Alteration of GABAergic synapses and gephyrin clusters in the thalamic reticular nucleus of GABAA receptor α3 subunit-null mice
Abstract
Multiple GABAA-receptor subtypes are assembled from α, β and γ subunit variants. GABAA receptors containing the α3 subunit represent a minor population with a restricted distribution in the CNS. In addition, they predominate in monoaminergic neurons and in the nucleus reticularis thalami (nRT), suggesting a role in the regulation of cortical function and sleep. Mice with a targeted deletion of the α3 subunit gene (α30/0) are viable and exhibit a subtle behavioural phenotype possibly related to dopaminergic hyperfunction. Here, we investigated immunohistochemically the consequences of the loss of α3 subunit for maturation of GABAA receptors and formation of GABAergic synapses in the nRT. Throughout postnatal development, the regional distribution of the α1, α2, or α5 subunit was unaltered in α30/0 mice and the prominent α3 subunit staining of nRT neurons in wildtype mice was not replaced. Subcellularly, as seen by double immunofluorescence, the α3 and γ2 subunit were clustered at postsynaptic sites in the nRT of adult wildtype mice along with the scaffolding protein gephyrin. In α30/0 mice, γ2 subunit clustering was disrupted and gephyrin formed large aggregates localized at the cell surface, but unrelated to postsynaptic sites, indicating that nRT neurons lack postsynaptic GABAA receptors in mutant mice. Furthermore, GABAergic terminals were enlarged and reduced in number, suggesting a partial deficit of GABAergic synapses. Therefore, GABAA receptors are required for gephyrin clustering and long-term synapse maintenance. The absence of GABAA-mediated transmission in the nRT may have a significant impact on the function of the thalamo-cortical loop of α30/0 mice.
Introduction
GABAA receptors mediate most of the fast phasic and tonic inhibition in the mammalian central nervous system (Moss & Smart, 2001; Farrant & Nusser, 2005). The existence of multiple GABAA receptor subtypes, with distinct functional and pharmacological properties, is based on differential assembly from a large family of constituent subunit genes (Mohler et al., 2002; Sieghart & Sperk, 2002; Rudolph & Möhler, 2004). GABAA receptors containing the α3 subunit represent approximately 10–15% of total GABAA receptors in adult brain (McKernan & Whiting, 1996). While these receptors are expressed in multiple regions at all levels of the neuraxis, they also comprise the major GABAA receptor subtype present in monoaminergic and basal forebrain cholinergic neurons, as well as in the nucleus reticularis thalami (nRT) (Gao et al., 1993; Fritschy & Mohler, 1995; Gao et al., 1995; Pirker et al., 2000). α3-GABAA receptors are therefore likely to contribute to GABAergic control over a broad range of behavioural and cognitive states. To gain insight into the function of α3-GABAA receptors, mice carrying a targeted deletion of the α3 subunit gene (α30/0 mice) have been generated (Yee et al., 2005). These mice are viable and fertile and show no gross morphological anomaly in the brain. However, they are hyperactive and exhibit a sensory-motor deficit most likely related to dopamine hyperfunction. The lack of a severe phenotype of α30/0 mice suggests that specific compensatory changes occur to substitute for the missing α3-GABAA receptors.
In the present study, we have focused on potential changes in GABAA receptor expression and in the organization of GABAergic synapses in α30/0 mice. Using immunohistochemistry, we investigated first potential changes in the regional distribution of major GABAA receptor α subunit variants during postnatal development, because α3-GABAA receptors are particularly abundant during ontogeny (Fritschy et al., 1994). Second, we investigated whether GABAergic synapses are altered in nRT neurons, which express a limited repertoire of GABAA receptor subunits, consisting mainly of α3, β3, and γ2 subunits (Bentivoglio et al., 1991; Fritschy & Mohler, 1995; Browne et al., 2001). These receptors mediate phasic inhibition between nRT neurons and are essential for the modulation of rhythmic firing by thalamocortical neurons in the ventrobasal complex (Huntsman et al., 1999). At the subcellular level, α3-GABAA receptors in the nRT are clustered at postsynaptic sites along with gephyrin (Baer et al., 2000; Sassoè-Pognetto et al., 2000). Therefore, changes in the subunit expression pattern and subcellular distribution of GABAA receptors and gephyrin were sought as surrogates for postsynaptic alterations in nRT neurons of α30/0 mice. Presynaptic changes involving GABAergic terminals were monitored by staining for the vesicular GABA transporter (VGAT).
The results demonstrate that the expression of other major GABAA receptor subtypes remains unaffected throughout the brain during postnatal development and in adult α30/0 mice. As a consequence, α3-GABAA receptors are not replaced in nRT neurons, disrupting clustering of postsynaptic proteins and leading to a reduction of presynaptic terminals. The loss of GABAA receptors in nRT neurons may have a significant impact on the function of the thalamo-cortical loop.
Materials and methods
Animals
Wildtype and α30/0 mice (see Yee et al., 2005; for characterization) were maintained C57BL/6J background (B6.129 × 1-Gabra3tm2Uru/Uru) and bred at the University of Zurich. As the α3 subunit gene is located on the X chromosome, mutant mice were either hemizygote male or homozygote female obtained from heterozygous/hemizygous breeding pairs or from α30/0 breeding pairs. Mice were genotyped by PCR analysis of tail biopsies. All experiments were approved by the veterinary office of the canton of Zurich and were performed in accordance with the European Community Council Directive (86/609/EEC).
Immunohistochemistry
The regional distribution of GABAA receptor subunits was investigated by immunoperoxidase staining of transverse or parasagittal sections prepared from perfusion-fixed brain tissue, as described previously (Fritschy et al., 1998). Adult (n = 3 per genotype) and juvenile mice [postnatal day (P) 0, P4–5, P7, P10, P14, P21; n = 4–5 mice per time-point and genotype] were analysed. Briefly, mice were anaesthetized with pentobarbital (Nembutal; 50 mg/kg, i.p.) or with ice (P0, P4) and rapidly perfused through the ascending aorta with 4% paraformaldehyde in 0.15 m phosphate buffer (pH 7.4). The brains were removed, postfixed in the same solution, and incubated in sodium citrate buffer (pH 4.5). They were then irradiated in a microwave oven (650 W, 90 s), cryoprotected with 10% dimethylsulfoxide in PBS, and cut from frozen blocks with a sliding microtome. Sections were processed free-floating for immunohistochemistry. Guinea pig antibodies against the α1, α2, α3, or α5 subunit (see Fritschy & Mohler, 1995; Yee et al., 2005; for characterization) and rabbit antibodies against the α4 and α6 subunit (gift from Dr W. Sieghart, Vienna; see Sassoè-Pognetto et al., 2000; Kralic et al., 2006; for characterization) were used. Sections were incubated overnight at 4 °C in primary antibodies dissolved in Tris-saline buffer containing 2% normal goat serum and 0.2% Triton-X100. Biotinylated secondary antibodies (1 : 300; Jackson Immunoresearch, West Grove, PA, USA) were applied for 30 min, followed by the Vectastain Elite kit (Victor Laboratories, Burlingame, CA, USA) and incubation with diaminobenzidine as chromogen. Sections were mounted on gelatin-coated glass slides, air-dried, dehydrated and coverslipped.
The analysis of postsynaptic GABAA receptor α2, α3, or γ2 subunit and gephyrin (mAb7a, Synaptic Systems, Göttingen, Germany) clusters was performed by double immunofluorescence staining of cryosections prepared from fresh-frozen tissue, as described (Fritschy et al., 1998). Seven adult mice per genotype were used. When required, presynaptic GABAergic terminals were detected using a rabbit antibody against the VGAT (Synaptic Systems). In some experiments, cell bodies in the nRT were labelled with parvalbumin (mouse monoclonal antibody, SWant, Bellinzona, Switzerland). Sections were incubated overnight at 4 °C in a mixture of primary antibodies derived from different species. Each antibody was then detected using the appropriate IgGs coupled to different fluorochromes (Alexa 488, Molecular Probes, Eugene, OR; Cy3, Cy5, Jackson Immunoresearch). Finally, sections were washed again, air-dried and coverslipped with buffered glycerol (pH 9.2).
Data analysis
Sections processed for immunoperoxidase staining were analysed by light microscopy (Axioskop Zeiss AG, Jena, Germany). For illustration, they were digitized with a high-resolution colour camera (Zeiss Axiocam). Sections from immunofluorescence staining were visualized by confocal laser scanning microscopy (Zeiss LSM510 Meta), using sequential acquisition of the different channels to avoid cross-talk between fluorochromes. Typically, stacks of 8–12 images (512 × 512 pixel, pixel size 70–150 nm) spaced by 0.3–0.5 µm were recorded, using a 100× objective with a numerical aperture 1.4. Images were processed with the software Imaris (Bitplane, Zurich, Switzerland). The numerical density of VGAT-positive terminals was quantified using three animals per genotype, using automatic 3D target recognition in a stack of ten confocal sections (Surpass module in Imaris version 4.2). The dimension of terminals was determined in single 2D confocal images using a threshold segmentation algorithm based on labelling intensity (min 90 on a 255 grey-level scale) and size (min 0.1 µm2) (MCID-M5 image analysis software; Imaging Research, St. Catherine, ON, Canada). Statistical analysis was performed on cumulative distribution plots (Kolmogorow–Smirnov) for size and with a nonparametric t-test (Kruskal–Wallis) for numerical density of terminals.
Results
Immunohistochemical staining of the six GABAA receptor α subunit variants performed in sections from adult mice with subunit-specific antisera confirmed earlier studies in rat and mice (Fritschy & Mohler, 1995; Jones et al., 1997; Pirker et al., 2000; Peng et al., 2002; Kralic et al., 2006) that only the α3 subunit-immunoreactivity is present in the nRT (not shown). In α30/0 mice, as reported previously (Yee et al., 2005), staining for the α3 subunit was completely abolished, and no other α subunit variant, including the α4, α5, and α6 subunit, was detected in the nRT. These initial observations suggested that a deficit of postsynaptic GABAA receptors might occur in adult nRT. For the rest of this study, we have therefore focused on the α subunit variants that contribute, at least in part, to postsynaptic GABAA receptors (α1, α2, α3, α5) in brain.
Unaltered distribution of GABAA receptor subunits during development of α30/0 mice
To establish whether a transient alteration in expression of postsynaptic GABAA receptors occurs in immature α30/0 mice, the regional distribution of the α1, α2, and α5 subunit was analysed during postnatal development. As shown at P7, the pattern of expression of these subunits was highly similar in both genotypes (Fig. 1), indicating that it was not affected by the lack of α3 subunit expression in mutant mice. In particular, all regional and areal boundaries revealed by the selective distribution of these three subunits in wildtype mice were identical in α30/0 mice. In cerebral cortex, where each of these subunits exhibits an area-specific laminar distribution, no change was apparent in mutants. The lack of alteration was clearly evident in all regions strongly stained for the α3 subunit in wildtype, such as layers V–VI of primary visual cortex, the entorhinal cortex, the nRT, the superior colliculus, or the spinal trigeminal nucleus. The same results were obtained at the other stages of development examined (P0, P4–5, P10, P14, and P21; data not shown). Altogether, these results indicated that the α3 subunit is not replaced by another α subunit variant in α30/0 mice and its absence has no detectable influence on the distribution of other major GABAA receptor subunits.
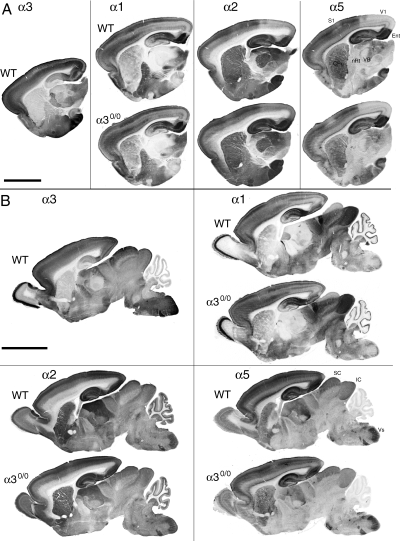
Normal distribution of the GABAA receptor α1, α2, and α5 subunit in the brain of α30/0 mice compared to wildtype, as illustrated in parasagital sections of P7 mice processed for immunoperoxidase staining with subunit specific antisera. For comparison, the distribution of the α3 subunit is shown in wildtype. (A) On lateral sections, the area- and laminar-specific distribution of GABAA receptor subtypes in the neocortex, forming sharp boundaries is clearly visible and is identical in both genotypes. Likewise, the subunit-specific distribution in basal ganglia and thalamus remains unchanged, including in all regions expressing the α3 subunit in wildtype. All sections from wildtype and mutant mice are from the same animals. (B) More medially, the olfactory bulb, tectum, cerebellum, and brainstem again show no change in subunit expression. The conserved subunit-specific pattern is particularly clear in the spinal trigeminal nucleus (Vs), for example. All sections are from the same wildtype and mutant mouse brains. CPu, striatum; Ent, entorhinal cortex; IC, inferior colliculus; S1, primary somatosensory cortex; SC, superior colliculus; V1, primary visual cortex.. Scale bars, 1 mm (A); 2 mm (B).
Maturation of GABAA receptor α subunit-IR in the nRT
To understand better the formation of GABAA receptors in the nRT, their distribution was monitored in detail during development in wildtype mice (Fig. 2). The α1 subunit was not detectable in the nRT at any age examined (not shown). As reported previously in rats, its expression increased gradually in relay nuclei, mainly during the third and fourth postnatal week. In contrast, α3 subunit immunoreactivity was prominent already at birth in the nRT, clearly outlining the boundaries of the nucleus (Fig. 2A). A weaker staining was evident in the ventrobasal complex (VB) and laterodorsal nucleus (LD). This distribution pattern did not change during development, except that α3 subunit staining intensity increased further in the nRT by P10 (Fig. 2C) and thereafter. In contrast, α3 subunit staining in VB and LD was decreased at P10 compared to P0 and P5. The α2 subunit, which is very prominent in VB during the first postnatal week (Fig. 2D–F), was virtually absent from the nRT at any age examined. Finally, the α5 subunit, which is not expressed in the thalamus of adult mice, was transiently detectable in the nRT at P5 (Fig. 2G–I). It subsided thereafter to become undetectable at P10. These results indicate that the α3 subunit represents the predominant GABAA receptor subtype in the nRT at every stage of postnatal development, with a minor contribution of the α5 subunit around P5.
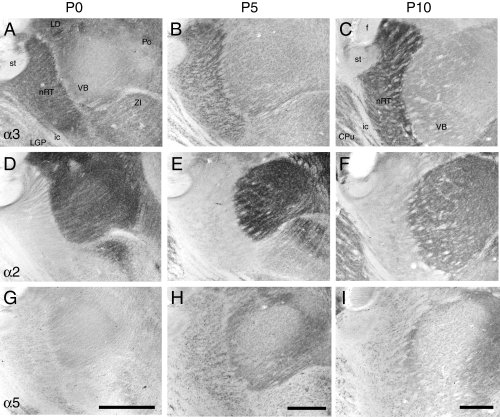
Changes in GABAA receptor α2, α3, and α5 subunit immunoreactivity in thalamus during postnatal development of wildtype mice, as illustrated in parasagital sections processed for immunoperoxidase staining at P0, P5 and P10. (A–C) The α3 subunit is most abundant in the nRT already at P0 and its staining gradually increases thereafter, while decreasing in the ventrobasal complex (VB) and laterodorsal nucleus (LD). (D and F) The α2 subunit staining is strongest at birth in these nuclei, and decreases gradually thereafter with a distinct temporal pattern among nuclei. It never becomes apparent in nRT. (G–I) The α5 subunit staining is very low at P0 and at P10, and exhibits a transient increase at P5, notably in the nRT. CPu, striatum; f, fornix; ic, internal capsule; LGP, globus pallidus, lateral part; Po, posterior nucleus of the thalamus; st, stria terminalis; ZI, zona incerta. Scale bars, 200 µm.
Selective loss of GABAA receptor and gephyrin clusters in the nRT of α30/0 mice
An α subunit variant is necessary for assembly and cell-surface expression of GABAA receptors (Fritschy et al., 1997; Jones et al., 1997; Sur et al., 2001). Postsynaptic GABAA receptors are also required for postsynaptic clustering of gephyrin (Essrich et al., 1998; Schweizer et al., 2003; Kralic et al., 2006). In view of the predominance of α3-GABAA receptors in the nRT, the formation of postsynaptic GABAA receptor and gephyrin clusters might thus be affected in α30/0 mice. To verify this hypothesis, double immunofluorescence was performed in adult mice (Fig. 3). In wildtype mice, clusters of α3 and γ2 subunit immunofluorescence colocalized with gephyrin readily were detected in the nRT (Fig. 3A and B), as reported previously (Baer et al., 1999). In α30/0 mice, no clusters were seen and gephyrin formed large aggregates measuring up to 15 µm (Fig. 3C and D). Staining for the γ2 subunit was increased intracellularly, clearly revealing the soma of individual nRT neurons (Fig. 3D), suggesting a deficit in membrane targeting and intracellular retention of this subunit. As expected from the immunoperoxidase staining experiments, no staining was seen for the α1, α2 (Fig. 3E), and α5 subunit in the nRT of either wildtype or mutant mice. To determine whether the large gephyrin aggregates formed in mutant mice are localized postsynaptically, presynaptic terminals were visualized by staining for VGAT (Fig. 3F and G). In wildtype mice, an apposition between VGAT-positive boutons and gephyrin clusters was clearly evident (Fig. 3F). The number of such appositions was much reduced in α30/0 mice and most gephyrin aggregates were not in contact with a presynaptic terminal (Fig. 3G). As a control for the regional specificity of these synaptic changes, sections from α30/0 mice were examined in the dentate gyrus, where the α3 subunit is almost not expressed in wildtype mice. As expected, the distribution of γ2 subunit and gephyrin clusters was indistinguishable in the dentate gyrus among the two genotypes (Fig. 3H and I), confirming that the formation of gephyrin aggregates in nRT neurons is due to the absence of GABAA receptors in α30/0 mice.
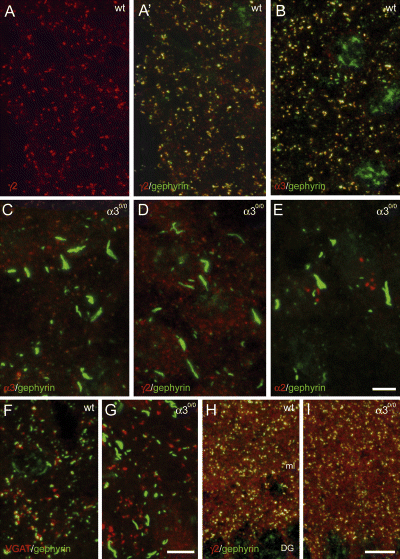
Selective disruption of GABAA receptor and gephyrin clustering in the nRT of α30/0 mice, as visualized by double immunofluorescence staining in adult brain sections. In some sections (A′, B, H and I), a nonspecific nuclear staining for gephyrin (green) is present. (A) Clustered distribution of the γ2 subunit (red) and gephyrin (green) in a wildtype mice; A′ shows the colocalization between both proteins in the overlay of the two fluorochromes. (B) A similar picture is seen for the α3 subunit (red), which shows clusters colocalized with gephyrin (green). (C–E) In α30/0 mice, gephyrin clusters (green) are disrupted and replaced by large aggregates. The remaining γ2 subunit staining (D) becomes detectable intracellularly, outlining the somata of nRT neurons. No α2 subunit immunoreactivity (E) becomes detectable in the nRT of α30/0 mice. (F) Postsynaptic localization of gephyrin (green), apposed to presynaptic terminals labelled for VGAT (red) in wildtype mice. (G) Most gephyrin aggregates formed in α30/0 mice are not apposed to a GABAergic terminal. (H and I) Unaltered distribution and clustering of the γ2 subunit (red) and gephyrin (green) in the dentate gyrus (DG) of α30/0 mice. ml, molecular layer of the DG. Scale bars, 5 µm (A–E); 5 µm (F and G) and 10 µm (H and I).
To understand better the alteration in subcellular distribution of gephyrin, the soma and dendrites of nRT neurons were visualized by immunofluorescence for the calcium-binding protein parvalbumin, which is abundantly expressed in the nRT. In wildtype mice, clusters of α3 subunit staining (Fig. 4A) and gephyrin (not shown) were seen on the soma and dendrites of individual nRT neurons. In α30/0 mice, gephyrin clusters were replaced by aggregates present mainly in the soma and in proximal dendrites and were located close to the cell surface (Fig. 4B). In contrast, the distribution of VGAT-positive terminals was less affected, although the size of individual terminals appeared larger and their number reduced (Fig. 4C and D). These observations were confirmed by a quantitative analysis in three mice per genotype, revealing a 25% decrease in areal density of VGAT-positive boutons in mutant mice compared to control (Fig. 4E; Kruskal–Wallis, P < 0.05), whereas their size was significantly increased in the nRT of α30/0 mice (Fig. 4F; Kolmogorov–Smirnov; P < 0.001).
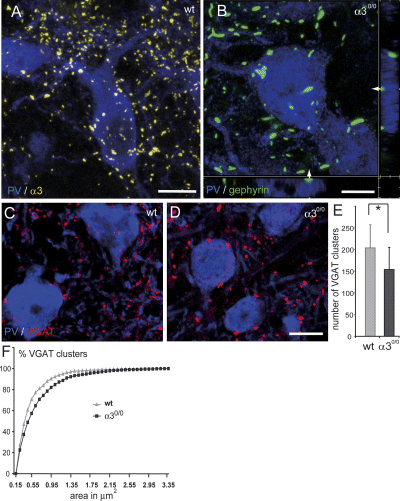
Alterations of GABAergic synapses in the nRT of α30/0 mice, as seen by double immunofluorescence staining for parvalbumin (PV; blue) with α3 (yellow), gephyrin (green), or VGAT (red). (A) Distribution of α3 subunit clusters (yellow) on the soma and dendrites of a PV-positive nRT neuron, as seen in a 3D reconstruction of a stack of 30 confocal sections spaced by 0.4 µm. (B) Gephyrin aggregates (green) in mutant mice are distributed along the surface of nRT neurons labelled for PV, as seen in x–y, x–z, and y–z projections from a stack of confocal images. The side projections are taken at the level indicated by the arrows. (C and D) Alterations of GABAergic terminals, as seen by staining with VGAT (red). A decrease in density and an increase in size are evident when comparing a section from wildtype (C) and mutant (D) mice. (E) Decreased density of VGAT-positive terminals, as counted in a 3D stack of ten confocal images spaced by 0.5 µm (mean ± SD; n = 3 mice per genotype; *P < 0.05). (F) Cumulative probability plot distribution of size (surface area) of VGAT-positive terminals illustrating the shift towards a greater proportion of large terminals in mutant mice. Scale bars, 10 µm (A); 5 µm (B) and 10 µm (C and D).
In summary, the loss of the α3 subunit in the nRT prevents the formation of GABAA receptors and alters postsynaptic clustering of gephyrin, resulting in a reduction of presynaptic boutons.
Discussion
The present results demonstrate that the absence of the α3 subunit is not compensated for by up-regulation or redistribution of GABAA receptor subtypes containing the α1, α2, or α5 subunit in α30/0 mice. In nRT neurons, which selectively express the α3 subunit, none of the six α-subunit variants was detectable immunohistochemically in adult mutant mice. As a consequence, clustering of GABAA receptor and gephyrin was disrupted in these cells, providing indirect evidence that these neurons do not express any other GABAA receptor subtype interacting with gephyrin at postsynaptic sites. These observations confirm that GABAA receptors are not assembled in the absence of an α subunit variant (Fritschy et al., 1997; Vicini et al., 2001; Fritschy et al., 2006) and that gephyrin depends on the presence of GABAA receptors for postsynaptic clustering (Essrich et al., 1998; Schweizer et al., 2003; Alldred et al., 2005; Kralic et al., 2006). Furthermore, a partial reduction of VGAT-positive GABAergic terminals occurs in the absence of postsynaptic GABAA receptors, accompanied by an enlargement of remaining terminals. Synaptic GABAA receptor-mediated transmission therefore contributes to long-term maintenance of GABAergic terminals in adult nRT neurons.
As α30/0 mice exhibit no major deficit in brain morphology and have a subtle behavioural phenotype (Yee et al., 2005), compensatory mechanisms are likely activated in these mice during development to maintain proper function of neuronal circuits that are normally modulated by α3-GABAA receptors. Most remarkably, these compensatory mechanisms do not include a detectable alteration of other GABAA receptor subtypes, unlike that observed in mutant mice lacking more abundant GABAA receptor subtypes, such as the α1 or δ subunit (Korpi et al., 2002; Peng et al., 2002; Kralic et al., 2006). In particular, the α4 subunit, which mediates tonic inhibition in thalamic relay nuclei, remains undetectable in the nRT of adult α30/0 mice. The missing α3 subunit is also not replaced transiently during postnatal development, when the relative abundance of α3-GABAA receptors is higher than in adulthood. The absence of morphological abnormality in the brain of α30/0 mice suggests that α3-GABAA receptors are not essential for mediating the proposed role of GABA as a paracrine regulator of brain development (Behar et al., 2000; Maric et al., 2001; Ben-Ari, 2002; Demarque et al., 2002). Furthermore, the expression of the α5 subunit remains transient around P5 in nRT neurons in α30/0 mice, indicating that the missing α3 subunit cannot be substituted for by the α5 subunit. This conclusion extends previous observations, notably in α10/0 mice, that a missing GABAA receptor α subunit is not replaced by another α subunit variant expressed in the same cell (Kralic et al., 2006).
The disruption of gephyrin clustering and the formation of intracellular aggregates was noted also in thalamic and cerebellar neurons of α10/0 mice (Kralic et al., 2006), indicating that postsynaptic targeting and clustering of gephyrin requires the presence of GABAA receptors (or probably glycine receptors). These gephyrin aggregates might therefore represent a morphological marker of neurons lacking postsynaptic GABAA receptors. Here, we extend these conclusions by showing that gephyrin aggregates are located in the soma and dendrites of nRT neurons that have otherwise a normal morphology. The shape of these aggregates is strongly reminiscent of those formed by gephyrin in recombinant expression systems that lack interaction partners, such as collybistin, for cell-surface targeting of gephyrin (Kins et al., 2000; Harvey et al., 2004). This observation is intriguing, because it means either that gephyrin interaction with GABAA receptors is required for cell-surface targeting, or that gephyrin is not stably anchored at synaptic sites in neurons lacking GABAA receptors and forms such aggregates prior to degradation. It is of note, however, that no gephyrin aggregates were reported in the brain of γ20/0 mice, which lack postsynaptic GABAA receptor and gephyrin clusters throughout the brain, but die within a few days after birth (Essrich et al., 1998; Baer et al., 1999). It is therefore possible that aggregates seen in α10/0 and α30/0 mice are formed slowly and become apparent only in adult animals.
The presence of numerous GABAergic terminals in the nRT of α30/0 mice shows that GABAergic synaptogenesis occurs normally even in the absence of GABAA receptors. However, it cannot be excluded that the presence of the α5 subunit during a short time-window possibly contributes to GABAergic synaptogenesis in mutant mice. The maintenance of GABAergic terminals in the absence of GABAA receptor-mediated transmission also occurs in the cerebellum of α10/0 mice (Fritschy et al., 2006). Ultrastructural analysis will be required to demonstrate directly the presence of GABAergic synapses on nRT neurons in mutant mice. It is unclear whether the enlarged size of these terminals represents a potential compensatory mechanism to increase GABA release as no immunohistochemical evidence for extrasynaptic GABAA receptors was obtained in this study. However, a functional analysis remains to be carried out to settle this issue.
Functional implications
The nRT is an important gate regulating the function of the thalamo-cortical loop and it likely contributes to bottom-up and top-down processing in multiple sensory and motor modalities (reviewed in Pinault, 2004). It is reciprocally connected to first and higher order relay nuclei and receives collaterals from layer VI corticothalamic axons (Jones, 2002). The crucial role of GABAA receptors in the nRT for regulating rhythmic firing and synchronization of thalamocortical neurons was first demonstrated in β30/0 mice, which, like α30/0 mice, lack GABAA receptors in the nRT (Huntsman et al., 1999). However, these mice have a severe phenotype and a curtailed survival, and exhibit major changes in GABAA receptor expression in other brain areas (Ramadan et al., 2003), which limits their use for studying the role of the nRT in thalamocortical function.
The nRT has been proposed to serve as pacemaker for generating oscillations in the spindle frequency range and is critically involved in inhibition of relay neurons during absence seizures (reviewed in Fuentealba & Steriade, 2005; Steriade, 2005). It also plays a general modulating or resetting role of thalamocortical oscillations that underlie discrete conscious events and regulate arousal states. The functional consequences of loss of GABAergic inhibition in the nRT of α30/0 mice are difficult to predict, in particular as GABAergic control of layer VI corticothalamic neurons, which mainly express α3-GABAA receptors, is also likely impaired. Furthermore, tight-junction coupling between nRT neurons (Landisman et al., 2002) might contribute to stabilize the activity of the entire population of nRT neurons in the absence intranuclear inhibition. Finally, compensatory adaptations involving other key mechanisms of synaptic inhibition in nRT neurons, notably expression of GABAB receptor and/or low threshold (T-type) Ca2+ channels might occur.
However, pharmacological blockade of intrareticular inhibition facilitates spike-wave discharges from thalamocortical cells (Sohal & Huguenard, 2003). Increased propensity or pharmacological sensitivity of α30/0 mice to exhibit absence seizures may therefore be expected. Furthermore, thalamic dysrhythmia, a pathological condition underlying negative and positive symptoms in neurological and psychiatric disorders (reviewed in Llinas et al., 2005) can emerge as a consequence of excessive inhibition of thalamocortical neurons. The subtle phenotype described so far in α30/0 mice (Yee et al., 2005) might reflect the fact that the thalamocortical system might operate normally under baseline conditions. The effects of excessive and uncoordinated inhibition of thalamocortical cells hypothesized above might become evident only after a specific challenge or under pathophysiological conditions, suggesting that α30/0 mice represent a potential animal model of thalamocortical dysrhythmia.
Acknowledgements
This study was supported by the Swiss National Science Foundation (grant No. 3100A0–108260 to JMF and 3100A0–102113 to UR). We would like to thank Dr Werner Sieghart (University of Vienna) for a generous gift of antisera against the GABAA receptor α4 and α6 subunit, Corinne Sidler and Franziska Parpan for excellent technical assistance, and Ruth Keist for her essential contribution to the generation and breeding of α30/0 mice.
Abbreviations
-
- nRT
-
- nucleus reticularis thalami
-
- P
-
- postnatal day
-
- VB
-
- ventrobasal complex
-
- VGAT
-
- vesicular GABA transporter