Resting EEG sources correlate with attentional span in mild cognitive impairment and Alzheimer's disease
Abstract
Previous evidence has shown that resting delta and alpha electroencephalographic (EEG) rhythms are abnormal in patients with Alzheimer's disease (AD) and its potential preclinical stage (mild cognitive impairment, MCI). Here, we tested the hypothesis that these EEG rhythms are correlated with memory and attention in the continuum across MCI and AD. Resting eyes-closed EEG data were recorded in 34 MCI and 53 AD subjects. EEG rhythms of interest were delta (2–4 Hz), theta (4–8 Hz), alpha 1 (8–10.5 Hz), alpha 2 (10.5–13 Hz), beta 1 (13–20 Hz), and beta 2 (20–30 Hz). EEG cortical sources were estimated by low-resolution brain electromagnetic tomography (LORETA). These sources were correlated with neuropsychological measures such as Rey list immediate recall (word short-term memory), Rey list delayed recall (word medium-term memory), Digit span forward (immediate memory for digits probing focused attention), and Corsi span forward (visuo-spatial immediate memory probing focused attention). A statistically significant negative correlation (Bonferroni corrected, P < 0.05) was observed between Corsi span forward score and amplitude of occipital or temporal delta sources across MCI and AD subjects. Furthermore, a positive correlation was shown between Digit span forward score and occipital alpha 1 sources (Bonferroni corrected, P < 0.05). These results suggest that cortical sources of resting delta and alpha rhythms correlate with neuropsychological measures of immediate memory based on focused attention in the continuum of MCI and AD subjects.
Introduction
Quantitative analysis of electroencephalographic (EEG) rhythms in awake resting subjects is a low-cost and useful neurophysiological approach to the study of Alzheimer's disease (AD; Maurer & Dierks, 1992; Szelies et al., 1992; Leuchter et al., 1993; Schreiter-Gasser et al., 1993). When compared to normal elderly subjects, AD patients were characterized by excessive delta (0–4 Hz) and theta (4–7 Hz) rhythms, and a significant decrement of posterior alpha (8–12 Hz) and beta (13–30 Hz) rhythms (Dierks et al., 1993, 2000; Huang et al., 2000; Ponomareva et al., 2003; Babiloni et al., 2004a; Jeong, 2004; Prichep et al., 2005). These EEG abnormalities were associated with altered regional cerebral blood flow (rCBF)/metabolism and with global cognitive function as evaluated by Mini Mental State Examination (MMSE; Sloan et al., 1995; Rodriguez et al., 1998, 1999a, b; Jeong, 2004). Similar EEG abnormalities have been also reported in subjects with mild cognitive impairment (MCI), a clinical state between elderly normal cognition and dementia in which subjects present memory complaints and objective evidence of cognitive impairment (Zappoli et al., 1995; Elmstahl & Rosen, 1997; Huang et al., 2000; Jelic et al., 2000; Koenig et al., 2005; Babiloni et al., 2006a).
Keeping in mind these data, a logical question is ‘which is the functional meaning of abnormal EEG rhythms in AD?’ It has been reported that, in AD patients, early pathological processes include loss of cholinergic basal forebrain neurons projecting to hippocampus and fronto-parietal areas, and that alpha and slower EEG rhythms can be modulated by these neurons (Helkala et al., 1996; Holschneider et al., 1999; Mesulam et al., 2004). In contrast, brainstem cholinergic innervation of the thalamus would be relatively spared in AD (Mash et al., 1985; Geula & Mesulam, 1989, 1996, 1999; Mesulam et al., 2004). It has been also reported that cholinergic and serotoninergic neurons are the most important factors responsible for the block of spindles and delta rhythms during arousal, with crucial effects on attentional, motivational, and memory functions (Dringenberg, 2000; Dringenberg et al., 2002). Finally, delta rhythms increase in AD patients as a function of atrophy in mesial-temporal, posterior, and/or frontal cortical areas (Fernandez et al., 2003; Babiloni et al., 2006b). Abnormal delta rhythms can be observed for other kinds of brain alterations such as cerebral vascular, traumatic, and tumour lesions (Harmony et al., 1993; Murri et al., 1998; de Jongh et al., 2003; Hensel et al., 2004). The distribution of abnormal delta rhythms depends on the type of lesions; namely, localized delta rhythms increase for underlying lesions of white-matter connection (‘disconnection’ delta), whereas diffuse delta increases by thalamic and troncoencephalic lesions (Gloor et al., 1977).
The functional meaning of EEG abnormalities in AD is enlightened by studies investigating the relationship between EEG rhythms and cognition in humans (i.e. attention, memory). It has been shown that a good cognitive performance is predicted by high alpha power and low theta power in the prestimulus period (Neubauer & Freudenthaler, 1995; Klimesch, 1999). Successful encoding processes would depend on the increase of the frontal theta power, reflecting the functional mode of loops including basal forebrain, hippocampus and cerebral cortex (Klimesch, 1999). Later, successful retrieval processes into semantic or episodic long-term memory would depend on the decrease of posterior alpha power, reflecting the functional mode of thalamo-cortical and cortico-cortical feedback loops (Klimesch, 1999). As a general rule, the stronger the prestimulus alpha power, the stronger its power reduction during the stimulus processing, and the better the cognitive performance (Neubauer et al., 1995; Klimesch, 1999).
In this study, we tested the hypothesis that cortical sources of resting EEG rhythms are correlated with attentional and/or memory functions across the continuum of subjects with MCI and AD, in line with the idea that these rhythms are an important substrate of higher functions in aged people with cognitive decline. We specifically probed attentional and/or memory functions, as AD is considered as a disease mainly affecting cholinergic and hippocampus systems underlying these functions (Helkala et al., 1996; Holschneider et al., 1999; Mesulam et al., 2004).
As a preliminary control hypothesis, we selected the cortical sources of EEG rhythms that correlated with subjects' global cognitive status as revealed by MMSE. The cortical generators of EEG rhythms were estimated by low-resolution brain electromagnetic source tomography (LORETA; Pascual-Marqui & Michel, 1994; Pascual-Marqui et al., 1999, 2002), which belongs to a large family of tomographic procedures based on EEG linear inverse estimation (Valdes et al., 1998; Fuchs et al., 2001; Gross et al., 2001; Pascual-Marqui et al., 2002; Sekihara et al., 2005). In precedence, LORETA has been validated for the study of ageing in the same experimental condition and setting of the present investigation (Anderer et al., 2003; Dierks et al., 2000; Huang et al., 2002; Saletu et al., 2002; Babiloni et al., 2004a; Goforth et al., 2004; Babiloni et al., 2006a, 2006b, 2006c; 2006d, 2006e).
Materials and methods
Subjects and diagnostic criteria
Thirty-four MCI subjects, 53 AD patients and 31 normal elderly (Nold) matched controls were enrolled. They were not consecutive cases, as we had to balance as much as possible age, education, and gender across the groups. Furthermore, we could include only MCI and AD subjects with a certain degree of general cognitive status (see in the following). Part of the individual data sets was used for previous EEG rhythms with absolutely other aims, namely the study of resting EEG sources as a function of age, genotypes, atrophy, and different kinds of dementia processes (Babiloni et al., 2004a, 2006a, 2006b, 2006c, 2006d, 2006e).
Diversely, the present study focused on the relationship between cortical EEG rhythms and attentional/memory functions in MCI and AD subjects. All experiments were performed with the informed and overt consent of each participant or caregiver, in line with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the standards established by the Author's Institutional Review Board.
The present inclusion and exclusion criteria for MCI were based on previous seminal studies (Rubin et al., 1989; Albert et al., 1991; Flicker et al., 1991; Zaudig, 1992; Petersen et al., 1995, 1997; Devanand et al., 1997; Petersen et al., 2001) and aimed at selecting elderly persons with objective cognitive deficits, especially in the memory domain, who did not meet criteria for a diagnosis of dementia or AD. The inclusion criteria for the MCI subjects included (i) objective memory impairment on neuropsychological evaluation, as defined by performances ≥ 1.5 standard deviation below the mean value of age and education-matched controls for a test battery including Memory Rey list (immediate recall and delayed recall), Digit forward, and Corsi forward tests; (ii) normal activities of daily living as documented by the history and evidence of independent living, and (iii) clinical dementia rating score of 0.5. The exclusion criteria for MCI included (i) mild AD, as diagnosed by the procedures described below; (ii) evidence of concomitant dementia such as frontotemporal, vascular dementia, reversible dementias (including pseudo-depressive dementia), fluctuations in cognitive performance, and/or features of mixed dementias; (iii) evidence of concomitant extra-pyramidal symptoms; (iv) clinical and indirect evidence of depression as revealed by Geriatric Depression Scale scores higher than 13; (v) other psychiatric diseases, epilepsy, drug addiction, alcohol dependence, and use of psychoactive drugs including acetylcholinesterase inhibitors or other drugs enhancing brain cognitive functions; (vi) current or previous uncontrolled or complicated systemic diseases (including diabetes mellitus) or traumatic brain injuries, and (vii) use of cholinergic drugs.
Probable AD was diagnosed according to NINCDS-ADRDA (McKhann et al., 1984). Patients underwent general medical, neurological and psychiatric assessments and were also rated with a number of standardized diagnostic and severity instruments that included MMSE (Folstein et al., 1975), Clinical Dementia Rating Scale (Hughes et al., 1982), Geriatric Depression Scale (Yesavage et al., 1982), Hachinski Ischemic Scale (Rosen et al., 1980), and Instrumental Activities of Daily Living scale (Lawton & Brodie, 1969). Neuroimaging diagnostic procedures (CT or MRI) and complete laboratory analyses were carried out to exclude other causes of progressive or reversible dementias, in order to have a homogenous AD patient sample. The exclusion criteria included, in particular, any evidence of (i) frontotemporal dementia diagnosed according to the criteria of The Lund & Manchester Groups (1994); (ii) vascular dementia as diagnosed according to the NINDS-AIREN criteria (Roman et al., 1993); (iii) extra–pyramidal syndromes; (iv) reversible dementias (including pseudodementia of depression), and (v) Lewy body dementia according to the criteria of McKeith et al., 1999). The detection of the vascular component in dementia and MCI was accounted based on previous theoretical guidelines from our network (Frisoni et al., 1995; Galluzzi et al., 2005). Of note, acetylcholinesterase inhibitors, benzodiazepines, antidepressants and/or antihypertensives were suspended for approximately 24 h before EEG recordings. This did not insure a complete washout of the drug, but the drug condition become comparable across the patients. A complete washout of drugs would have required suspension of the treatment for too long with high risks for the patients.
The Nold subjects were recruited mostly among nonconsanguineous patients' relatives. All Nold subjects underwent physical and neurological examinations as well as cognitive screening. Subjects affected by chronic systemic illnesses, subjects receiving psychoactive drugs, and subjects with a history of present or previous neurological or psychiatric disease were excluded. All Nold subjects had a GDS score lower than 14 (no depression).
Table 1 summarizes demographic and clinical data of the recruited Nold, MCI, and AD subjects. Four anova analyses using the factor Group (Nold, MCI and AD) were computed to evaluate the presence or absence of statistically significant differences among the Nold, MCI and AD for age, education, gender, and MMSE. No statistically significant differences for age (P > 0.66), education (P > 0.38), and gender (P > 0.11) were found. On the contrary, as expected, the anova analysis for MMSE showed a statistically significant difference (F2,115 = 107; P < 0.0001), indicating that the MMSE values were higher in the Nold compared to the MCI group (P < 0.0002) and in the MCI compared to the AD group (P < 0.0001). However, age and education were used as covariates in the statistical evaluation of cortical sources of EEG rhythms, to remove possible confounding effects.
| Nold(n = 31) | MCI(n = 34) | AD(n = 53) | P-value (anova) | |
|---|---|---|---|---|
| Age (years) | 74.1 ± 1.4 | 72.7 ± 0.9 | 74 ± 1.1 | 0.66 |
| Gender (F/M) | 15F/16M | 18F/16M | 38F/15M | 0.38 |
| MMSE | 28 ± 0.3 | 25.8 ± 0.3 | 20.4 ± 0.5 | 0.0001 |
- Data are presented as means ± SEM. P-values ranged from 0 to 1 with cut-off for significance of 0.05.
Neuropsychological measures
For the correlation with EEG rhythms, the following four neuropsychological measures were collected in all MCI and AD subjects, Rey list immediate recall, Rey list delayed recall, Digit span forward, and Corsi span forward. Of note, we were unable to recruit a sufficient number of Nold subjects for the collection of the mentioned neuropsychological measures, as many of them refused to take these tests. This made the analysis of the relationship between neuropsychological measures and the characteristics of EEG rhythmicity in the Nold subjects not viable compared to the MCI and AD subjects, an important issue for future research.
In the Rey list immediate recall test, 15 words are read to the subject one by one (one word at a second). Afterwards, the subject is asked to immediately repeat all words he/she can remember. This procedure is repeated five times (namely, reading of the 15 words to the subject and the immediate repetition by the subject). The score is the total number of recalled words, ranging from zero to 75. The Rey list immediate recall basically probes short-term memory for words.
The Rey list delayed recall test starts 15 min after the end of the Rey list immediate recall test. The subject is newly asked to repeat as many words as he can recall from the mentioned Rey list. The score is the total number of recalled words, ranging from 0 to 15. The Rey list delayed recall test basically probes medium-term memory for words.
In the Digit span forward test, a first sequence of two numbers (one number at a second) is read to the subject. The subject is asked to repeat them immediately. If the subject performs well, a sequence of three numbers (one number at a second) is read to the subject. The test continues until either the subject fails two consecutive sequences of the same length or the subject repeats the sequence of ten numbers. The score is determined by the length of the longer correctly repeated sequence, ranging from zero to nine numbers. The Digit span forward test basically probes immediate memory for digits, which is based on focused attention.
In the Corsi span forward test, a wood tablet with ten cubes is positioned in front of the subject. A clinician touches with the index a first sequence of two cubes (one cube every 2 s). The subject is asked to repeat that motor sequence immediately. If the subject performs well, then a successive sequence of three cubes is shown. The test continues until either the subject fails two consecutive sequences of the same length or the subject repeats the sequence of ten cubes. The score is determined by the length of the longer correctly repeated sequence, ranging from zero to ten cubes. The Corsi span forward test basically probes immediate visuo-spatial memory, which is based on focused attention.
EEG recordings
EEG data were recorded by specialized clinical units in wake rest state (eyes-closed) in late morning hours from 19 electrodes positioned according to the International 10–20 System (i.e. Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, O2; 0.3–70 Hz filtering bandpass). A specific reference electrode was not imposed to all recording units of this multicentric study, as preliminary data inspection and LORETA source analysis were carried out after EEG data were re-referenced to a common average reference. To monitor eye movements, the horizontal and vertical electrooculogram (0.3–70 Hz bandpass) was simultaneously recorded. All data were digitized in continuous recording mode (5 min of EEG; 128–256 Hz sampling rate, the sampling rate being fixed in each recording research unit of this multicentric study). In order to keep constant the level of vigilance, an operator controlled on-line the subject and the EEG traces, alerting the subject any time there were signs of behavioural and/or EEG drowsiness.
The duration of the EEG recording (5 min) allowed the comparison of the present results with several previous AD studies using either EEG recording periods shorter than 5 min (Buchan et al., 1997; Pucci et al., 1999; Szelies et al., 1999; Rodriguez et al., 2002; Babiloni et al., 2004a, 2006a; 2006b, 2006c, 2006d, 2006e) or shorter than 1 min (Dierks et al., 1993, 2000). Longer resting EEG recordings in AD patients would have reduced data variability but would have increased the possibility of EEG ‘slowing’ because of reduced vigilance and arousal.
The recorded EEG data were analysed and fragmented off-line in consecutive 2-s epochs. For standardization purposes, preliminary analysis of all data was centralized in one research unit. The EEG epochs with ocular, muscular, and other types of artifact were preliminary identified by a computerized automatic procedure. EEG epochs with sporadic blinking artifacts (less than 15% of the total) were corrected by an autoregressive method (Moretti et al., 2003). Two independent experimenters, blind to the diagnosis, manually confirmed the EEG segments accepted for further analysis.
Spectral analysis of the EEG data
The spectral analysis of the EEG data was computed with the original LORETA software (http://www.unizh.ch/keyinst/NewLORETA/LORETA01.htm) from the common averaged EEG potentials. A digital FFT-based power spectrum analysis (Welch technique, Hanning windowing function, no phase shift) computed power density of the EEG rhythms with 0.5 Hz frequency resolution. The output of the procedure was the EEG cross-spectra. The following standard band frequencies were studied; delta (2–4 Hz), theta (4–8 Hz), alpha 1 (8–10.5 Hz), alpha 2 (10.5–13 Hz), beta 1 (13–20 Hz), and beta 2 (20–30 Hz). They were chosen on the basis of the previous relevant EEG studies on dementia (Leuchter et al., 1993; Jelic et al., 1996; Besthorn et al., 1997; Chiaramonti et al., 1997; Rodriguez et al., 1999a, 1999b) and have been successfully used in recent studies on AD of this consortium (Babiloni et al., 2004a, 2006a, 2006b, 2006c, 2006d, 2006e). Sharing of a frequency bin by two contiguous bands is a widely accepted procedure (Leuchter et al., 1993; Cook & Leuchter, 1996; Jelic et al., 1996; Besthorn et al., 1997; Pucci et al., 1997; Nobili et al., 1998; Holschneider et al., 1999; Kolev et al., 2002). Furthermore, this fits the theoretical consideration that near EEG rhythms may overlap at their frequency borders (Klimesch, 1996, 1999; Klimesch et al., 1997, 1998; Babiloni et al., 2004b, 2004c, 2004d, 2004e, 2004f).
Choice of the fixed EEG bands did not account for individual alpha frequency (IAF) peak, defined as the frequency associated with the strongest EEG power at the extended alpha range (Klimesch, 1999). However, this should not affect the present results, as most of the subjects had IAF peaks within the alpha 1 band (8–10.5 Hz). In particular, mean IAF peak was 9.1 Hz (± 0.2 standard error, SEM) in Nold subjects, 9.2 Hz (± 0.2) in MCI subjects, and 8.3 Hz (± 0.2) in AD patients. To control for the effect of IAF on the EEG comparisons among these three groups, the IAF peak was used as a covariate (together with age, education and gender) for further statistics.
Cortical source analysis of the EEG rhythms by LORETA
The EEG cross-spectra were given as an input to the proper option of the original LORETA software for the EEG source analysis (Pascual-Marqui & Michel, 1994, Pascual-Marqui et al., 1999, 2002). LORETA is a functional imaging technique belonging to a family of linear inverse solution procedures (Valdes et al., 1998) modelling 3-D distributions of the cortical source patterns generating scalp EEG data (Pascual-Marqui et al., 2002). With respect to the dipole modelling of cortical sources, no a priori decision of the dipole position is required by the investigators in LORETA estimation. In a previous review paper, it has been shown that LORETA was quite efficient when compared to other linear inverse algorithms like minimum norm solution, weighted minimum norm solution or weighted resolution optimization (Pascual-Marqui et al., 1999). Furthermore, independent validation of the LORETA solutions has been provided by recent studies (Yao & He, 2001; Phillips et al., 2002). Finally, LORETA has been successfully used in recent EEG studies on pathological ageing (Dierks et al., 2000; Babiloni et al., 2004a). LORETA computes 3-D linear solutions (LORETA solutions) for the EEG inverse problem within a three-shell spherical head model including scalp, skull, and brain compartments. The brain compartment is restricted to the cortical grey matter/hippocampus of a head model coregistered to the Talairach probability brain atlas and digitized at the Brain Imaging Center of the Montreal Neurological Institute (Talairach & Tournoux, 1988). This compartment includes 2394 voxels (7-mm resolution), each voxel containing an equivalent current dipole.
LORETA can be applied to EEG data collected with a spatial sampling of 10–20 system as routinely tested worldwide with 19 electrodes. In fact, several previous studies have shown that brain rhythms in the wake state are generated by largely distributed cortical sources that can be accurately investigated by the standard 10–20 system and LORETA (Anderer et al., 2000, 2003, 2004; Isotani et al., 2001; Mulert et al., 2001; Winterer et al., 2001; Laufer & Pratt, 2003a, 2003b; Veiga et al., 2003; Babiloni et al., 2004a, 2006a, 2006b, 2006c, 2006d, 2006e).
The LORETA solutions consisted of voxel spectral density of estimated z-current density values able to predict EEG spectral data at scalp electrodes. These solutions are reference-free, in that one obtains the same LORETA source distribution for EEG data referenced to any reference electrode including common average. A normalization of the data was obtained by normalizing the current density at each voxel with the power density averaged across all frequencies (0.5–45 Hz) and across all 2394 voxels of the brain volume. This procedure reduced intersubjects variability as previously shown (Nuwer, 1988; Leuchter et al., 1993; Babiloni et al., 2004a, 2006a, 2006b, 2006c, 2006d, 2006e). After the normalization, the LORETA solutions lost the original physical dimension and were represented by an arbitrary unit scale. Other methods of normalization using the principal component analysis are effective for estimating the subjective global factor scale of the EEG data (Hernández et al., 1994). These methods are not available in the LORETA package and were not used in this study.
Solutions of the EEG inverse problem are underdetermined and ill-conditioned when the number of spatial samples (electrodes) is lower than that of the unknown samples (current density at each voxel). To account for that, the cortical LORETA solutions predicting scalp EEG spectral power density were regularized to estimate distributed rather than punctual EEG source patterns (Pascual-Marqui & Michel, 1994, Pascual-Marqui et al., 1999, 2002). In line with the low spatial resolution of the LORETA technique, we combined solutions within each of the following regions of the brain model coded into the Talairach space: frontal, central, temporal, parietal, occipital, and limbic. The Brodmann areas forming each of these regions of interest (ROIs) are listed in Table 2 . Figure 1 shows these six ROIs on LORETA brainmap.
| ROI | Brodmann areas |
|---|---|
| Frontal | 8, 9, 10, 11, 44, 45, 46, 47 |
| Central | 1, 2, 3, 4, 6 |
| Parietal | 5, 7, 30, 39, 40, 43 |
| Temporal | 20, 21, 22, 37, 38, 41, 42 |
| Occipital | 17, 18, 19 |
| Limbic | 31, 32, 33, 34, 35, 36 |
- LORETA solutions were combined within each ROI (frontal, central, parietal, occipital, temporal and limbic).
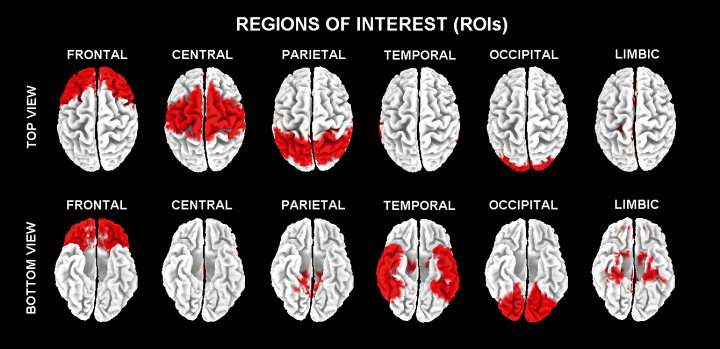
Visualization (top view and bottom view) of frontal, central, parietal, temporal, occipital and limbic areas on LORETA brainmap.
The main advantage of the regional analysis of LORETA solutions was that our modelling could disentangle rhythms of contiguous cortical areas. For example, the rhythms of the occipital source were disentangled with respect to those of the contiguous parietal and temporal sources, etc. This was made possible by the fact that this method solves the linear inverse problem by taking into account the well-known effects of the head as a volume conductor. With respect to other procedures of data reduction, this type of lobar approach may represent an important reference for multimodal comparisons with structural and functional neuroimaging methods (SPECT, PET, surface EEG/MEG topography). Finally, it can be stated that the present approach represents a clear methodological improvement compared to EEG computerized spectral analysis at surface electrodes. Indeed, the EEG potentials collected at each scalp electrode are strongly affected by head volume conductor effects with contamination at individual electrodes from far-field generator sources.
Statistical analyses
Statistical comparisons were performed by anova for repeated measurements. Mauchley's test evaluated the sphericity assumption and the correction of the degrees of freedom was made by Greenhouse-Geisser procedure. Duncan test was used for posthoc test comparisons (P < 0.05).
For the evaluation of difference between MCI and AD for the tests in question (Rey list immediate recall, Rey list delayed recall, Digit span forward, and Corsi span forward), four anova analyses having the scores of the mentioned neuropsychological measures as dependent variables were performed. These anova analyses used the factor Group (MCI, AD).
For the evaluation of the control hypothesis (sensitivity of the present LORETA approach), the LORETA solutions from Nold, MCI, and AD subjects were used as an input for an anova analysis. Age, gender, education and IAF were used as covariates in line with our previous EEG studies (Babiloni et al., 2004a, 2006a, 2006b, 2006c, 2006d, 2006e). The anova analysis used the factors Group (Nold, MCI, AD; independent variable), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2), and ROI (central, frontal, parietal, occipital, temporal, limbic). The control hypothesis would be confirmed by the following three statistical results; (i) a statistical anova effect including the factor Group (P < 0.05); (ii) a posthoc test indicating statistically significant differences of the LORETA solutions with the patterns Nold < MCI < AD or Nold > MCI > AD (Duncan test, P < 0.05), and (iii) a statistically significant correlation in all subjects as a single group among the MMSE score and the LORETA solutions fitting these patterns. Of note, the analysis of the mentioned correlation allowed evaluating whether the LORETA solutions are sensitive to global cognitive status across Nold, MCI and AD subjects as revealed by MMSE.
As mentioned above, the experimental hypothesis regarded the correlation between neuropsychological measures and regional EEG sources in MCI and AD subjects. This hypothesis would be confirmed by statistically significant correlations (Pearson test, Bonferroni corrected, P < 0.05) among neuropsychological measures and the amplitude of the LORETA solutions sensitive to the subject's cognitive status (see first statistical analysis; namely, the LORETA solutions fitting the patterns Nold < MCI < AD or Nold > MCI > AD and having a statistically significant correlation with the MMSE score).
Results
Neuropsychological results
Table 3 reports the mean (± SEM) of the score of Rey list immediate recall, Rey list delayed recall, Digit forward, and Corsi forward tests in MCI and AD subjects. The scores of the four mentioned neuropsychological measures were used as an input for corresponding anova analyses including the factor Group (MCI, AD). The anova analyses for the Rey list immediate recall (F1,85 = 13.1, P < 0.0005), for the Rey list delayed recall (F1,85 = 15.1, P < 0.0003), for the Digit span forward (F1,85 = 11.2, P < 0.001), and for the Corsi span forward (F1,85 = 8.1, P < 0.005) showed a main statistical effect for the factor Group. Similar results were obtained using independent t-test (Rey list immediate recall, t = 3.61, P < 0.0005; Rey list delayed recall, t = 3.89, P < 0.0003; Digit span forward, t = 3.35, P < 0.001; Corsi span forward, t = 2.85; P = 0.005). The results indicated that the score to these tests was significantly lower in AD patients than in MCI subjects.
| Test scores | P-value (anova) | ||
|---|---|---|---|
| MCI | AD | ||
| Rey list immediate recall | 24.5 ± 1.5 | 17.9 ± 1.1 | 0.0005 |
| Rey list delayed recall | 3.2 ± 0.4 | 1.3 ± 0.3 | 0.0003 |
| Digit forward | 5.3 ± 0.2 | 4.6 ± 0.2 | 0.0001 |
| Corsi forward | 4.4 ± 0.1 | 3.5 ± 0.2 | 0.005 |
- Data are presented as mean ± SEM. P-values compared with ranged from 0 to 1 with cut-off for significance of 0.05.
Topography of the EEG cortical sources as estimated by LORETA
For illustrative purposes, Fig. 2 maps the grand average of the LORETA solutions (i.e. relative current density at cortical voxels) modelling the distributed EEG sources for the investigated rhythms in Nold, MCI, and AD groups. The Nold group presented alpha 1 sources with the maximal values of amplitude distributed in parieto-occipital regions. Delta, theta, and alpha 2 sources had moderate amplitude values when compared to alpha 1 sources. Finally, beta 1 and beta 2 sources were characterized by lowest amplitude values. Compared to Nold group, AD group showed an amplitude increase of widespread delta sources, along with a dramatic amplitude reduction of parieto-occipital alpha 1 sources. With respect to Nold and AD groups, MCI group showed intermediate magnitude of delta and alpha 1 sources.
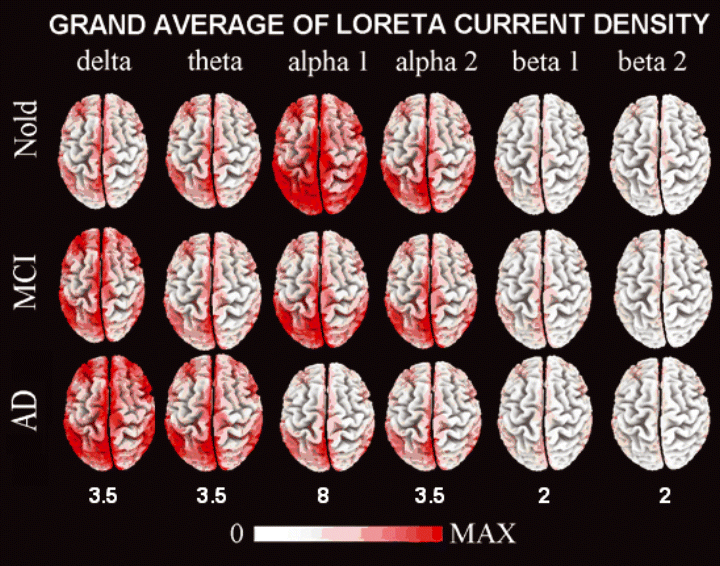
LORETA, low resolution brain electromagnetic tomography. Grand average of LORETA solutions (i.e. normalized relative current density at the cortical voxels) modelling the distributed EEG sources for delta, theta, alpha 1, alpha 2, beta 1, and beta 2 bands in Nold, MCI and AD groups. The left side of the maps (top view) corresponds to the left hemisphere. Color scale – all power density estimates were scaled based on the averaged maximum value (i.e. alpha 1 power value of occipital region in Nold). The maximal value of power density is reported under each column.
Statistical analysis of the EEG cortical sources estimated by LORETA
Figure 3 shows mean regional LORETA solutions (distributed EEG sources) relative to a statistical anova interaction (F50,2875 = 5.7, MSe = 0.63, P < 0.0001) among the factors Group (Nold, MCI, AD), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2), and ROI (central, frontal, parietal, occipital, temporal and limbic). In the figure, regional solutions had the shape of EEG relative power spectra. Notably, profile and magnitude of these spectra in Nold, MCI, and AD groups differed across diverse cortical macro-regions, thus supporting the idea that scalp EEG rhythms are generated by a distributed pattern of cortical sources. The planned posthoc testing showed that (i) the source patterns Nold < MCI < AD was fitted by occipital and temporal delta sources (P < 0.04 to P < 0.00004) and (ii) the source patterns Nold > MCI > AD was fitted by occipital and temporal alpha 1 sources (P < 0.02 to P < 0.000001).
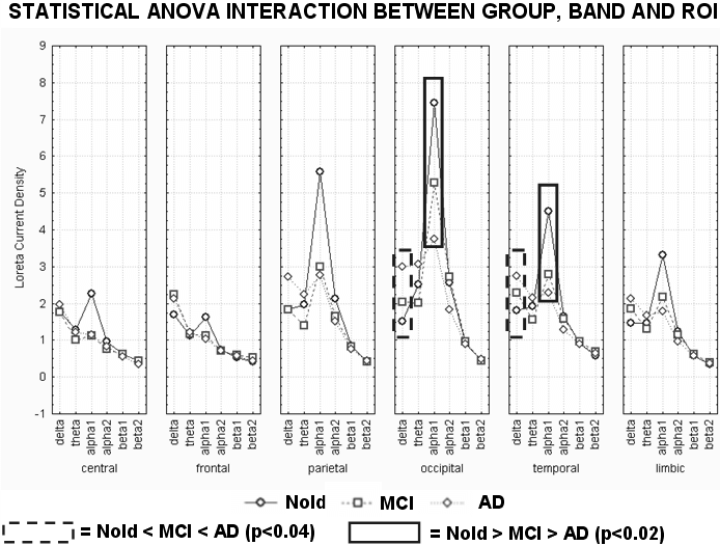
Regional LORETA solutions (mean across subjects) relative to a statistical anova interaction among the factors Group (Nold, MCI, AD), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2), and ROI (central, frontal, parietal, occipital, temporal, limbic). This anova design used the normalized relative current density values at ROI level as a dependent variable. Subjects' age, education and individual alpha frequency peak (IAF) were used as covariates. Regional solutions modelled the EEG relative power spectra as revealed by a sort of ‘virtual’ intracranial macroelectrodes on the macrocortical regions of interest. The rectangles indicate the cortical regions and frequency bands in which LORETA solutions presented statistically significant patterns Nold < MCI < AD or Nold > MCI > AD (P < 0.05, planned Duncan posthoc testing). See Materials and methods for further details.
These four regional solutions were correlated with the MMSE score in all subjects as a whole group (Pearson test; Bonferroni correction for four repetitions of the test gave the threshold P < 0.0125 to obtain the Bonferroni corrected P < 0.05). Figure 4 shows scatterplots among individual regional solutions and MMSE scores of the statistically significant correlations (P < 0.0125). Of note, the regression lines of all subjects as whole group and of the MCI and AD groups separately were reported. The MMSE score negatively correlated with occipital (r = −0.33, P = 0.0003) and temporal (r = −0.32, P = 0.0004) delta sources Furthermore, the MMSE score positively correlated with occipital (r = 0.29, P = 0.001) and temporal (r = 0.31, P = 0.0006) alpha 1 sources. Of note, we also correlated the mentioned four regional LORETA solutions with MMSE score in the Nold, MCI and AD groups separately. There was no statistically significant correlation (P > 0.25). Probably, this result is related to the very small range of the MMSE score within the single groups. These four LORETA sources were considered as specifically sensitive to pathological ageing across the recruited Nold, MCI, and AD subjects.
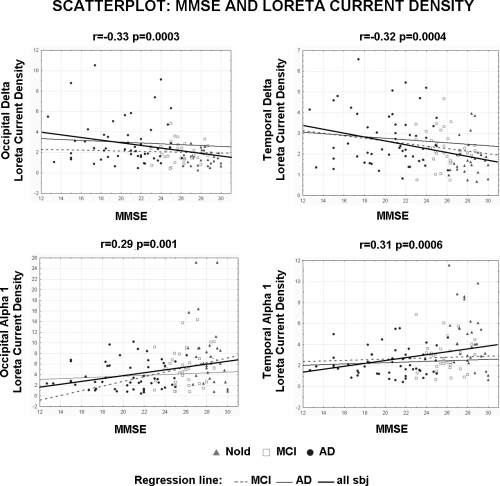
Scatterplots among the individual regional solutions and the MMSE score in all Nold, MCI and mild AD subjects as a single group. These solutions refer to the EEG sources showing statistically significant patterns Nold < MCI < AD or Nold > MCI > AD namely occipital delta source as well as parietal, occipital and temporal alpha 1 sources. Of note, the regression lines of all subjects as whole group and of the MCI and AD groups separately were reported.
The mentioned four regional LORETA sources were then used as an input for the correlation with the neuropsychological measures in MCI and AD subjects as a whole group. In particular, the amplitude of the mentioned four sources were correlated with the following four neuropsychological measures; Rey list immediate recall and Rey list delayed recall, Digit forward and Corsi forward (Pearson test; Bonferroni correction for four sources × four tests gave the threshold P < 0.003 to obtain the Bonferroni corrected P < 0.05). The statistically significant results were as follows; (i) a negative correlation between the occipital delta sources and the Corsi forward score (r = −0.41, P = 0.00007); (ii) a negative correlation between the temporal delta sources and the Corsi forward score (r = −0.34, P = 0.001), and (iii) a positive correlation between the occipital alpha 1 sources and the Digit forward score (r = 0.34, P = 0.001). Figure 5 shows the scatterplots of these statistically significant correlations. Of note, the regression lines of all subjects as whole group and of the MCI and AD groups separately were reported. These scatterplots support the view that the statistical correlations were not due to the effects just in one of the groups.
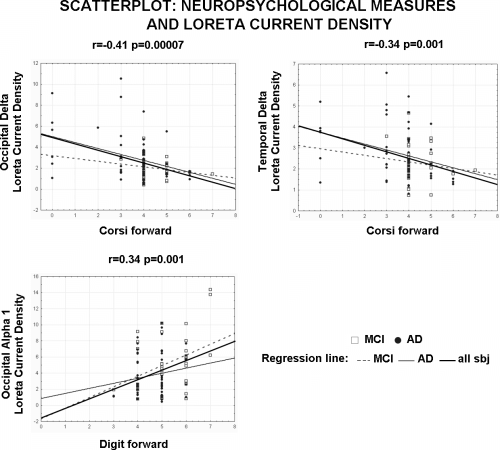
Scatterplots among the individual solutions and neuropsychological measures in MCI, and AD subjects as a single group. Of note, the regression lines of all subjects as a whole group and of the MCI and AD groups separately were reported.
As a further control of the above view, the mentioned three regional LORETA sources were used as an input for the correlation with the neuropsychological measures in MCI and AD subjects as separate groups. Bonferroni correction for three sources × four tests × two groups gave the threshold P < 0.002 to obtain the Bonferroni corrected P < 0.05. Marginal statistical correlations were observed between the occipital delta sources and the Corsi forward score (r = −0.19, P = 0.2 for MCI subjects; r = −0.39, P = 0.004 for AD subjects), between the temporal delta sources and the Corsi forward score (r = −0.13, P = 0.4 for MCI subjects; r = −0.33, P = 0.015 for AD subjects), and between the occipital alpha 1 sources and the Digit forward score (r = 0.37, P = 0.02 for MCI subjects; r = 0.17, P = 0.2 for AD subjects). These results were likely due to the fact that the correlation analysis on each single group reduced the degrees of freedom, the range of the values to be correlated, and then the sensitivity of the statistical computation.
Control analyses
To cross-validate the LORETA differences among the Nold, MCI, and AD groups, the analysis was directly repeated on the EEG data used as an input for the LORETA analysis. The same frequency bands of interest of the LORETA analysis were considered, namely delta (2–4 Hz), theta (4–8 Hz), alpha 1 (8–10.5 Hz), alpha 2 (10.5–13 Hz), beta 1 (13–20 Hz), and beta 2 (20–30 Hz). Five ROIs were considered. These ROIs included, respectively (i) C3, Cz and C4 electrodes for the central region; (ii) F3, Fz and F4 electrodes for the frontal region; (iii) P3, Pz and P4 electrodes for the parietal region; (iv) O1, O2 electrodes for the occipital region, and (v) T3, T4, T5, T6 for the temporal region. Compared to the LORETA results, the limbic region was excluded due to its deep location. The same kind of normalization of the LORETA solutions was used for the EEG spectral solutions of this control analysis. The spectral power density at each electrode was normalized to the spectral power density averaged across all frequencies (0.5–45 Hz) and across all electrodes. The values of normalized spectral power density of the electrodes belonging to the same ROI were averaged at each of the six frequency bands of interest.
The results of the control data analysis were used as inputs for an anova analysis. The values of the normalized, regional spectral power density served as a dependent variable. The anova factors (levels) were Group (Nold, MCI, AD), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2), and ROI (central, frontal, parietal, occipital, temporal). Subjects' age, education, gender and IAF were used as covariates. This anova design showed a statistical interaction (F40,2300 = 8.43; MSe = 1.35; P < 0.0001) between factors Group, Band and ROI (see Fig. 6). Of note, normalized, regional spectral power density showed a clearly lower spatial resolution compared to normalized regional LORETA solutions. According to the statistical results obtained in the LORETA sources analysis, the planned Duncan posthoc testing assessed the progressive differences in the normalized regional spectral power density across the three groups, that is Nold > MCI > AD or Nold < MCI < AD. In that line, occipital alpha 1 and parietal alpha 2 sources showed stronger amplitude in Nold compared to MCI group (P < 0.02) and in MCI compared to mild AD group (P < 0.00001). Furthermore, the planned Duncan posthoc showed that occipital delta sources was lower in Nold compared to MCI and AD groups (P < 0.02). On the whole, these anova results confirmed the differences among Nold, MCI and AD in line with the results of the LORETA analysis.
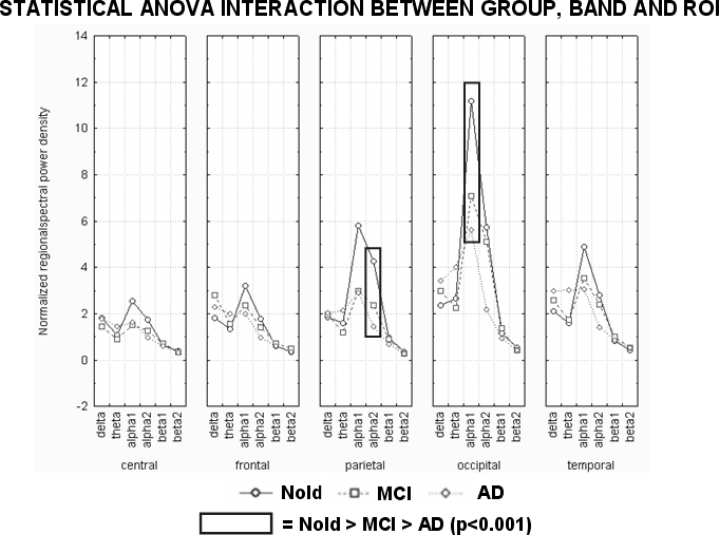
Normalized regional EEG spectral power density (mean across subjects) relative to a statistical anova interaction among the factors Group (Nold, MCI, AD), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2), and ROI (central, frontal, parietal, occipital, temporal). This control anova design was focused on the EEG data used as an input for the LORETA analysis, in order to cross validate the LORETA solutions. The anova design used the normalized regional EEG spectral power density as a dependent variable. Subjects' age, education, gender and individual alpha frequency peak (IAF) were used as covariates. The rectangles indicate the cortical regions and frequency bands in which normalized regional EEG spectral power density presented statistically significant patterns Nold < MCI < AD or Nold > MCI > AD (P < 0.05, planned Duncan posthoc testing).
Discussion
Control analysis validated the procedures for subjects' selection and EEG data analysis
A preliminary control analysis allowed the modelling of resting EEG sources (LORETA solutions) sensitive to global cognitive status (MMSE score) in the recruited Nold, MCI, and AD subjects. Delta (2–4 Hz) and alpha 1 (8.0–10.5 Hz) sources at occipital and temporal areas had an intermediate magnitude in MCI subjects when compared to AD and Nold subjects. The magnitude of these four cortical EEG sources showed linear correlations with the MMSE score (global cognitive level) across all Nold, MCI, and AD subjects considered as a single group. These results suggest that delta and alpha cortical EEG rhythms change in pathological ageing as a function of the global cognitive level (Babiloni et al., 2006a) and are globally in line with previous evidence showing an enhancement of the delta rhythms in MCI and AD compared to Nold subjects (Prichep et al., 1994; Jelic et al., 2000; Grunwald et al., 2001; Wolf et al., 2003; Babiloni et al., 2004a, 2006b) and a magnitude decrease of the alpha rhythms in AD and MCI compared to Nold subjects (Dierks et al., 1993, 2000; Jelic et al., 1996, 2000; Rodriguez et al., 1999a, 1999b; Huang et al., 2000; Grunwald et al., 2001; Frodl et al., 2002; Babiloni et al., 2004a, 2006a; Moretti et al., 2004).
As an exception, our procedure could not disclose EEG changes at beta frequencies in AD vs. Nold subjects (Claus et al., 2000, 1998). This may be due to the use of quite large frequency bands for beta 1 (13–20 Hz) and beta 2 (20–30 Hz), together with the average of LORETA solutions across large cortical regions. Of note, the procedure served to reduce the mismatch between the limited number of EEG channels (i.e. 19) and the high number of LORETA voxels (i.e. 2394).
From a methodological viewpoint, the validation of the procedures for subjects' selection and EEG data analysis corroborated the original results of the present study, namely those on the correlations among indexes of attentional and memory functions and EEG sources of delta and alpha rhythms. Of note, inhibitors of acetylcholinesterase were administrated only to AD patients (drug dose practically equal in all). Therefore, it is unlikely that the cholinergic therapy per se could induce a marked linear correlation between indexes of memory/attentional functions and the mentioned cortical EEG rhythms across MCI and AD subjects. Rather, it is probable that the present findings reflect in some way cerebral atrophy due to neurodegenerative processes. However, the available data sets made it possible to evaluate just the correlative nature of the relationship among EEG rhythms, neurodegenerative processes, and cognitive functions.
Visuo-spatial immediate memory correlates with delta EEG sources in MCI and AD subjects
A statistically significant negative correlation (Bonferroni corrected, P < 0.05) was observed between the Corsi span forward score and the amplitude of occipital and temporal delta sources (as revealed by LORETA solutions) across MCI and AD subjects. A crucial question is then ‘Why did index of visuo-spatial immediate memory correlate with delta but not alpha rhythms in the continuum of MCI and AD subjects? A tentative answer to this question stems upon a different sensitivity to brain atrophy of the subcortical and cortical systems that produce and modulate delta and alpha rhythms. During slow-wave sleep, corticofugal slow oscillations (< 1 Hz) are effective in grouping thalamic-generated delta rhythms (1–4 Hz) and spindling activity (7–14 Hz) rhythms (Steriade, 2003). Delta would dominate EEG rhythms and alpha (approximately 8–12 Hz) would be low in amplitude. In the case of brain arousal, spindles, high and low components of the delta rhythms are blocked by the inhibition of reticulo-thalamic (7–14 Hz), thalamo-cortical (1–4 Hz), and intracortical (< 1 Hz) oscillators. These rhythms are replaced by fast oscillations (beta and gamma) induced by forebrain (nucleus basalis) cholinergic inputs to the hippocampus and cortex as well as by thalamocortical projections (Steriade et al., 1996; Steriade, 2003). In the wake rest, alpha would dominate the EEG rhythms and delta would be low in amplitude (Steriade & Llinas, 1988; Brunia, 1999; Pfurtscheller & Lopez da Silva, 1999). The relative amplitude of delta and alpha rhythms during the mentioned sleep and awakening would suggest a sort of ‘reciprocal inhibition’ between their generators.
Keeping in mind this theoretical framework, the observed correlation between visuo-spatial immediate memory and occipital delta sources could be interpreted on the basis of two main mechanisms. The first tentative mechanism is the loss of cholinergic basal forebrain neurons projecting to the hippocampus and posterior cortical connections (Helkala et al., 1996; Holschneider et al., 1999; Mesulam et al., 2004). These neurons, together with serotoninergic neurons, would explain the replacement of spindles and delta rhythms by fast EEG rhythms during wakefulness (Dringenberg, 2000; Dringenberg et al., 2002). Several lines of evidence have shown that experimental lesions of the basal forebrain increased the amplitude of slow EEG rhythms (Stewart et al., 1984, 1984; Buzsaki et al., 1988; Ray & Jackson, 1991). The same was true for clinical lesions to the basal forebrain as those observed in AD subjects (Dierks et al., 1993, 2000; Rodriguez et al., 1999a, 1999b; Huang et al., 2000; Babiloni et al., 2004a; Mesulam et al., 2004; Moretti et al., 2004; Babiloni et al., 2006b). These lesions were found to relatively spare brainstem cholinergic innervation of the thalamus (Mash et al., 1985; Geula & Mesulam, 1989, 1996, 1999; Tanaka et al., 2003; Mesulam et al., 2004).
The second tentative mechanism explaining the negative correlation between occipital and temporal delta sources and visuo-spatial immediate memory would rely on the loss of cortical neurons normally impinged by the cholinergic inputs. Cortical atrophy would reflect the impairment of input/output cortical information flows to the cortex in MCI and AD subjects. This would cause a cortical ‘disconnection mode’ that would pathologically disclose the cortico-fugal EEG rhythms triggering thalamo-cortical delta rhythms. This explanation is in line with recent evidence showing that in mild to moderate AD patients, the amount of cortical atrophy in MRI measurements significantly correlates with memory, verbal fluency, and executive performance (Capizzano et al., 2004; Gootjes et al., 2004; Tullberg et al., 2004). Indeed, a strict relationship links together cortical atrophy, increase of slow EEG rhythms, and relative cortical hypoperfusion (Stigsby et al., 1981; Brenner et al., 1986; Rae-Grant et al., 1987; Dossi et al., 1992; Kwa et al., 1993; Steriade, 1994; Passero et al., 1995; Nobili et al., 1998; Rodriguez et al., 1999a). Furthermore, previous studies have reported the effects of early mesial-temporal degeneration on hippocampus-cortical connectivity in MCI and AD subjects (Killiany et al., 1993) and the effects of hippocampal-entorhinal atrophy on posterior delta rhythms (Fernandez et al., 2003).
Both explanations should be, however, considered as tentative explanations that require further empirical support. For example, interpretation of delta sources in terms of disinhibited thalamo cortical oscillations is very hypothetical based on surface EEG data. Furthermore, in some cases cortical atrophy can cause a general reduction of EEG amplitude rather than increase of delta. Finally, the notion of a relationship between ‘disconnection’ and immediate spatial attention in ageing will have to be corroborated with measurements of functional cortical connectivity (Thatcher et al., 2005).
The reasons why alpha rhythms were not significantly correlated with Corsi span forward are unknown at the present early stage of research. It can be speculated that the lack of a significant correlation is merely due to the relatively low number of subjects (i.e. correlation trend between Corsi span forward and the amplitude of temporal alpha 1 sources, P = 0.06). Alternatively, this might be intrinsically due to the fact that the performance in question is mainly related to the immediate visual–spatial perceptive impression of the stimuli within posterior cortical areas, which would be mainly subserved by the cholinergic enhancement of thalamo-cortical inputs (Furey et al., 2000) rather than by the cortico-cortical re-entrant functional loops that especially modulate alpha rhythms (Nunez et al., 2001).
Immediate digit memory correlates with alpha EEG sources in MCI and AD subjects
A positive correlation was shown among the Digit span forward score and the occipital alpha 1 sources (Bonferroni corrected, P < 0.05). From a physiological point of view, alpha rhythms are mainly modulated by thalamo–cortical and cortico–cortical interactions facilitating or inhibiting (i) the transmission of sensorimotor information among subcortical and cortical pathways and (ii) the retrieval of semantic information from cortical storage (Brunia, 1999; Pfurtscheller & Lopez da Silva, 1999; Steriade & Llinas, 1988). Furthermore, a large amount of scalp alpha rhythms in the resting condition is usually considered as a sign of brain wakeful idling and inhibitory processes. Within the extended alpha band (8–13 Hz) lower frequencies would be mainly related to attentional components, whereas higher frequencies would reflect processing of task-specific sensorimotor or semantic information (Steriade & Llinas, 1988; Rossini et al., 1991; Klimesch, 1996; Klimesch et al., 1997, 1998). At rest condition, voltage of the alpha rhythms would be inversely correlated with cortical excitability and emerging attention in relation to novelty and salience of the stimulus. For this reason, it has been suggested that amplitude of alpha rhythms and corresponding cortical excitability reflect arousing inputs of cholinergic forebrain to cerebral cortex (Ricceri et al., 2004).
In the present study, the correlations between cortical alpha sources and psychometric score were observed with immediate memory functions rather than with short- and medium-term memory functions requiring storage information for tens of seconds (namely, Rey list tests). Indeed, immediate memory functions are mainly based on the prestimulus functional brain state (as reflected by resting alpha rhythms) with respect to short- and medium-term memory functions, which mainly depend on the functional brain state during the on-going stimulation lasting several seconds. In that sense, it is probable that on-going event-related alpha rhythms would have been significantly correlated with the short- and medium-term memory functions, in line with previous investigations (Neubauer et al., 1995; Klimesch, 1999).
The present results complement previous evidence showing that alpha rhythms constitute an important neural substrate for human cognition (Klimesch et al., 1997, 1999; Klimesch et al., 1998, 2001, 2003, 2004; Vogt et al., 1998; Jensen et al., 2002; Sauseng et al., 2002; Bauer et al., 2006; Medendorp et al., 2006; Jokisch & Jensen, 2007; Palva & Palva, 2007; Tuladhar et al., 2007). Specifically, they complement previous evidence reporting that posterior and central alpha rhythms parametrically increased in power with memory load during short-term retention phase of a Sternberg's task (Jensen et al., 2002; Tuladhar et al., 2007). Furthermore, parieto-occipital alpha rhythms increased in power during the retention phase of face identities when compared to the retention phase of face orientations (Jokisch & Jansen, 2007). The present results also lend support to the idea that the high power of prestimulus alpha rhythms reflects back-ground cortical inhibitory oscillations that can allow a poststimulus information processing suitable for memorization but not for fine perceptual discrimination (Worden et al., 2000; Ergenoglu et al., 2004; Thut et al., 2006; Hanslmayr et al., 2005b; Kelly et al., 2006). In the prestimulus period, the high power of alpha rhythms predicts a good cognitive performance, namely completing a specific function or task with fewer errors and greater efficiency (Neubauer et al., 1995; Klimesch, 1999). For example, successful retrieval of information from semantic or episodic long-term memory would depend on the decrease of posterior alpha power, reflecting the functional mode of thalamo-cortical and cortico-cortical feedback loops (Klimesch, 1999; Pfurtscheller & Lopez da Silva, 1999). A general rule is as follows; the stronger prestimulus alpha power the better the cognition/memorization (Neubauer et al., 1995; Klimesch, 1999), although challenging evidence has been presented (Worden et al., 2000; Thut et al., 2006). To test the hypothesis that prestimulus alpha rhythms are concerned with cognitive performance, previous studies have tried to enhance them by repetitive transcranial magnetic stimulation at the frequency of alpha rhythms (Klimesch et al., 2003). Similar effects have been observed enhancing the power of alpha rhythms by neurofeed-back in real time (Hanslmayr et al., 2005a). Results of those studies have shown that cognitive performance improved only in the subjects in whom the mentioned procedures enhanced the power of prestimulus alpha rhythms (Klimesch et al., 2003; Hanslmayr et al., 2005a). Power of EEG alpha rhythms and cognition might be also modulated by the use of flickering visual stimulation at approximately 10 Hz. Previous evidence in humans has documented that brief visual flicker stimuli at 10 Hz, given before trigrams, specifically enhanced both 10-Hz occipital EEG power and recognition memory; this did not happen at adjacent (approximately 9 and 12 Hz) stimulation frequencies (Williams, 2001). Also, it has been shown that brief 10-Hz flickering visual stimulation specifically induced an increment of recognition memory in healthy elderly subjects (Williams et al., 2006).
Why were delta rhythms not significantly correlated with Digit span forward? Again, a definitive explanation is unknown at the present early stage of research. We can speculate that it might be due either to the relatively low number of subjects (i.e. correlation trend between Digit span forward and the amplitude of temporal delta sources, P = 0.03) or to the fact that an immediate repetition of digits after auditory stimuli implies some sort of subvocal rehearsal, which requires effective cortico-cortical re-entrant functional loops (i.e. Wernicke-Broca circuit) modulating alpha rhythms (Nunez et al., 2001) rather than immediate auditory perception subserved by the cholinergic enhancement of thalamo-cortical inputs.
Conclusions
Are cortical sources of resting EEG rhythms correlated with memory functions across MCI and AD subjects? A statistically significant negative correlation (Bonferroni corrected, P < 0.05) was observed between the Corsi span forward score and the amplitude of occipital or temporal delta sources across MCI and AD subjects. Furthermore, a positive correlation was shown among the Digit span forward score and the occipital alpha 1 sources (Bonferroni corrected, P < 0.05). These results suggest that cortical sources of resting delta and alpha rhythms correlate with neuropsychological measures of immediate memory based on focused attention in the continuum of MCI and AD subjects. They also prompt future studies for the combination of measurements of cerebral atrophy, EEG functional connectivity, and indexes of immediate memory as well as for the evaluation of EEG rhythms during attention and memory tasks.
Acknowledgements
We thank, Francesca Bergami, Tania Carfagna, Nicola Girtler, and Katiuscia Sosta for their precious help in the development of the present study. We thank also Prof Fabrizio Eusebi for his continuous support. The research was granted by Association Fatebenefratelli for Research (AFaR).
Abbreviations
-
- AD
-
- Alzheimer's disease
-
- EEG
-
- electroencephalography
-
- IAF
-
- individual alpha frequency
-
- LORETA
-
- low resolution brain electromagnetic tomography
-
- MCI
-
- mild cognitive impairment
-
- MMSE
-
- Mini Mental State Examination
-
- Nold
-
- normal elderly
-
- ROIs
-
- regions of interest




