Repetition suppression of induced gamma band responses is eliminated by task switching
Abstract
The formation of cortical object representations requires the activation of cell assemblies, correlated by induced oscillatory bursts of activity > 20 Hz (induced gamma band responses; iGBRs). One marker of the functional dynamics within such cell assemblies is the suppression of iGBRs elicited by repeated stimuli. This effect is commonly interpreted as a signature of ‘sharpening’ processes within cell-assemblies, which are behaviourally mirrored in repetition priming effects. The present study investigates whether the sharpening of primed objects is an automatic consequence of repeated stimulus processing, or whether it depends on task demands. Participants performed either a ‘living/non-living’ or a ‘bigger/smaller than a shoebox’ classification on repeated pictures of everyday objects. We contrasted repetition-related iGBR effects after the same task was used for initial and repeated presentations (no-switch condition) with repetitions after a task-switch occurred (switch condition). Furthermore, we complemented iGBR analysis by examining other brain responses known to be modulated by repetition-related memory processes (evoked gamma oscillations and event-related potentials; ERPs). The results obtained for the ‘no-switch’ condition replicated previous findings of repetition suppression of iGBRs at 200–300 ms after stimulus onset. Source modelling showed that this effect was distributed over widespread cortical areas. By contrast, after a task-switch no iGBR suppression was found. We concluded that iGBRs reflect the sharpening of a cell assembly only within the same task. After a task switch the complete object representation is reactivated. The ERP (220–380 ms) revealed suppression effects independent of task demands in bilateral posterior areas and might indicate correlates of repetition priming in perceptual structures.
Introduction
One of the most basic forms of mnemonic functioning is repetition priming, an implicit form of memory that can be behaviourally observed in improved performances after stimulus repetition (Tulving & Schacter, 1990). A robust neuronal correlate of repetition priming is the ‘repetition suppression’ of neuronal activity elicited by primed material (Desimone, 1996). Recently, it was suggested that this reduction mirrors the ‘sharpening’ of a cortical stimulus representation, i.e. a mechanism providing a sparser and more efficient network (Wiggs & Martin, 1998; Grill-Spector et al., 2006).
Repetition suppression has been observed at multiple levels of analysis: for single cortical neurons (Li et al., 1993), haemodynamic responses (Henson et al., 2000; Fiebach et al., 2005) and event-related potentials (ERPs) (Penney et al., 2001; Henson et al., 2004). In particular, an ERP component at ∼200–400 ms after stimulus onset shows reduced amplitudes after stimulus repetition. Although these electroencephalogram (EEG) studies provide useful insights into the neuronal mechanism of repetition priming, ERPs do not disclose the whole spectrum of relevant electro-cortical activity (Gruber et al., 2002, 2004b). In particular, so-called ‘induced’ responses which occur with jitters in latency from one trial to the next are averaged out in the ERP (Eckhorn et al., 1990). One extensively studied form of induced brain activity are oscillatory bursts in the gamma frequency range (∼20–100 Hz) at ∼250 ms after stimulus onset (induced gamma band responses; iGBRs; Tallon-Baudry & Bertrand, 1999; Bertrand & Tallon-Baudry, 2000; Keil et al., 2001; Tallon-Baudry, 2003; Kaiser & Lutzenberger, 2005). iGBRs are considered as a correlate of the transient integration of widespread and functionally specific brain areas, a crucial mechanism for the establishment of a cortical object representation (Singer, 1999; Engel & Singer, 2001). Such cortical object representations have been shown to be ‘sharpened’ after stimulus repetition (Gruber & Müller, 2002; Gruber et al., 2004a). Additionally, it was shown that iGBRs mirror sharpening within ‘semantic’ cell assemblies, whereas ERP effects can be associated with suppression effects within perceptual networks (Fiebach et al., 2005; Gruber & Müller, 2005). These studies focused on the influence of stimulus properties on the priming-related processes. Top-down influences on these mechanisms remain largely unexplored. Therefore, the present study examined the influence of task demands on repetition-related iGBR modulations. In particular, we contrasted iGBRs when the same task was performed on initial and repeated object presentations (no-switch condition) with repetitions in the context of a task switch (switch condition). Furthermore, evoked electro-cortical responses were analysed. We expected to replicate the previously reported iGBR suppression (sharpening) in the no-switch condition. Additionally, we assumed that in order to fulfil the new task demands after a task switch, a large part of the object representation has to be reactivated. Thus, we expected no iGBR suppression after a task switch.
The repetition-sensitive ERP component at ∼200–400 ms might be mainly related to activity of perceptual networks, and thus it should be modulated independent of task demands.
In order to learn more about the cortical distribution of the assumed networks, all responses were modelled in source space using VARETA (Variable Resolution Electromagnetic Tomography; Gruber et al., 2006).
Materials and methods
Participants
Fourteen healthy, right-handed university students (nine female; 19–29 years, mean 22.2 ± 0.8 years) received class credits for participation. All had normal or corrected-to-normal visual acuity. Informed consent was obtained from each participant. The study conformed with the Code of Ethics of the World Medical Association.
Stimuli and procedure
Stimuli were 440 coloured pictures of concrete objects taken from a standard picture library (Hemera Photo-Objects Volume 1, Hemera Technologies, Gatineau, Quebec Canada). The pictures depicted living or non-living entities, which were either bigger or smaller than a shoebox (110 pictures per category). The four categories were matched for averaged luminance and number of pixels. Stimuli were presented on a black background in the centre of a 19-inch computer screen placed 1.5 m in front of the participants (frame rate 70 Hz). The pictures covered an average visual angle of 5.5 × 5.5°. All trials started with a cue (400 ms), which instructed the participant as to the upcoming task. The cue (approximately 2.1 × 0.5°) was either the word NATUR (German for ‘nature’) or KARTON (German for ‘box’). The ‘nature’ cue indicated that volunteers had to make a ‘living/non-living’ decision regarding the depicted object; the ‘box’ cue required a ‘bigger/smaller than a shoebox’ evaluation. After the presentation of the cue, a randomized 500–800-ms baseline period during which a fixation cross (0.5 × 0.5°) was presented was followed by the picture (depicted for 700 ms). Picture onset was synchronized to the vertical retrace of the monitor. The stimulus was then replaced by the fixation cross, which remained on screen for another 800 ms and was followed by a blank screen (900 ms). Participants were instructed to react as fast and as accurately as possible and to press one key for a ‘living’ or ‘smaller than a shoebox’ stimulus, and another key for a ‘non-living’ or ‘bigger than a shoebox’ stimulus. Key-to-task allocations were counterbalanced across participants. Furthermore, the volunteers were instructed to avoid eye movements and blinking during the display of the fixation cross or a stimulus.
Stimulus sequence
From the stimulus pool of 440 pictures, 432 pictures were randomly chosen for the experiment. The remaining pictures were used for training purposes. Stimuli were presented either twice, with one or two intervening items (with the number of intervening items being randomized) or they were presented once (fillers). Filler items were introduced to avoid expectations regarding the second presentation and were not further analysed. Recurring pictures were either repeated with the same task requirements (no-switch condition) or with a different task (switch condition). In particular, in the no-switch condition 72 objects were preceded by the cue ‘box’ in the first and second presentation (144 trials), or by the cue ‘nature’, respectively (144 trials). In the switch condition 72 images were preceded by the cue ‘nature’ and repeated with the cue ‘box’ (144 trials) or vice versa (144 trials). Furthermore, 72 objects were presented only once with the cue ‘box’ or ‘nature’, respectively (144 fillers). This design results in 720 trials, which were presented with a randomized task order. To allow for resting intervals, the experiment was divided into six blocks of 120 trials. Before the experiment was started, participants performed a training session in order to become accustomed to the key-to-task allocations.
Figure 1 depicts an excerpt of the stimulus sequence. Together with the dimensions of task switch (no-switch and switch) and repetition (1st and 2nd presentation), the experimental set-up resulted in a four-level factorial design. Importantly, stimulus repetition was task-irrelevant in both conditions. Thus, the experimental design can be regarded as an implicit (or indirect) assessment of repetition-related memory effects [see Gruber & Müller (2006) and Henson et al. (2002) for a similar definition and for a comparison of indirect and direct assessments of memory in stimulus-repetition paradigms].
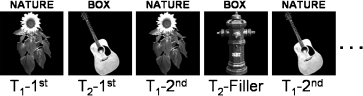
Excerpt of the stimulus sequence. Task 1 (T1; indicated by the cue ‘NATURE’) required a ‘living/non-living’ decision. Task 2 (T2, ‘BOX’) entailed a ‘bigger/smaller than a shoebox’ classification. The sunflower is repeated without task switch (no-switch condition). The repetition of the guitar is an example of the task switch condition. Fillers are interspersed to exclude expectancy effects.
Data analysis: behavioural data
Only reaction times between 300 and 1200 ms after stimulus onset were considered to be correct responses. Reaction times shorter or longer than that period were seen as false alarms or missed responses. Furthermore, a response was classified as being incorrect when different classifications were made for initial and repeated presentations. Behavioural data were analysed by means of a four-level repeated measurements anova with the factor CONDITION (1st and 2nd presentations with and without task switch). Only correct responses were analysed in this and all further analyses described below.
Electrophysiological recordings
EEG was recorded continuously from 128 Ag/AgCl electrodes with a BioSemi Active-Two amplifier system (BioSemi, Amsterdam, The Netherlands). To monitor for eye movements and blinks the horizontal and vertical electrooculogram (EOG) was recorded. EEG and EOG were sampled at 512 Hz. Two additional electrodes (CMS-Common Mode Sense and DRL-Driven Right Leg) were used as reference and ground (see http://www.biosemi.com/faq/cms&drl.htm for details). For further offline analysis the Average Reference was used. The EEG was segmented to obtain epochs starting 500 ms prior and 1500 ms following picture onset. Artefact correction was performed by means of ‘statistical correction of artifacts in dense array studies’ (SCADS; Junghöfer et al., 2000). This procedure is widely accepted in the field and has been applied and described in several publications (e.g. Gruber et al., 1999; Keil et al., 1999). Using this approach, three subjects were excluded owing to excessive artefact numbers. For the remaining 11 subjects the average rejection rate was 20% resulting in ∼115 trials per condition (note that approximately 4% of the trials had to be rejected owing to the above mentioned behavioural criteria, 16% owing to recording artefacts).
Data analysis: spectral changes
Spectral changes in oscillatory activity were analysed by means of Morlet wavelets with a width of seven cycles per wavelet. This procedure is described in detail elsewhere (e.g. Bertrand & Pantev, 1994; Tallon-Baudry & Bertrand, 1999). In brief, the method provides a time-varying magnitude of the signal in each frequency band, leading to a time-by-frequency (TF) representation of the data. Importantly, given that induced gamma band responses occur with a jitter in latency from one trial to another (Eckhorn et al., 1990), they tend to cancel out in the averaged evoked potential. Thus, TF amplitude is averaged across single-trial frequency transformations, allowing analysis of non-phase-locked components. Furthermore, as we focused on the non-phase-locked components of the signal, the evoked response (i.e. the ERP) was subtracted from each trial before frequency decomposition (for a similar procedure, see: Gruber et al., 2002, 2004b; Fiebach et al., 2005; Gruber & Müller, 2005).
In order to identify the latency and frequency range of induced spectral peaks, mean baseline-corrected spectral amplitude (baseline: 400–100 ms prior to stimulus onset) across all electrodes and across all experimental conditions was represented in a TF plot for the 30–100-Hz range. For further analysis a time window of maximum induced gamma amplitudes (200–300 ms after stimulus onset; see Results, Fig. 2A) was used. Owing to inter-individual differences in the gamma peak frequency, for each subject the wavelet designed for the frequency of his/her maximal amplitude in the defined gamma range was chosen. Electrodes for further analysis were chosen based on a spherical-spline interpolated topographical distribution (Perrin et al., 1988) of the gamma peak averaged across all conditions (these electrodes are indicated in Fig. 2B). Subsequently, we calculated a repeated-measurement anova with a four-level factor of CONDITION (1st and 2nd presentations with and without task switch). It is noteworthy that our approach, namely to average all experimental conditions to define the relevant peaks, seems inevitably to avoid biasing the choice of the individual peak wavelets, and thus the comparisons between conditions in the subsequent anova[see Fiebach et al. (2005) for a similar approach].
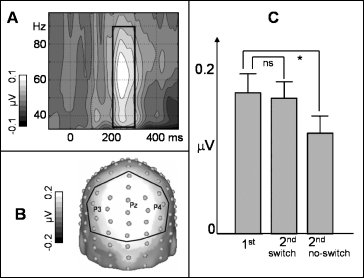
(A) Grand mean baseline-corrected TF plot (across all experimental conditions) and across all electrodes (the box indicates the induced gamma peak). (B) Grand mean spherical-spline interpolated amplitude map (averaged across all conditions) for the induced gamma peak at 200–300 ms. (C) Mean induced gamma peak amplitudes (+SEM) at parieto-occipital electrodes (as indicated in B) subdivided according to the experimental conditions. Note: initial presentations with and without subsequent task switch are averaged in the bar chart. *P < 0.05.
Furthermore, in order to examine the influence of stimulus–response associations on the present results, we have analysed the variations of iGBRs depending on the laterality of the two consecutive responses. In particular, we have extracted second presentations, which required a response with the same hand in both presentations. Additionally, second presentations which required a change of the responding hand as opposed to the first presentation were extracted. The resulting data was analysed by means of a paired t-test (2nd presentations with vs. 2nd presentations without changes of the response hand) for a similar time range, frequency range and electrode range as described above. If a simple stimulus–response association were to have a major influence on our results, we would expect higher iGBRs in the ‘changed hand’ as opposed to the ‘same hand’ case (after the 2nd presentation).
Evoked gamma oscillations were analysed by means of spectral decompositions of the averaged and unfiltered evoked response. For statistical analysis a time window from 70 to 120 ms after stimulus onset and a frequency range from 35 to 45 Hz was analysed by means of the anova model described above. The evoked peak was identified on the basis of a TF plot averaged across posterior electrode sites [see Herrmann et al. (2004b) for a similar approach and Fig. 3B for locations of the electrodes used].
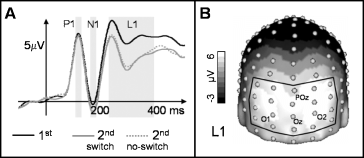
(A) Grand mean baseline corrected ERPs for initial (solid, black) and repeated object presentation (switch and no-switch condition) at a posterior regional mean (the utilized electrodes are outlined in B). Boxes indicate time windows as used for mean amplitude statistics (P1, N1, L1). (B) Grand mean spherical-spline interpolated amplitude map (averaged across all conditions) for the L1 component (220–380 ms).
To exclude baseline differences between conditions as an alternative explanation for our results, we have tested the baseline uncorrected evoked and the induced frequency range with the identical anova model described above, in a time window from 400 to 100 ms prior to stimulus onset.
Data analysis: ERP
A 25-Hz low-pass filter was applied to the data before all ERP analysis. Based on previous findings regarding the repeated presentation of pictures similar to those used in the present study (Gruber & Müller, 2002; Gruber et al., 2004a) and the baseline-corrected grand mean evoked potential (baseline: 400–100 ms prior to stimulus onset) at posterior electrode sites (see Fig. 3), three ERP components were defined: two early components, P1 (105–135 ms) and N1 (155–185 ms), and a later component, L1 (220–380 ms). Mean amplitudes averaged across the respective time windows and posterior recording sites were analysed using the repeated-measurement anova model described above.
Wherever appropriate, P-values were adjusted by Greenhouse–Geisser corrections in all anovas. Planned comparisons were conducted in order to analyse relevant differences by means of paired t-tests. In particular, we contrasted second presentations with the corresponding initial presentations separately for the task switch and the no-switch condition. To validate the robustness of the data, initial presentations with and without subsequent switch were compared as well. No significant effect was expected here. Means and standard errors (SE) are presented throughout.
Data analysis: source modelling (VARETA)
To reconstruct the estimated sources of the observed effects in the time and frequency domain an adaptation of VARETA was used. A detailed description of this approach can be found elsewhere (Bosch-Bayard et al., 2001; Trujillo-Barreto et al., 2004; Gruber et al., 2006). In brief, VARETA estimates the spatially smoothest generator estimates compatible with the observed scalp topographies. Furthermore, the method places anatomical constraints upon the allowable solutions. In particular, the generators of the EEG data inside the brain are mapped by using a three-dimensional (3D) grid of points (or voxels) that represent possible sources of the signal and for which the probability for grey matter is different from zero (based on the average probabilistic brain atlas produced by the Montreal Neurological Institute; Evans et al., 1993). To uncover the sources of iGBR peaks, single trials were first transformed into the frequency domain. Subsequently, single trial estimates of the primary current densities in source space (i.e. at the pre-defined 3D grid locations) are computed for individually selected gamma peaks (see above). In order to localize differences in activation between first and repeated presentations, statistical comparisons were carried out by means of dependent anova one-way statistical designs. Corresponding statistical parametric maps (SPMs) were constructed based on the output of the one-way anovas. Activation threshold correction to account for spatial dependencies between voxels is calculated by means of random field theory (Worsley et al., 1996). Subsequently, the SPMs were projected onto coronal, axial and sagittal planes. To uncover the generators of relevant ERP components, a similar approach was applied to averaged ERPs.
Regarding all SPMs, the results were thresholded at a significance level of P < 0.01.
Results
Behavioural data
Participants' correct responses were on average 96.3 ± 2.1%. The averaged reaction time for initial picture presentations was 729 ± 37 ms. Repeated presentations after a task switch resulted in a mean reaction time of 709 ± 36 ms; in the ‘no-switch’ situation participants reacted at 646 ± 32 ms. Statistical analysis resulted in a significant main effect of CONDITION (F3,30 = 25.1, P < 0.0001). Planned comparisons revealed a significant priming effect in the no-switch condition (t10 = 9.9, P < 0.0001). No significantly faster reaction times were observed in the cases in which the second presentation required a different response as compared with the first (switch condition).
Spectral changes
Figure 2A depicts the baseline-corrected TF plot averaged across all experimental conditions, all 128 electrodes and 11 subjects. Spectral amplitudes in the induced gamma range show a clear peak in a time window from 200 to 300 ms after stimulus onset in a frequency range between approximately 30 and 90 Hz (see box in Fig. 2A). The topographical distribution of a gamma peak showed a maximum at parieto-occipital electrode sites (see Fig. 2B). For further analyses the regional mean indicated in Fig. 2B was used. The anova resulted in a significant main effect of CONDITION (F3,30 = 5.2, P = 0.01), which is depicted in Fig. 2C. Note that for the sake of clarity initial presentations, which were succeeded by a task switch, and initial presentations without subsequent switch are averaged in the bar chart (no significant difference was found for first presentations with and without subsequent switch). For the no-switch condition, a planned comparison revealed a significant amplitude decrease (suppression) from first to second presentations (t10 = 2.5, P < 0.05). No significant change of iGBR peak amplitudes was found for second presentation in the switch condition.
Furthermore, the comparison of the iGBR elicited by 2nd presentations with vs. 2nd presentations without changes of the response hand revealed no significant effect (t10 < 1).
Spectral amplitudes in the evoked gamma range were characterized by a peak in a time window from approximately 70 to 120 ms and a frequency range between approximately 35 and 45 Hz. However, the analysis of these peaks revealed no significant effects.
Importantly, tests for baseline differences (400–100 ms prior to stimulus onset) between conditions revealed no significant effects for the evoked and the induced gamma band response, respectively (all F-values < 1).
Event related potential
Figure 3A depicts posterior regional mean ERPs for all conditions (as with the iGBR peak, initial presentation with and without subsequent switch revealed no significant differences and were averaged). We found no significant effects for the P1 and N1 components. The topographical distribution of the late component L1 (220–380 ms) is depicted in Fig. 3B. Statistical analysis of the posterior regional mean (see Fig. 3B) resulted in a significant effect of CONDITION (F3,30 = 24.2, P < 0.0001), reflecting generally lower amplitudes for repeated as compared with initial presentations in the switch condition (t10 = 6.4, P < 0.0001) and in the no-switch condition (t10 = 5.36, P < 0.001; see Fig. 3A).
Source modelling (VARETA)
Figure 4A shows the projection of voxels that exhibited significant repetition-related differences in the no-switch condition for the iGBR peak (200–300 ms) onto axial, coronal and sagittal planes. We found widespread source modulations of the suppression effect in the right superior parietal lobe spreading to occipital areas, the left superior occipital gyrus, right inferior temporal gyrus, and in the left inferior frontal gyrus.
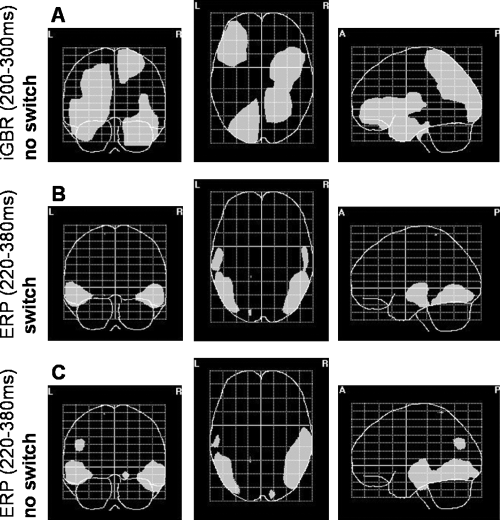
(A) Voxels which exhibit significant repetition-related effects (P < 0.01) in the induced gamma range (200–300 ms) in the no-switch condition projected onto coronal, axial and sagittal planes. Note: as no significant differences were found for the switch condition, the respective SPMs are not shown. (B and C) As in A, for repetition-related differences of the L1 ERP component in the switch and no-switch condition.
As no significant repetition-related effects were found for primed objects presented under different task instructions, no inverse solution is presented for the switch condition.
Figure 4B and C show the projection of significant voxels of the inverse solutions corresponding to the L1 component of the ERP. We found a similar pattern of repetition-related effects for both the switch (Fig. 4B) and the no-switch (Fig. 4C) condition. Source modulations in bilaterally distributed middle temporal, inferior temporal and lateral occipitotemporal areas can be seen.
Discussion
The present study was designed to investigate the influence of task demands on repetition-related modulations of iGBRs at the scalp surface and in source space. In particular, participants performed either a ‘living/non-living’ or a ‘bigger/smaller than a shoebox’ classification on repeated pictures of everyday objects. We contrasted repetition-related iGBR effects after the same task was used for initial and repeated presentations (no-switch condition), to repetitions after a task switch occurred (switch condition). In order to complement our findings in the induced high-frequency range, we have analysed reaction times, evoked gamma activity and event-related potentials (ERPs).
When the same task was used for initial and repeated presentations, we found significantly faster reaction times after the second presentation. Thus, in the no-switch condition behavioural data verified that our experimental set-up was suitable to fulfil the criteria of repetition priming. This priming effect was abolished in the switch condition, i.e. when first and second presentations required different classifications.
Interestingly, iGBRs were found to be the only electrophysiological marker of brain activity, which co-varied with the observed pattern of behavioural results. Therefore, they will be discussed first.
Induced activity
In line with previous studies, in which pictures of everyday objects were used as stimuli (Gruber et al., 2002, 2006), iGBRs showed a topographically widespread distribution with a maximum at parieto-occipital recording sites peaking at approximately 200–300 ms after stimulus onset. This transient induced high-frequency burst is commonly interpreted as a correlate of activated contents of long-term memory, which have to be integrated in order to establish a cortical object representation of a stimulus and which consist of perceptual, semantic and task-related features (Gruber & Müller, 2006; Busch et al., 2006).
iGBR amplitudes were significantly suppressed after the repeated presentation of an object on which the same classification task had to be applied. This result corroborates the findings of previous studies examining high-frequency correlates of repetition priming (Gruber & Müller, 2002; Gruber et al., 2004a) and is regarded as an index of the ‘sharpening’ of a primed cortical object representation. Sharpening results in a more efficient processing within such networks, and thus in facilitated behavioural responses (Wiggs & Martin, 1998; Grill-Spector et al., 2006). The sources computed for the repetition suppression effect were found to be located in widespread areas spanning from the occipital to parietal lobes, inferior temporal gyri and frontal areas. This widespread distribution is in line with the understanding that iGBRs reflect cortical object representations, which are composed of many different features including visual, verbal and semantic information in dispersed cortical regions (Tallon-Baudry & Bertrand, 1999). Furthermore, the source distribution is in accordance with a previous report on the generators of iGBRs during object recognition (Gruber et al., 2006).
The tomographical distribution of the suppression effect has an important implication for our understanding of the ‘sharpening’ process described above. It is not just a single cortical area that drops out of the cortical representation of a stimulus. Rather, the whole and distributed ‘induced gamma band network’ undergoes a tuning mechanism.
A possible alternative explanation for the present findings might be that initial as compared with repeated picture presentations were attracting more attention. Indeed, iGBRs are known to be modulated by attention (Gruber et al., 1999; Müller et al., 2000; Müller & Gruber, 2001; Müller & Keil, 2004). However, in the present task the occurrence of first and second presentations was unpredictable. Thus, subjects had to pay the same amount of attention to every picture presentation. Given an average correct response rate of about 96% for both first and second presentations, it is very unlikely that the attentional effort is reduced after the first presentation. Furthermore, filler items, which were presented only once, were introduced to diminish expectancy effects regarding the second presentation.
Most interestingly, the presentation of primed (i.e. repeated) objects after a task switch revealed no repetition-related effects in the induced gamma range. It is likely that large parts of the network have to be reactivated in order to fulfil the requirements imposed by a new task. Note that the observed disruption of iGBR suppression after a task switch cannot be attributed to differences in difficulty of the two tasks, because task order was randomized across the experiment, i.e. the ‘shoebox’ task could be followed by the ‘nature’ task and vice versa.
A similar elimination of suppression effects after a task switch was reported in a recent functional magnetic resonance imaging study by Dobbins et al. (2004). These authors reported reduced cortical activity in prefrontal, parietal, occipito-temporal and fusiform regions for repeated relative to initial object presentations, which was disrupted after task switching. In contrast to our interpretation, Dobbins et al. claimed that the reduction of cortical activity is not a result of a ‘sharpening’ mechanism, but rather results from rapid response learning regarding the previous task. However, Dobbins and colleagues did use a task switch during which retrieval remained directed towards the same object properties. In particular, they switched from a ‘bigger than a shoebox’ to a ‘smaller than a shoebox’ decision. By contrast, we implemented a task switch situation in which the retrieval of semantic information was necessary to conduct the new task. Thus, it is unlikely that our effects are caused by rapid response learning. This argument is underpinned by the fact that we found no significant differences between repetitions that required a response with the same hand as opposed to repetitions in which the responding hand had to be changed.
Thus, gamma repetition effects are neither a merely automatic consequence of repeated perceptual processing nor do they simply depend on stimulus–response associations. Rather, the dynamics within an ‘iGBR network’ depend on the task requirements and are use-dependent. This interpretation is in line with some aspects of a recent theoretical model, which claims that induced gamma oscillations signify the ‘utilization’ of a cortical network during information processing (Herrmann et al., 2004b).
It should be mentioned that only 1–2 items intervening between first and second presentations were used here. Thus, the dynamics within activated parts of long-term memory were only assessed during a relative brief period of time. Although repetition-suppression effects in the induced gamma band range were found up to ten intervening items (Fiebach et al., 2005), the present study should be replicated with longer lags between repetitions in order to clarify the temporal robustness of the observed effects.
Evoked activity
Confirming previous findings (Herrmann et al., 2004a), the evoked gamma response (35–45 Hz, 70–120 ms) was characterized by a narrow posterior topographical distribution. Recently, it was suggested that this form of oscillatory brain activity reflects an early match of incoming information to memory contents (Herrmann et al., 2004b). However, we did not find a repetition- (i.e. memory-) related modulation of the evoked gamma response. Our findings are, rather, in line with the suggestion that the early evoked gamma oscillation is linked to the perceptual processing of lower-level features (Karakas & Basar, 1998), which are a prerequisite for the activation of an object representation as reflected in the iGBR [see Eckhorn et al. (1990) for a similar argument].
Regarding the ERP we have found an amplitude decrease in a latency range from approximately 220 to 380 ms after stimulus onset (L1), which replicates previous studies regarding picture repetition (Gruber & Müller, 2002; Penney et al., 2003; Gruber et al., 2004a). In contrast to the iGBR, the L1 suppression occurred in both the switch and the no-switch condition. A number of previous publications have pointed towards the functional complementarities of high-frequency oscillations and ERPs (Tallon-Baudry et al., 1997; Gruber et al., 2004b; Gruber & Müller, 2005). In a recent study, which examined the influences of stimulus familiarity on neuronal correlates of repetition priming, we were able to demonstrate that the ERP repetition effect may be caused by ‘neuronal priming’ mainly in perceptual networks, whereas iGBR modulations might be related to ‘neuronal priming’ in semantic or associative networks (Gruber & Müller, 2005). This interpretation can be directly conveyed to our understanding of the present results. The ‘iGBR network’ contains mainly higher-level features, which cannot be suppressed after a task switch. By contrast, networks consisting of lower-level features that are not directly task-relevant are suppressed regardless of the task requirement (i.e. in the switch and in the no-switch condition). This understanding of the L1 effect is underpinned by the tomographical distribution of the suppression effect in repetition-sensitive perceptual areas (Rugg et al., 1995; Pegna et al., 2004).
Conclusion
In the present experimental design iGBR modulations were the sole electrophysiological marker that co-varied with the observed pattern of behavioural data. The sharpening of a cell assembly – as indicated by iGBR suppression – might be one underlying neuronal mechanism of repetition priming. After a task switch ‘neuronal priming’ effects of iGBR amplitudes were eliminated, as large parts of the object representation needed to be reactivated in order to perform the new task. ERP suppression effects might signify ‘neuronal priming’ in structures which process lower-level stimulus features and which do not directly relate to the behavioural outcome.
Acknowledgements
We are grateful to Uwe Hassler for programming and Renate Zahn for help in data acquisition. This research was supported by a grant from the Deutsche Forschungsgemeinschaft (GR2684/2-1).
Abbreviations
-
- EEG
-
- electroencephalogram
-
- EOG
-
- electrooculogram
-
- ERP
-
- event-related potential
-
- iGBR
-
- induced gamma band response
-
- SPMs
-
- statistical parametric maps
-
- TF
-
- time-by-frequency




