electrically induced LTP. This effect is long lasting as it was still present 24 h after the acquisition (Fig. 1G), and it is strictly related to associative processes as it was not present in the unpaired group. It should be noted that the lack of effects in unpaired subjects demonstrates that arousal and/or stress processes did not significantly affect electrically induced LTP.
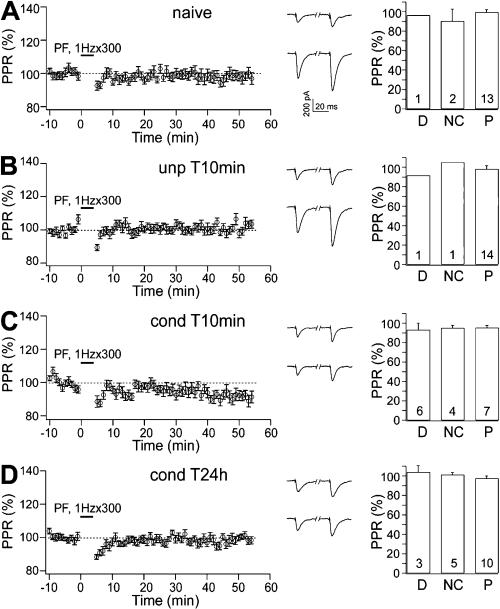
Fear- and electrically induced LTP were postsynaptic in origin. (A–D) The pre- or postsynaptic nature of LTP was tested by analysing PPR in (A) naïve, (B) unpaired T10min, (C) conditioned T10min and (D) conditioned T24h groups. (Left panels) PPR was analysed before and after 1 Hz stimulation. PPRs were unchanged in all the behavioural groups. (Centre panels) Representative traces of EPSCs obtained by paired PF stimuli with 100-ms intervals were taken 5 min before (upper) and 50 min after (lower) the repetitive stimulation. (Right panels) In the same behavioural groups, PPR was separately analysed for the cells that showed depression (D), no change (NC) or potentiation (P). PPRs were unchanged in all these cells. Numbers in the bars indicate the number of cells.
Fear conditioning shifted the direction of synaptic plasticity induced by 1-Hz stimuli
The data displayed in Fig. 1 are averaged values and therefore they do not provide information on the behaviour of individual neurons within each group. In order to better understand the distribution of the synaptic strength after LTP induction in terms of individual cells, we separately analysed the frequency distribution of the change in direction of EPSC amplitude. Cells were sorted into three groups; ‘not changed’, ‘depressed’ and ‘potentiated’, according to whether EPSC amplitudes changed < 10%, decreased to < 90%, or increased to > 110% of baseline responses, respectively (Crepel & Jaillard, 1991) (Fig. 2A–D and Table 2).

Fear conditioning shifted the direction of synaptic plasticity induced by 1 Hz stimulation. (A–D) Distribution of synaptic strengths measured 50 min after the delivery of 1 Hz stimuli in (A) naïve, (B) unpaired T10min, (C) conditioned T10min and (D) conditioned T24h groups. (Left panels) Cell number in the four behavioural groups is plotted against the normalized EPSC amplitude (% of baseline). (Right panels) On the basis of the changes in EPSC amplitude, cells were sorted into three groups: ‘depressed’ (D), ‘not changed’ (NC) and ‘potentiated’ (P). In the conditioned groups there was both a decrease in potentiation and an increase in depression.
| Group | LTP experiment | LTD experiment | ||||
|---|---|---|---|---|---|---|
| D (%) | NC (%) | P (%) | D (%) | NC (%) | P (%) | |
| Naïve | 6 | 13 | 81 | 71 | 12 | 17 |
| UnpT10min | 6 | 11 | 87 | 35 | 24 | 41 |
| CondT10min | 35 | 24 | 41 | 39 | 22 | 39 |
| CondT24h | 17 | 27 | 56 | 69 | 22 | 9 |
- D, depressed; NC, not changed; P, potentiated.
Fifty minutes after the delivery of the 1-Hz stimulation, EPSC amplitude distributions were similar for naïve and unpaired animals (Fig. 2A and B) with 81 and 87%, respectively, of the neurons being potentiated. Conditioned T10min and T24h groups showed similar amplitude distributions to each other, but only 41 and 56% of cells were potentiated (Fig. 2C and D). These values were much lower than in the naïve and unconditioned groups. In both naïve and unpaired groups, 6% of cells showed depression (Fig. 2A and B). The percentage of depressed cells was increased to 35 and 17% in conditioned T10min and T24h groups (Fig. 2C and D). Therefore, in conditioned animals the overall decrease in the level of potentiation represents not just a decline in the extent of potentiation in individual cells but also an increase in the proportion of cells that underwent a reversal in the direction of plasticity in favour of depression (Table 2).
Fear- and electrically induced LTP were both mediated by postsynaptic mechanisms
Two forms of LTP, pre- and postsynaptic in terms of the site of induction and expression, have been identified in vitro at PF-PC synapses (Salin et al., 1996; Lev-Ram et al., 2002; Boyden et al., 2004; Jorntell & Hansel, 2006). On the other hands, fear-induced LTP is postsynaptic in origin (Sacchetti et al., 2004). In fact, the PPR of PF-PC responses is unchanged after fear conditioning. PF-PC EPSCs are characterized by paired-pulse facilitation (Konnerth et al., 1990), which is attributed to residual Ca2+ in the presynaptic terminal that facilitates transmitter release. Changes in the PPR associated with changes in synaptic strength suggest the involvement of a presynaptic component (Zucker, 1989). An unchanged PPR indicates a postsynaptic origin. However, there is a specific caveat about the application of PPR analysis at the PF-PC synapse as PF-PC EPSCs with multiple peaks (polyphasic EPSCs) can be observed in slices from animals older than 15 days (Isope et al., 2004). Therefore, in our previous study, in addition to PPR we analysed asynchronous quantal events evoked by stimulating PF-PC synapses in the presence of Sr2+ (Sacchetti et al., 2004). This analysis confirmed the postsynaptic nature of the LTP induced by fear conditioning. However, by using a molecular approach it has been demonstrated that the stimulation of PF at 1 Hz induced LTP that has no significant presynaptic component (Lev-Ram et al., 2002; Kakegawa & Yuzaki, 2005), in line with PPR data (Lev-Ram et al., 2002). Therefore, in the present study we employed PPR to verify that under our experimental conditions the mechanisms that mediate these two forms of potentiation have no presynaptic components. Two-way repeated-measures anova (4 × 2), with four behavioural groups as between-subjects variable and two time intervals as within-subject variable, showed no significant differences among the four groups (F3,67 = 1.276, P = 0.289) and between the two time intervals (F1,67 = 1.525, P = 0.221). There was also no significant interaction (F3,67 = 1.230, P = 0.306; (Fig. 3A–D, left panels). Thus, the plasticity induced by fear conditioning and by the 1-Hz protocol are both postsynaptically expressed.
Granule cells form synapses onto PCs by way of their ascending axons and the PFs. PF-PC synapses and granule cell ascending axon–PC synapses exhibit different presynaptic release probability and thus different PPRs (Sims & Hartell, 2005, 2006). The absolute PPR values that we obtained for each group were 1.455 ± 0.043 (n = 18) in naïve subjects; in unpaired T10min, 1.458 ± 0.036 (n = 16); in conditioned T10min, 1.486 ± 0.048 (n = 18); and in conditioned T24h, 1.510 ± 0.034 (n = 19). One-way anova showed no differences in the absolute baseline PPRs among the groups (F3,67 = 0.40, P = 0.747). These data indicate that there was no difference in the proportion of PF-PC synapses and granule cell–ascending synapses recruited in each group.
To examine whether the different forms of plasticity observed in the conditioned groups might have some presynaptic component, we separately calculated PPRs for the populations of cells that showed potentiation, depression or no change, respectively. In all these groups, PPRs were not changed after 1-Hz stimulation (Fig. 3A–D, right panels), suggesting that all the forms of plasticity were entirely postsynaptically expressed.
LTD was affected in the short but not long term by aversive stimulation
The classical LTD induced by paired PF and CF stimulation is also postsynaptic in origin (Ito, 2001; but see Casado et al., 2002). The recent demonstration that 1 Hz PF stimulation in the absence of concurrent CF activity (Coesmans et al., 2004) produces a form of LTP that reverses LTD and vice versa (Kakegawa & Yuzaki, 2005) indicates that the two types of plasticity may interact. To examine whether LTD elicited by conjunctive PF and CF stimulation is affected by learned fear, we applied PF and CF stimulation at 1 Hz for 5 min (Karachot et al., 1994) in each of the four groups of animals. In a series of control experiments, we monitored EPSC amplitudes at the baseline stimulation rate of 0.1 Hz for at least 40 min. As shown in Fig. 4A, EPSC amplitudes remained stable. Conjunctive CF and PF stimuli were then applied to slices prepared from naïve, unpaired and conditioned animals. We adjusted PF stimulus intensities to induce EPSC amplitudes ranging between 300 and 500 pA. By this approach, we can detect, when present, any significant depression induced by CF and PF stimulation. One-way anova showed no differences among the absolute EPSC amplitudes measured 5 min before the repetitive stimulation (F3,78 = 1.930, P = 0.132), thus confirming the uniformity of PF responses before LTD induction (see also Table 3). After the LTD protocol, two-way anova (4 × 2), with four behavioural groups as between-subjects variable and two time intervals as within-subject variable, showed significant differences in the EPSC amplitudes among the four groups (F3,78 = 3.396, P = 0.022) and between the two time intervals (F1,78 = 8.866, P = 0.004). The Newman–Keuls test showed no difference between naïve and conditioned T24h groups (P > 0.05; Fig. 4B and E), and between unpaired T10min and conditioned T10min groups (P > 0.05; Fig. 4C and D), but LTD in the latter two groups was significantly smaller than that in the former two (P < 0.05; see Table 3). These results indicate that LTD wais affected immediately, but not 24 h, after behavioural manipulations. At the short time period, LTD was impaired in both conditioned and unpaired animals, thus suggesting that the effects on LTD induction may be due to arousal and/or stress phenomena arising from acoustic and/or nociceptive stimuli.
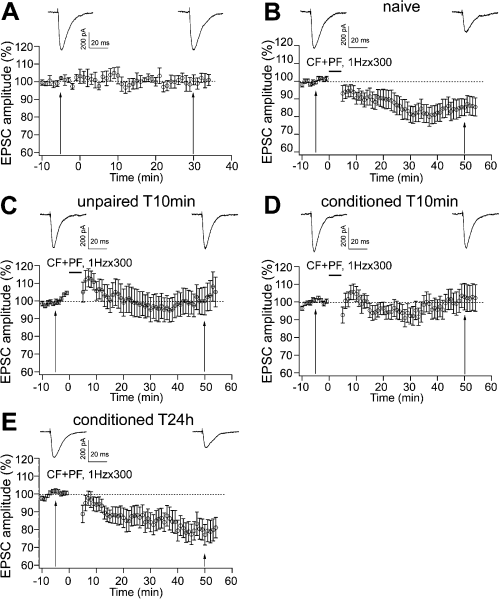
LTD was affected 10 min, but not 24 h, after the behavioural training in conditioned and unpaired subjects. (A) In the absence of the LTD induction protocol, PF EPSCs were stable in response to baseline frequency of PF activation. (B) After 300 conjunctive stimuli of PFs and CFs at 1 Hz, LTD was present in naïve animals. (C and D) LTD was impaired in the unpaired T10min group and the conditioned T10min group. (E) Twenty-four hours after training, LTD was observed in the conditioned T24h group. Representative traces above each graph were taken 5 min before and 50 min after the repetitive CF and PF stimulation. Data are expressed as mean ± SEM.
| Group | LTP experiment | LTD experiment | ||||||
|---|---|---|---|---|---|---|---|---|
| −5 min | 50 min | −5 min | 50 min | |||||
| Amplitude (pA) | n | Amplitude (pA) | n | Amplitude (pA) | n | Amplitude (pA) | n | |
| Naïve | 129.3 ± 9.8 | 16 | 187.8 ± 20.6 | 16 | 434.1 ± 15.8 | 24 | 358.7 ± 29.7 | 24 |
| UnpT10min | 171.9 ± 10.4 | 16 | 278.1 ± 30.4 | 16 | 385.8 ± 15.7 | 17 | 375.4 ± 25.0 | 17 |
| CondT10min | 148.9 ± 12.1 | 17 | 157.4 ± 21.3 | 17 | 406.2 ± 11.6 | 18 | 405.2 ± 26.0 | 18 |
| CondT24h | 169.8 ± 10.9 | 18 | 205.0 ± 20.6 | 18 | 419.74 ± 13.30 | 23 | 324.84 ± 23.96 | 23 |
- Data are presented as means ± SEM. n = number of animals in the group.
The direction of synaptic plasticity was shifted in unpaired and conditioned subjects
To understand the distribution of the shift in the synaptic strength following LTD induction in individual cells within each behavioural group, we used the same method as for LTP experiments to analyse separately the frequency distributions of EPSC amplitude. On the basis of the change in EPSC amplitude, we sorted cells into three groups; ‘not changed’, ‘depressed’ and ‘potentiated’, according to whether EPSC amplitudes changed < 10%, decreased to < 90% or increased to > 110% of baseline responses, respectively (Crepel & Jaillard, 1991; Table 2).
Fifty minutes after CF and PF stimulation, the frequency distributions of individual response amplitudes were similar for naïve and conditioned T24h groups (Fig. 5A and D), with 71 and 69% of the neurons, respectively, being depressed. Also, the unpaired and conditioned T10min groups showed similar distributions, with 35 and 39% of cells being depressed (Fig. 5B and C), but with a much lower representation than the former two groups (Table 2). From these data it is clear that following the LTD protocol most cells displayed depression in naïve and conditioned T24h groups, while in animals that experienced aversive stimuli 10 min before killing there was a decrease in the number of cells showing depression.
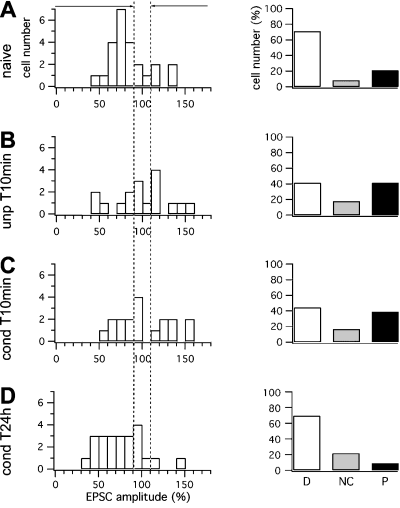
Aversive stimuli decreased the number of cells showing depression after 1 Hz stimulation in conditioned and unpaired animals. (A–D) Distribution of the cells that showed depression, no change or potentiation after conjunctive stimuli in (A) naïve, (B) unpaired T10min, (C) conditioned T10min and (D) conditioned T24h animals. (Left panels) Cell number in the four behavioural groups is plotted against the normalized EPSC amplitude. (Right panels) In unpaired T10min and conditioned T10min groups there was a decrease in depressed cells and an increase in potentiated cells.
Postsynaptic processes mediated the forms of plasticity elicited by the LTD induction protocol
As for the LTP experiments, we analysed PPRs to assess the pre- or postsynaptic nature of the different types of plasticity induced by CF and PF stimulation. Two-way anova (4 × 2) showed no significant differences among the four groups (F3,74 = 2.521, P = 0.064) or between the two time intervals (F1,74 = 0.936, P = 0.336; Fig. 6A–D, left panels). There was also no interaction (F3,74 = 2.474, P = 0.068). These results confirmed that LTD was postsynaptically expressed in all groups.
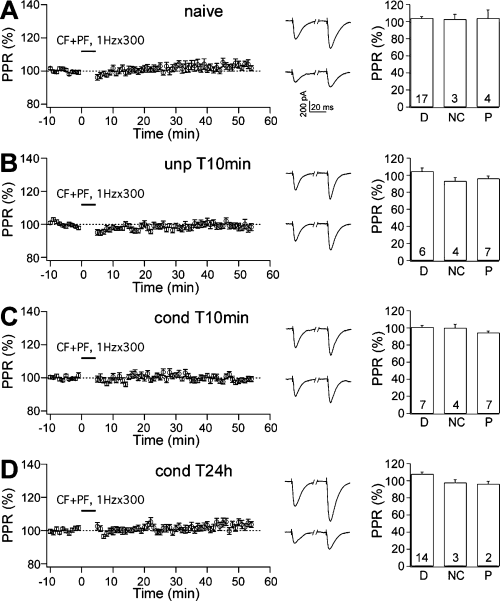
PPR was unchanged after the LTD induction protocol. (A–D, left panels) PPR was not changed after LTD induction in (A) naïve, (B) unpaired T10min, (C) conditioned T10min and (D) conditioned T24h animals. (Centre panels) Representative traces of EPSCs obtained by paired PF stimuli with 100-ms intervals were taken 5 min before (upper) and 50 min after (lower) the repetitive stimulation. (Right panels) In each behavioural group, PPR was unchanged in cells showing depression (D), no change (NC), and potentiation (P). Numbers in the bars indicate the number of cells.
To establish whether presynaptic changes may have contributed to responses in subgroups of cells, we again performed a separate analysis on the PPR for the cells that showed depression, potentiation and no change, respectively (Fig. 6A–D, right panels). PPR was not changed in any group, confirming that any changes were postsynaptically expressed.
Discussion
The present results show that LTP electrically induced at the PF-PC synapse is decreased after fear learning, and this effect is (i) long-lasting, as it is present 24 h after the acquisition; and (ii) specifically related to learning mechanisms as potentiation was unaffected in animals that received the same amount but temporally separated CS and US. Therefore, fear- and electrically induced LTP share common mechanisms. Furthermore, the delivery of repetitive stimuli that normally induce LTP, to fear-conditioned animals, also elicits a form of LTD that might protect PCs from being overstimulated. On the other hand, the electrical induction of LTD is only affected in the short term in both conditioned and unpaired subjects.
Cerebellar LTP and fear memory
In the conditioned groups, electrically induced LTP was remarkably reduced with only a small fraction of cells displaying a potentiation. This occlusion demonstrates that fear conditioning activates transduction signals that are not available for subsequent electrical recruitment. This occlusion approach has been commonly used to investigate whether the plasticity obtained in vitro by electrical stimulation is a reliable model for studying the mechanisms of learning and memory (Martin et al., 2000). This effect was found after the acquisition trial, i.e. during the consolidation process. Thus, synaptic strengthening that occurs during fear consolidation engages signal transduction pathways that are in common with those activated by electrical stimulation. The occlusion lasts at least 24 h, in line with the persistence of the increased freezing response and with the previously reported time course of fear-induced potentiation in cerebellum (Sacchetti et al., 2004). In line with the present results, a similar LTP occlusion has been found after a fear paradigm in amygdala (Tsvetkov et al., 2002) and hippocampus (Sacchetti et al., 2001, 2002b). Thus, LTP might be a common mechanism for associative memory in the brain.
LTP is induced in vitro in the cerebellar cortex by a repetitive stimulation of PFs without a concomitant activation of CF (Lev-Ram et al., 2003; Coesmans et al., 2004; Kakegawa & Yuzaki, 2005). If, as we propose, electrically and behaviourally induced LTP share common mechanisms, it is possible that fear-induced LTP is also induced by the sole activation of the PF pathway. It is known that this input carries both acoustic and nociceptive information to the cerebellar cortex (see Sacchetti et al., 2005). During fear learning, CS and US could activate two separate PF pathways impinging on the same PCs (Heck et al., 2007).
Electrically induced LTP occurs following 1-Hz stimulation under conditions that limit intracellular Ca2+ elevation (Sakurai, 1990; Lev-Ram et al., 2003; Coesmans et al., 2004; Kakegawa & Yuzaki, 2005), including PC hyperpolarization (Crepel & Jaillard, 1991). These conditions can be achieved in vivo during learning by activating the GABAergic interneurons contacting the PC. These synapses are formed by stellate and basket cells, both of them being activated by PF inputs (Ito, 1984).
Following the LTP protocol, together with a decrease in the number of PCs showing LTP we also observed in conditioned animals an increase in the number of cells displaying depression. This phenomenon may be similar to that reported in a study showing that LTD is induced in vitro by 1-Hz PF stimulation alone at relatively high stimulus strength (Hartell, 1996). This paradigm led to accumulation of dendritic Ca2+ and thus to LTD (Hartell, 1996). Although we have taken measures to limit intracellular Ca2+ transients, by employing small PF-PC responses and including 10 mm BAPTA in the intracellular solution, if local dendritic Ca2+ is not sufficiently chelated, residual LTD might still occur. An alternative explanation for the presence of LTD could be that some residual LTD is unmasked when LTP is occluded.
The physiological role of the depression we observed might be to reduce fear-induced LTP that may have saturated synaptic transmission, thus enabling these synapses to form new memories. Alternatively, it may represent a neuroprotective mechanism that prevents an overstimulation of already potentiated PF-PC synapses, in line with the hypothesis proposed by De Schutter (1995) and with recent findings (Kimura et al., 2005; Welsh et al., 2005).
Cerebellar LTD and LTP
The last part of our work was aimed at testing the effects of fear conditioning on LTD evoked by the conjunctive stimulation of PFs and CFs. This type of LTD has been proposed to be the substrate of motor learning (Marr, 1969; Albus, 1971; Ito, 1984; Kim & Thompson, 1997). This hypothesis has been strongly supported by a series of experiments on the adaptation of the vestibulo-ocular reflex and on eye-blink conditioning (Ito, 2001). Our experiments suggest a different model for learned fear. We found that LTD is unchanged at 24 h after conditioning. This means that the transduction pathways leading to LTP and to fear memory are independent of those of LTD.
LTD is decreased in the unpaired group where there is no fear learning. As these animals have been submitted to a stressful condition, it is likely that the stressful stimuli are responsible for the decreased LTD observed. This effect is specific for the LTD protocol as it is not observed after the LTP paradigm. Therefore, stressful stimuli might affect some molecular mechanisms necessary for LTD, but not LTP, induction. Interestingly, it has recently been demonstrated that a prolonged stressful stimulation led to the accumulation of cGMP, a molecule involved in LTD (Ito, 2001) but not LTP (Lev-Ram et al., 2002), in the cerebellum (Ryder et al., 2006). In the groups where LTD was affected, there was also an increase in the number of cells showing LTP. As previous studies showed that the LTD protocol elicits LTP when intracellular Ca2+ is chelated (Sakurai, 1990; Shibuki & Okada, 1992; Coesmans et al., 2004) it may be speculated that immediately after aversive manipulations some molecules, e.g. Ca2+-binding proteins, are activated in the PC to protect the cell from an excessive Ca2+ elevation in unpaired and conditioned animals. These mechanisms may be responsible for the switch from LTD to LTP following CF and PF stimulation. These neuroprotective processes should be related to acute stress and, thus, no longer necessary hours after the stressful stimuli, in line with the lack of effects we observed 24 h after the behavioural procedures.
Ten minutes after conditioning, LTD was affected in both conditioned and unpaired animals. Therefore, it is plausible to assume that the decreased LTD observed in these subjects is the consequence of the stressful stimuli. Obviously, we cannot rule out the possibility that at this time period in the conditioned animals LTD can also be affected by some short-term associative process. It is interesting to note that in the conditioned T10min animals the electrical induction of both LTP and of LTD was modified. We should stress, however, that not all the cells tested in the LTP experiment showed LTP occlusion, and not all the cells analysed in the second set of experiments presented an interference with LTD. It can be speculated that in the LTP experiment the cells that showed no LTP would be possible candidates for undergoing LTD in the LTD experiment while the cells showing LTD in the first experiment would be unlikely to further show LTD.
Recent studies have proposed that electrically induced LTP and LTD could reverse each other (Medina et al., 2002; Lev-Ram et al., 2003; Boyden et al., 2004; Coesmans et al., 2004; Jorntell & Hansel, 2006). However, in the present study we found that electrically induced LTP and LTD were differently affected by the fear paradigm. Thus, cerebellar LTP and LTD cannot be fully reciprocally reversing phenomena. LTP and LTD are considered reciprocally reversing phenomena in the hippocampus (Heynen et al., 1996) and motor cortex (Rioult-Pedotti et al., 2000). In these sites LTP and LTD are induced by stimulating the same synaptic locus at high (LTP) or low (LTD) frequency, and previous reports showed that in these two sites behavioural manipulations affected LTP and LTD in opposite directions, i.e. decreasing LTP and increasing LTD (Rioult-Pedotti et al., 2000; Diamond et al., 2005). On the other hand, in the cerebellum LTP is obtained by stimulating PFs alone (i.e. it is homosynaptically induced), while LTD requires both PF and CF activation (i.e. it is heterosynaptic). Thus, cerebellar LTP and LTD may be involved in different forms of information processing and therefore can be differentially affected by behavioural treatments. Recent studies of simple behaviours such as the adaptation of the vestibulo-ocular reflex indicate that multiple plasticity mechanisms contribute to cerebellum-dependent learning (Hansel et al., 2001; Boyden et al., 2004; Jorntell & Hansel, 2006). Multiple plasticity mechanisms may provide the flexibility required to store different types of memories and regulate the dynamics of cerebellar-dependent responses (Hansel et al., 2001; Boyden et al., 2004; Jorntell & Hansel, 2006).
Acknowledgements
The authors thank R. E. Sims for his useful assistance in the preliminary experiments and A. Renna for technical assistance. This work was supported by grants from MIUR, Italian Ministry of Health, European Community, ASI, Regione Piemonte and Fondazione San Paolo.
Abbreviations
-
- ACSF
-
- artificial cerebrospinal fluid
-
- BAPTA
-
- 1,2-bis-(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid
-
- CF
-
- climbing fibre
-
- CS
-
- conditioned stimulus
-
- EPSC
-
- excitatory postsynaptic current
-
- LTD
-
- long-term depression
-
- LTP
-
- long-term potentiation
-
- PC
-
- Purkinje cell
-
- PF
-
- parallel fibre
-
- PPR
-
- paired-pulse ratio
-
- US
-
- unconditioned stimulus