Failure of nicotine-dependent enhancement of synaptic efficacy at Schaffer–collateral CA1 synapses of AD11 anti-nerve growth factor transgenic mice
Abstract
Alzheimer's disease is a neurodegenerative disorder characterized by neuronal loss associated with a progressive impairment of cognitive functions. Early consequences of Alzheimer's disease include deficit of cholinergic signalling in particular regions controlling memory processes, such as the cortex and hippocampus, and accumulation of β-amyloid (Aβ) peptide in neuritic plaques. The cholinergic system depends for its integrity and function on nerve growth factor. Chronic nerve growth factor deprivation in transgenic mice (AD11) engineered to produce recombinant neutralizing anti-nerve growth factor antibodies leads to progressive age-dependent Alzheimer's-like neurodegenerative pathology similar to that found in patients with Alzheimer's disease, associated with a selective loss of cholinergic neurones in the basal forebrain. Here we show that in the hippocampus of 6-month-old AD11 mice, Aβ aggregates started appearing in the CA1 region. The accumulation of Aβ was associated with a loss of cholinergic function at CA3–CA1 synapses. Whereas in wild-type mice nicotine induced a persistent increase of synaptic efficacy via α7 nicotine acetylcholine receptors, in AD11 mice this alkaloid failed to modify synaptic strength. Moreover, nicotine failed to transiently enhance the frequency of spontaneous miniature glutamatergic currents (miniature excitatory postsynaptic currents) recorded from CA1 but not from CA3 pyramidal neurones of AD11 mice. However, in CA3 principal cells of AD11 mice, the potentiating effect of nicotine on miniature excitatory postsynaptic currents was prevented when Aβ peptide 1–42 was added to the extracellular solution. These data suggest that in AD11 mice, Aβ interferes with nicotine acetylcholine receptors at the level of presynaptic glutamatergic terminals, inhibiting their function possibly through calcium signalling via presynaptic α7 nicotine acetylcholine receptors.
Introduction
Nicotine acetylcholine receptors (nAChRs) are widely distributed within the brain where they contribute to the regulation of high cognitive functions. The hippocampus, a key structure in learning and memory processes, receives a large cholinergic innervation (Kasa, 1986) and is endowed with a variety of nAChRs (Alkondon & Albuquerque, 2004), which increase the release of several neurotransmitters and modulate synaptic plasticity processes (McGehee, 2002). Deficits in the cholinergic system produce impairment of cognitive functions, which are particularly relevant during senescence and in age-related neurodegenerative pathologies (Selkoe, 2002). The early loss of learning and memory functions in patients with Alzheimer's disease (AD) suggests that the initial targets of AD are synapses encoding new declarative memories in the cortex and hippocampus (Selkoe, 2002; Mattson, 2004). At the molecular and cellular level, memory impairment correlates with accumulation of soluble amyloid β (Aβ) protein in neuritic plaques and loss of cholinergic function followed by degeneration of cholinergic innervation (Liu et al., 2001). In particular, neuronal loss and selective vulnerability of basal forebrain cholinergic neurones constitute a histopathological hallmark of AD (Bartus et al., 1982). Basal forebrain cholinergic neurones critically depend on nerve growth factor (NGF) for their survival and differentiation (Hefti, 1986; Li et al., 1995; Molnar et al., 1998; Cattaneo et al., 1999; Debeir et al., 1999), and NGF has been proposed as a potential therapeutic agent to prevent degeneration of basal forebrain cholinergic neurones and age-related neurodegenerative disorders, on the basis of animal models (Capsoni et al., 2002a; De Rosa et al., 2005) and preliminary patient data (Tuszynski et al., 2005). Consistent with this view, the chronic deprivation of NGF in anti-NGF mice expressing recombinant neutralizing antibodies (Ruberti et al., 2000) leads to important deficits in the cholinergic function, in parallel with an age-dependent neurodegenerative pathology closely resembling AD (Capsoni et al., 2000). Hence, adult AD11 anti-NGF mice display a neurodegenerative phenotype characterized by impairment of spatial memory tasks associated with cholinergic atrophy, neuronal loss, τ hyperphosphorylation and insolubility, abnormalities of the neuronal cytoskeleton reminiscent of tangles (Capsoni et al., 2000, 2002b), Aβ plaques (Capsoni et al., 2002c) and deficit in cortical synaptic plasticity (Pesavento et al., 2002).
In this study we examined the ability of nAChRs to modulate glutamatergic synaptic transmission in hippocampal slices from 5- to 6-month-old anti-NGF mice. At this age, in the absence of a fully blown neurodegenerative phenotype, transgenic mice start accumulating Aβ in the CA1 region of the hippocampus. This was associated with a deficit of the cholinergic function. Thus, a brief application of nicotine failed to enhance synaptic efficacy at CA3–CA1 synapses. Moreover, this alkaloid was unable to transiently increase the frequency of spontaneous miniature glutamatergic events recorded from CA1 pyramidal cells. In the CA3 region of the hippocampus, which at this age is devoid of Aβ neuritic plaques, nicotine was still able to transiently potentiate spontaneous miniature events recorded from CA3 principal cells but this effect was blocked when exogenous Aβ 1–42 was added to the extracellular solution. This demonstrates that in AD11 mice the loss of cholinergic function at CA3–CA1 synapses is mediated by Aβ peptide.
Materials and methods
Slice preparation
Transverse hippocampal slices (300–350 µm thick) from adult wild-type (WT) and AD11 mice (5–6 months old) were prepared as previously described (Maggi et al., 2003). The procedure was in accordance with the regulations of the Italian Animal Welfare Act and was approved by the local authority veterinary service (Dr. P. Zucca). Briefly, animals were decapitated after being anaesthetized with an i.p. injection of urethane (2 g/kg). The brain was quickly removed from the skull and placed in an ice-cold solution containing (in mm): sucrose, 230; KCl, 3.5; NaH2PO4, 1.2; NaHCO3, 25; MgCl2, 1.3; CaCl2, 2; glucose, 25; ascorbic acid, 0.0004, saturated with 95% O2 and 5% CO2 (pH 7.3–7.4). After 1 h of recovery in oxygenated artificial cerebrospinal fluid [containing (in mm): NaCl, 130; KCl, 3.5; NaH2PO4, 1.2; NaHCO3, 25; MgCl2, 1.3; CaCl2, 2; glucose, 25; ascorbic acid, 0.0004, saturated with 95% O2 and 5% CO2 (pH 7.3–7.4)] an individual slice was transferred to the recording chamber where it was continuously superfused at 30–33 °C with artificial cerebrospinal fluid at a rate of 2–3 mL/min.
Electrophysiological recordings
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-mediated excitatory postsynaptic currents (EPSCs) were recorded at −60 mV from individual CA1 or CA3 pyramidal neurones using the patch-clamp technique in whole-cell configuration, voltage-clamp mode, at 30–33 °C. Patch electrodes, formed from thin borosilicate glass (Hilgenberg, Malsfeld, Germany), had a resistance of 4–6 MΩ when filled with an intracellular solution containing (in mm): Cs-methanesulphonate, 125; CsCl, 10; HEPES, 10; EGTA, 0.3; MgATP, 2; NaGTP, 0.3; QX-314, 5 (pH adjusted to 7.2 with CsOH). In current-clamp experiments the intracellular solution contained (in mm): KMeSO4, 135; KCl, 10; HEPES, 10; MgCl2, 1; Na2ATP, 2; Na2GTP, 0.4 (pH adjusted to 7.2 with KOH). In some experiments, bicuculline methiodide (10 µm) or picrotoxin (100 µm) was added to the bath solution to block GABAA receptors. Schaffer collaterals were stimulated with bipolar twisted NiCr-insulated electrodes placed in the stratum radiatum. Mossy fibres were stimulated with electrodes positioned in the dentate gyrus. The stimulus intensity was adjusted to activate only one or a few presynaptic fibres (minimal stimulation). Usually, paired pulses (50-ms interval, duration 100 µs) were used to test paired-pulse facilitation or depression. In most experiments, two converging inputs into the same pyramidal cell were alternatively activated (every 2 s).
The stability of the patch was checked by repetitively monitoring the input and series resistance during the experiments. Cells exhibiting more than 20% changes were excluded from the analysis. The membrane potential was corrected for the liquid junction potential of 10 mV.
Drugs were applied to the bath via a three-way tap system. The drugs used were tetrodotoxin (Latoxan, France); bicuculline methiodide; nicotine and α-bungarotoxin (α-BGT) (Sigma, Milan, Italy); QX-314 and picrotoxin (Tocris, UK), and Aβ 1–42 (Biosource, Camarillo, CA, USA). Nicotine was bath applied, for 3 min, at concentrations of 1–100 µm and nicotinic effects were evaluated 20 min after application.
If not otherwise stated, data are expressed as mean ± SEM and statistical comparisons were made with the use of a paired t-test. In the cases of non-parametric paired data a Wilcoxon signed rank test was used and a Kolmogorov-Smirnov test was applied to compare distributions (P < 0.05 was taken as significant). For statistical analysis, kyplot v.2.0 software was used.
Data acquisition and analysis
Data were acquired with the ltp114 software package for evoked responses (courtesy of W.W. Anderson, Bristol University, UK) and pclamp clampex 9.0 program (Axon Instruments, Foster City, CA, USA) for spontaneous miniature EPSCs. Current signals were transferred to a computer after digitization with an A/D converter (Digidata 1200, Axon Instruments). Data were sampled at 10 kHz, filtered with a cut-off frequency of 2 kHz and analysed off-line with the clampfit 9.0 program. Evoked EPSCs were discriminated from noise using a threshold criterion (five to six times the SD of the noise) and frequently visually checked. The mean EPSC amplitude was usually measured by averaging 100 responses including failures. The paired-pulse ratio (PPR) was calculated as the mean amplitude of the second EPSC over the first EPSC. The coefficient of variation (CV) was calculated as the ratio between the SD of EPSC amplitude and the mean.
Miniature EPSCs were analysed with the template search function of clampfit 9.
Immunohistochemical analysis
To evaluate the expression of Aβ in the hippocampus of 6-month-old WT and AD11 mice, six WT and six AD11 mice were anaesthetized with 10.5% chloral hydrate/saline (8 mL/g body weight) and then transcardially perfused with 4% paraformaldehyde in phosphate-buffered saline. Brains were removed, postfixed for 2 h at 4 °C and cryprotected in 30% sucrose overnight. Coronal sections (40 µm thick) were collected in 12-well plates containing phosphate-buffered saline and preincubated for 30 min at room temperature (22 °C) in 10% fetal calf serum in Tris buffered saline plus 0.3% Triton-X 100 (0.1 m Tris/HCl, 0.05 m NaCl, pH 7.4, plus 0.3% Triton-X 100). To evaluate the expression of Aβ, sections were incubated with the goat anti-Aβ antibody directed against the NH2 terminus of this peptide (1 : 100, Santa Cruz Biotechnology, Santa Cruz, CA, USA). After a 72-h incubation at 4 °C, the reaction was developed using biotinylated anti-goat antibodies and the ABC method developed with nitrobluetetrazolium and 5-bromo-4-chloro-3-indolyl phosphate toluidinium salt. Sections were collected on slides and observations were made using the motorized Eclipse 1000 microscope (Nikon Corp., Tokyo, Japan).
Results
Aβ deposits are mainly localized on the CA1 region in the hippocampus of AD11 mice
Immunocytochemical experiments were performed on the hippocampus from 6-month-old AD11 (n = 6) and WT (n = 6) mice using selective anti-Aβ antibodies. These revealed the presence of Aβ-positive dystrophic neuritis in AD11 but not in WT mice. Double immunohisotochemistry (Capsoni et al., unpublished data) showed that Aβ-positive dystrophic neurites were positive for both microtubule-associated protein 2 and α-internexin, two markers used to identify dendrites and axons, respectively. As illustrated in Fig. 1, Aβ immunostaining was localized mainly in the CA1 region and was merely detectable in the CA3 hippocampal area.
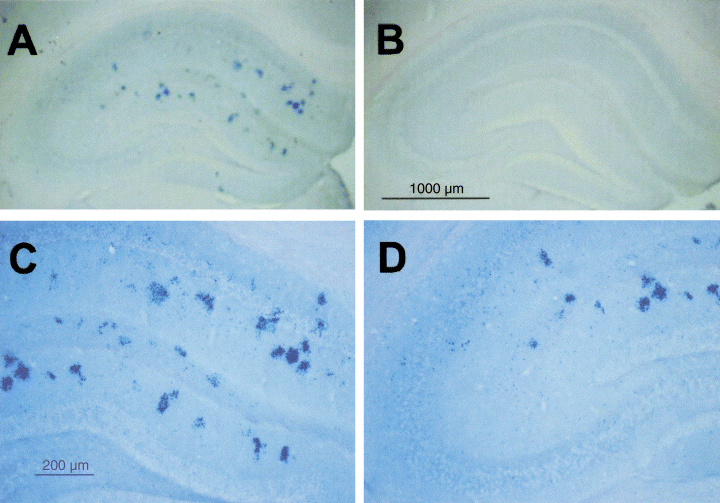
Distribution of Aβ deposits in AD11 mice. (A) Aβ-positive dystrophic neuritis in the hippocampus of 6-month-old AD11 mice. (B) Absence of Aβ-positive deposits in the hippocampus of age-matched wild-type mice. (C) Enlarged picture of the hippocampus of AD11 mice, showing the distribution of Aβ deposits in the CA1 region. (D) Aβ aggregates are not detectable in the CA3 region of hippocampus from AD11 mice. Scale bars: A and B, 1000 µm; C and D, 200 µm.
In previous studies (Wang et al., 2000) Aβ peptides have been shown to selectively bind to high-affinity α7 nAChRs leading to an impairment of cholinergic signalling (Liu et al., 2001; Dougherty et al., 2003). Therefore, the following experiments were undertaken to see whether Aβ may interfere with the cholinergic function in AD11 mice. In particular, we studied how activation of nAChRs by nicotine affects glutamatergic transmission in the CA1 hippocampal area. The observed effects have been compared with those obtained in the CA3 region which, at 6 months of age, is apparently free of Aβ immunostaining and aggregates.
In AD11 mice nicotine fails to modify synaptic efficacy at glutamatergic CA3–CA1 synapses
Whole-cell recordings in voltage-clamp mode were performed from 54 and 47 CA1 pyramidal cells in hippocampal slices obtained from WT and AD11 mice, respectively. The passive membrane properties of these cells did not significantly differ between WT and transgenic mice. In WT and AD11 mice, the resting membrane potential was −59.1 ± 2.1 and −58.2 ± 1.8 mV, input conductance 5.3 ± 0.3 and 5.1 ± 0.2 nS and membrane capacitance 111 ± 6 and 109 ± 8 pF, respectively. Action potentials evoked by short depolarizing current pulses did not differ in WT and AD11 mice. The amplitude of the spikes calculated from threshold was 50.3 ± 4.1 and 51.4 ± 2.8 mV, the half width 1.6 ± 0.2 and 1.4 ± 0.2 ms and spike threshold −39.3 ± 3.0 and −44.1 ± 1.9 mV for WT (n = 5) and AD11 (n = 8) mice, respectively. The values obtained from WT and AD11 mice were not significantly different (P > 0.5).
Minimal stimulation (see Materials and methods) of the Schaffer collaterals evoked in principal cells whole-cell synaptic currents which were intermingled with response failures. In agreement with a previous study on neonatal rats (Maggi et al., 2003), bath application of nicotine (1 µm for 3 min) to low-probability (P < 0.5) synapses in WT mice induced an increase in amplitude of EPSCs that was associated with a reduction of failure rate (Fig. 2A). This effect persisted for more than 40 min after nicotine application. Overall, in 22 cases, nicotine persistently increased the mean EPSC amplitude (successes plus failures), which varied from 4.13 ± 0.53 pA in control to 13.60 ± 2.57 pA at 20 min after nicotine application (P < 0.001; Wilcoxon test). The increase in amplitude was associated with a significant increase in the probability of success (Psuccess) which on average varied from 0.30 ± 0.03 to 0.63 ± 0.05 (P < 0.01; Fig. 2B). As expected for the increase in Psuccess, nicotine-induced potentiation of EPSCs was accompanied by a significant decrease of the CV (from 1.92 ± 0.15 to 1.04 ± 0.12, P < 0.01) and PPR (from 1.95 ± 0.13 to 1.52 ± 0.08, P < 0.01; Fig. 2C).
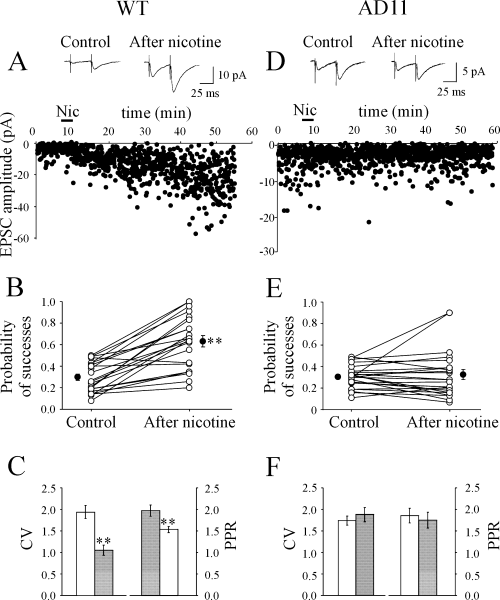
In AD11 mice nicotine (Nic) fails to potentiate low probability synapses. (A–C) Wild-type (WT) mice. (A) Average of successes plus failures (n = 100, insets) evoked in a CA1 pyramidal neurone by Schaffer collateral stimulation before (left) and 20 min after (right) nicotine application. Below, the peak amplitude of the first excitatory postsynaptic current (EPSC) (same neurone) is plotted against time before, during (bar) and after nicotine application. (B) Probability values for all individual experiments (n = 22; ○) in control condition and at 20 min after nicotine application. •, average values. Low probability synapses were persistently potentiated by nicotine. (C) Coefficient of variation (CV) (n = 22) and paired-pulse ratio (PPR) (n = 22) in control (open bars) and at 20 min after nicotine application (filled bars). Note depression of CV and PPR after nicotine. **P < 0.01. (D–F) AD11 mice. (D–F) as in (A–C). Note that nicotine did not affect the probability of successes, the CV and PPR (n = 23).
The possibility that the potentiating effect of nicotine on WT mice was not direct but was generated at the network level on GABAergic interneurones via disinhibition of principal cells was tested in experiments performed in the presence of the GABAA receptor blockers bicuculline (10 µm; n = 14) or picrotoxin (100 µm; n = 9). In these cases, nicotine was still able to potentiate synaptic responses increasing the probability of successes from 0.32 ± 0.03 to 0.52 ± 0.05 (P < 0.01; n = 23). These values did not significantly (P > 0.05) differ from those obtained in the absence of GABAA receptor antagonists.
In striking contrast with WT mice, nicotine failed to persistently change synaptic efficacy in the vast majority of CA1 pyramidal cells recorded from AD11 mice. Only in two cases out of 23 did nicotine induce a persistent facilitation of EPSCs indistinguishable from that observed in WT, whereas in the remaining 21 cases nicotine was ineffective. A representative example is shown in Fig. 2D. On average, the EPSC amplitude measured before and 20 min after application of nicotine was 2.76 ± 0.22 and 3.59 ± 0.77 pA, respectively (P = 0.89; Wilcoxon test; n = 23). In the same line, the mean P-value was 0.31 ± 0.02 in control and 0.33 ± 0.05 after nicotine application (Fig. 2E). Summary data for CV and PPR are presented in Fig. 2F. As shown in the figure, similar values of CV (P = 0.23; Wilcoxon test) and PPR (P = 0.38) were detected before and after nicotine application (1.74 ± 0.11 and 1.88 ± 0.16 for the CV and 1.83 ± 0.17 and 1.73 ± 0.18 for the PPR), suggesting that nicotine did not modify the probability of release. Similar results were obtained when bicuculline (10 µm) was present in the extracellular solution (the probability of successes varied from 0.42 ± 0.03 to 0.43 ± 0.06; P > 0.05; n = 6).
Differences in EPSC amplitude between WT and AD11 mice (4.13 ± 0.53 pA in WT vs. 2.76 ± 0.22 pA in AD11 mice) were not statistically significant (P > 0.5). Moreover, in both WT and AD11 mice similar stimulation voltages were applied to the Schaffer collaterals to evoke single fibre EPSCs (4.9 ± 0.2 V, n = 46 and 4.4 ± 0.2 V, n = 49, respectively; P = 0.1), suggesting the absence of significant changes in fibre excitability in anti-NGF transgenic mice.
These data demonstrate that whereas in WT mice nicotine directly potentiates glutamate release by activating nAChRs localized on Schaffer collaterals, it fails to produce any effect on CA3–CA1 connections in the hippocampus of AD11 mice.
The lack of nicotine-induced changes of synaptic efficacy in AD11 mice could be attributed to modifications in the number and/or functional properties of nAChRs (affinity, transduction mechanisms, etc.). In order to see whether a change in receptor affinity may account for the present results, additional experiments were performed using higher concentrations of nicotine (10 and 100 µm instead of 1 µm). Whereas in WT mice nicotine (10 µm) induced a potentiation of EPSCs (from 5.19 ± 1.06 to 9.48 ± 1.92 pA; P < 0.01; n = 7) associated with a significant increase in Psuccess (from 0.45 ± 0.04 to 0.74 ± 0.05; P = 0.01), in AD11 mice higher concentrations of nicotine still failed to modify synaptic currents (Fig. 3). The amplitude of EPSCs was 4.71 ± 1.25 and 3.92 ± 1.26 pA in control and at 20 min after nicotine application, respectively (n = 4). Similar results were obtained with 100 µm of nicotine (n = 8 for both WT and AD11 mice; Fig. 3).
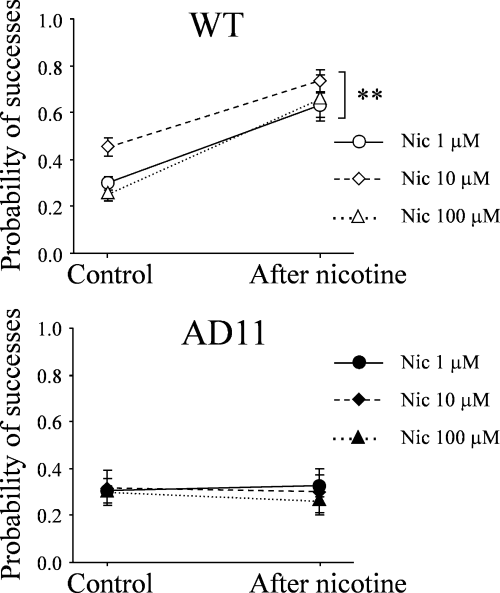
In AD11 mice high concentrations of nicotine (Nic) fail to modify the probability of glutamate release. Each point represents the mean probability of successes obtained in wild-type (WT) and AD11 mice in control conditions and at 20 min after application of different concentrations of nicotine. Nicotine (1 µm) (n = 22 and 23 in WT and AD11 mice, respectively), nicotine (10 µm) (n = 7 and 4 in WT and AD11 mice, respectively) and nicotine (100 µm) (n = 8 for both WT and AD11 mice). **P < 0.01.
In wild-type mice, nicotine-induced persistent changes of synaptic efficacy are mediated by α7 nicotine acetylcholine receptors
In an attempt to clarify the subtypes of nAChRs involved in the nicotine-induced increase in synaptic efficacy, in additional experiments from WT mice nicotine was applied in the presence of the selective α7 nAChR antagonist α-BGT (n = 9). Hippocampal slices were preincubated in α-BGT (100 nm for 1 h) and then transferred to the recording chamber where they were superfused with an extracellular solution containing the same concentration of α-BGT. In these conditions, the application of nicotine (1 µm for 3 min) did not produce any effect indicating, in agreement with previous findings from neonatal rats (Maggi et al., 2003), that α7 nAChR subtypes were responsible for its action. On average, the EPSC amplitude was 3.82 ± 0.48 and 4.88 ± 0.92 pA (P = 0.16) before and 20 min after nicotine, respectively. The average time course of the amplitude of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-mediated EPSCs, normalized to their control, before and after nicotine for all cells preincubated or not in α-BGT is represented in Fig. 4.
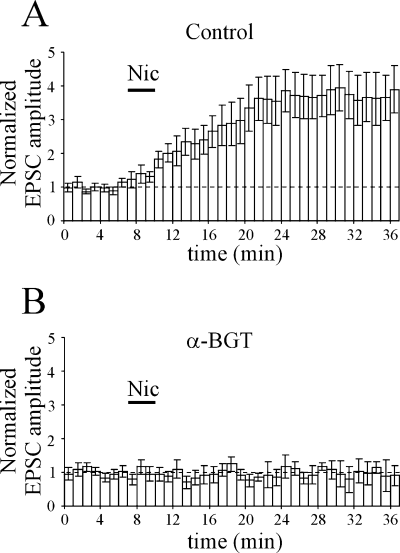
In wild-type mice, the nicotine (Nic)-induced increase in synaptic efficacy is mediated by α7 nicotine acetylcholine receptors. Average time course of the peak amplitude of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-mediated excitatory postsynaptic currents (EPSCs), normalized to their control (dashed line), before and after nicotine (bar) in the absence (A, n = 22) and presence (B, n = 9) of α-bungarotoxin (α-BGT) (100 nm). Note that α-BGT prevented nicotine-induced potentiation.
In wild-type but not AD11 mice, nicotine transiently increases the frequency of spontaneous miniature glutamatergic currents
In previous reports from the neonatal and adult rat hippocampus (Gray et al., 1996; Maggi et al., 2003), nicotine was shown to transiently increase the frequency of spontaneous miniature EPSCs (mEPSCs). Therefore, in the next set of experiments, we tested the effects of nicotine on mEPSCs recorded from CA1 principal cells in the presence of tetrodotoxin (1 µm) in both WT and AD11 mice. In WT mice, nicotine (1 µm for 3 min) transiently increased the frequency (from 0.86 ± 0.15 to 1.12 ± 0.17 Hz; n = 9, P < 0.01; Fig. 5A–C) but not the amplitude (from 7.40 ± 0.59 to 7.72 ± 0.75 pA, n = 9, P = 0.82, Wilcoxon test; Fig. 5B) of mEPSCs. The nicotine-induced up-regulation of mEPSC frequency was transient and lasted on average 11 ± 2 min (Fig. 5C).
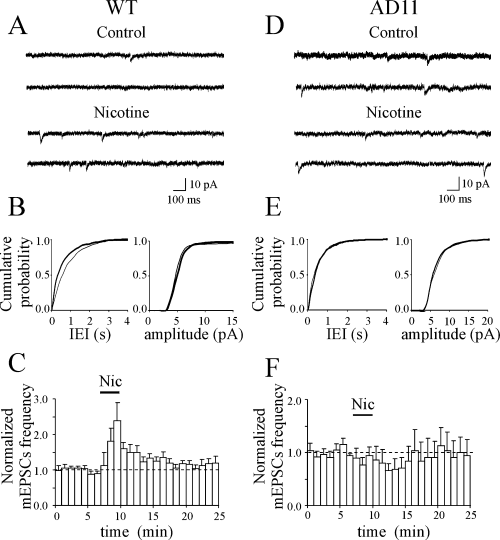
In AD11 mice, nicotine (Nic) fails to modify the frequency of spontaneous miniature excitatory postsynaptic currents (mEPSCs). (A) Representative traces of mEPSCs recorded from a CA1 principal cell [from wild-type (WT) mice] before and during nicotine application. (B) Cumulative distribution of interevent intervals (IEIs) and amplitude, before (thin line) and during (thick line) nicotine application [P < 0.01 for IEI and P > 0.05 for amplitude distributions, respectively, Kolmogorov-Smirnov test]. (C) Average time course of mEPSC frequency and amplitude normalized to control values (dashed lines) before, during (bar) and after nicotine application (n = 9). Note that nicotine transiently increased the frequency but not the amplitude of mEPSCs. (D–F) as in (A–C) but from AD11 mice (n = 7). Note that nicotine failed to modify the frequency and amplitude of mEPSCs. Cumulative distributions were not statistically different (P > 0.05 for both IEI and amplitude distributions, Kolmogorov-Smirnov test).
Differently, in CA1 principal cells from AD11 mice nicotine did not increase the frequency and amplitude of mEPSCs. On the contrary, nicotine tended to decrease the frequency of mEPSCs (from 0.98 ± 0.24 Hz in control to 0.64 ± 0.13 Hz after nicotine; P = 0.08; n = 7; Fig. 5D–F) although in a non-significant way. The amplitude of mEPSCs remained constant (7.90 ± 0.59 and 7.41 ± 0.63 pA before and after nicotine, respectively; P = 0.1; Fig. 5E).
High Ca2+ extracellular solution persistently increases Psuccess in both wild-type and AD11 mice
From the present experiments it is clear that nicotine was able to persistently enhance the probability of release in WT but not AD11 mice. Therefore, the following experiments were undertaken to see whether it would have been possible to increase probability of success Psuccess (particularly in AD11 mice) by changing the extracellular calcium concentration. To this aim, we increased [Ca2+/Mg2+]o from 2/1.3 to 4/1. In WT mice (n = 9), the high calcium solution produced an increase in Psuccess from 0.33 ± 0.04 to 0.68 ± 0.09 (P < 0.01; Fig. 6A and B). As shown in Fig. 6C, this was associated with an increase in the peak amplitude of EPSCs (from 3.22 ± 0.54 to 17.26 ± 7.50 pA; P < 0.01, Wilcoxon test) and a reduction of the CV (from 1.66 ± 0.16 to 0.83 ± 0.17; P < 0.01) and PPR (from 1.91 ± 0.14 to 1.27 ± 0.12; P < 0.01).
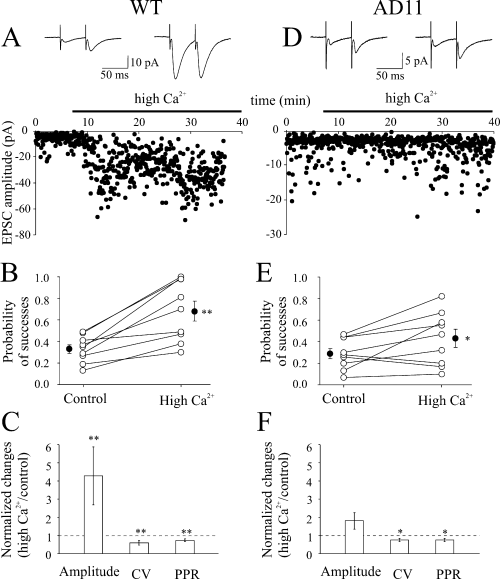
In the presence of a high calcium extracellular solution, changes in Psuccess are more pronounced in wild-type (WT) than in AD11 mice. (A–C) WT mice. (A) Average of successes plus failures (n = 100, insets) evoked in a CA1 pyramidal neurone by Schaffer collateral stimulation before (left) and during (right) bath application of a solution containing 4/1 mm[Ca2+/Mg2+]o. Below, the peak amplitude of the first excitatory postsynaptic current (EPSC) (same neurone) is plotted against time before and during (bar) superfusion of a high Ca2+-containing solution. (B) Probability values for all individual experiments (n = 9; ○) in control condition and in high Ca2+. (C) Each column represents the mean peak amplitude, coefficient of variation (CV) and paired-pulse ratio (PPR) values (normalized to controls, dashed lines) obtained in high calcium. (D–F) as in (A–C) but for AD11 mice (n = 9). *P < 0.05; **P < 0.01.
In previous work from neonatal rats we have found that CA3–CA1 synapses can undergo potentiation or depression according to the initial state of the synapses (low or high Psuccess, respectively;Maggi et al., 2004). Therefore, nicotine was applied to CA3–CA1 synapses after having increased Psuccess with high calcium. In agreement with Maggi et al. (2004), the alkaloid produced a reduction of synaptic efficacy (the probability of successes changed from 0.53 ± 0.15 to 0.36 ± 0.06, n = 3; data not shown).
In AD11 mice (n = 9) a high calcium-containing solution was still able to enhance Psuccess but to a minor extent (from 0.29 ± 0.05 to 0.43 ± 0.08; P < 0.05; Fig. 6D and E). In comparison with WT mice, the variation of Psuccess (expressed as percentage of control) was significantly smaller (66.0 ± 37.3 vs. 112.4 ± 20.7%, P < 0.05). The EPSC amplitude did not increase significantly, whereas CV and PPR were decreased (P < 0.05 for both; Fig. 6F).
However, in contrast with WT mice, in AD11 mice nicotine failed to modify synaptic efficacy, in spite of the small increase in Psuccess obtained in high calcium (Psuccess was 0.47 ± 0.11 and 0.45 ± 0.11 before and after nicotine, respectively; P = 0.71; n = 6). The amplitude of EPSCs did not change significantly before and after nicotine application (4.42 ± 1.11 and 3.58 ± 1.03 pA, respectively; P = 0.25; data not shown).
In both wild-type and AD11 mice nicotine fails to modify synaptic efficacy of glutamatergic mossy fibres–CA3 synapses
In WT mice, the bath application of nicotine (1 µm for 3 min), in the presence of bicuculline (10 µm) to block GABAA receptors, did not modify the amplitude of low probability synaptic responses evoked in CA3 pyramidal cells by stimulation of mossy fibres. In three cells (out of 11) nicotine significantly increased or decreased the number of successes, respectively (Fig. 7B). Overall, in 11 cases, the mean EPSC amplitude (successes plus failures) varied from 3.76 ± 0.57 pA in control to 4.09 ± 1.08 pA at 20 min after nicotine application, respectively (n = 11, P > 0.05; Fig. 7A). Similarly, nicotine failed to significantly change Psuccess, CV and PPR [on average P-values were 0.32 ± 0.05 and 0.36 ± 0.07 (n = 11, P > 0.05) before and at 20 min after nicotine application, respectively; Fig. 7B and C].
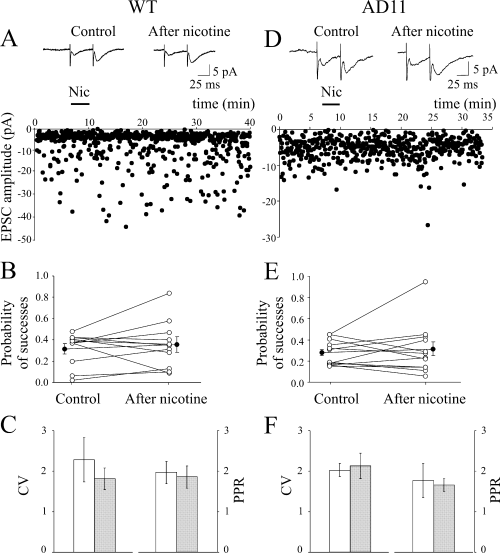
Nicotine (Nic) fails to potentiate mossy fibres–CA3 synapses in both wild-type (WT) and AD11 mice. (A–C) WT mice. (A) Average of successes plus failures (n = 100, insets) evoked in a CA3 pyramidal neurone by stimulation of granule cells in the dentate gyrus, before (left) and at 20 min after nicotine application (right). Below, the peak amplitude of the first excitatory postsynaptic current (EPSC) (same neurone) is plotted against time before, during (bar) and after nicotine application. (B) Probability values for all individual experiments (n = 11; ○) in control condition and at 20 min after nicotine application. •, average values. (C) Coefficient of variation (CV) and paired-pulse ratio (PPR) in control (open bars) and at 20 min after nicotine application (filled bars). (D–F) as in (A–C) but for AD11 mice (n = 12).
Similar data were obtained from AD11 mice. As in WT animals, nicotine was unable (except in one case) to change synaptic strength at mossy fibres–CA3 synapses (Fig. 7D). On average, the mean EPSC amplitude (successes plus failures) varied from 3.21 ± 0.50 pA in control to 4.22 ± 1.57 pA at 20 min after nicotine application, respectively (n = 12, P > 0.05). Likewise, no significant changes in the probability of successes, CV and PPR were observed after nicotine application (Fig. 7E and F).
The lack of nicotine effects on action potential-dependent glutamate release at mossy fibres–CA3 synapses in both WT and AD11 mice is in line with the reported inability of nicotine receptor agonists to modify presynaptic calcium at mossy fibres boutons (Vogt & Regehr, 2001). This can be attributed to the endogenous calbindin-dependent buffering capacity of mossy fibre terminals (Blatow et al., 2003) which would neutralize the rise of calcium through nAChRs and voltage-dependent calcium channels.
However, in the CA3 hippocampal region, nicotine is known to positively modulate the action potential-independent release of glutamate, possibly through different mechanisms (Gray et al., 1996; Sharma & Vijayaraghavan, 2003). The nicotine-induced increase in frequency of mEPSCs requires calcium influx through presynaptic nAChRs and modulation of calcium stores via calcium-induced calcium release mechanisms (Sharma & Vijayaraghavan, 2003). Therefore, in the next series of experiments, the action of nicotine was assessed on spontaneous miniature glutamatergic currents recorded in the CA3 hippocampal region in the presence of tetrodotoxin.
In CA3 principal cells from AD11 mice, nicotine potentiates spontaneous miniature excitatory postsynaptic currents, an effect that is prevented by the Aβ 1–42 peptide
In agreement with a previous work (Gray et al., 1996), in WT mice nicotine transiently increased the frequency of mEPSCs recorded in the presence of tetrodotoxin (1 µm) from CA3 pyramidal cells in six out of nine cases. Because of the high variability of mEPSC frequency and amplitude in the control condition, the effect of nicotine was evaluated after normalization to the control.
In WT mice, nicotine did not significantly affect the amplitude of miniature currents but up-regulated the mEPSC frequency of 62 ± 16% from 5.08 ± 0.57 to 8.58 ± 1.64 Hz (n = 6; P < 0.05, Wilcoxon test; Fig. 8B and C). This effect was transient, lasting on average 15.3 ± 2.4 min. Similar results were obtained from AD11 mice. In six out of nine pyramidal cells, nicotine transiently (16.5 ± 1.8 min) enhanced the mEPSC frequency of 48 ± 11% (from 5.58 ± 1.87 to 9.00 ± 3.38 Hz; n = 6; P < 0.05, Wilcoxon test; Fig. 8E and F) without significantly changing their amplitude (Fig. 8D and E).
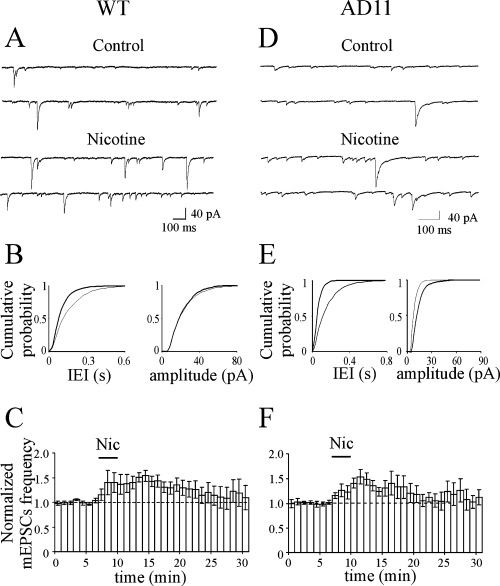
In the CA3 area nicotine (Nic) transiently enhances the frequency of spontaneous miniature excitatory postsynaptic currents (mEPSCs) in both wild-type (WT) and AD11 mice. (A) Representative traces of mEPSCs recorded from a CA3 principal cell (from WT mice) before and during nicotine application. (B) Cumulative distribution of interevent intervals (IEI) and amplitude, before (thin line) and during (thick line) nicotine application [P < 0.05 for IEI and P > 0.05 for amplitude distributions, respectively, Kolmogorov-Smirnov test]. (C) Average time course of mEPSC frequency and amplitude normalized to control values (dashed lines) before, during (bar) and after nicotine application (n = 6). Note that nicotine transiently increased the frequency but not the amplitude of mEPSCs. (D–F) as in (A–C) but for AD11 mice (n = 6; P < 0.01 for both IEI and amplitude distributions, Kolmogorov-Smirnov test).
Thus, it appears that, unlike what was observed when recording from the CA1 region of AD11 mice, in the CA3 region of AD11 mice the responses to nicotine were similar to those observed in control WT mice.
To see whether the nicotine-induced up-regulation of minature EPSC frequency in AD11 mice could be attributed to the lack of Aβ expression in the CA3 region, in the next series of experiments the effect of nicotine was tested after exogenously adding Aβ 1–42 peptide. This peptide, applied in the bath at a concentration of 100 nm, blocked the effects of nicotine on mEPSC frequency and had no significant effect on either the basal rate or amplitude of spontaneous mEPSCs (mEPSC frequency was 9.02 ± 3.32 Hz in control, 8.08 ± 2.99 Hz in the presence of Aβ and 6.82 ± 2.47 Hz in the presence of Aβ plus nicotine, respectively; n = 6; P > 0.05; Fig. 9A–C). Interestingly, a tendency toward depression was observed as for spontaneous events recorded in the CA1 hippocampal area. In four neurones, after washing out the peptide, the additional application of nicotine induced a significant increase of mEPSC frequency of 36.7 ± 17.2% in the absence of any change in amplitude (Fig. 9D). These data are consistent with the peptide acting on presynaptic nAChRs.
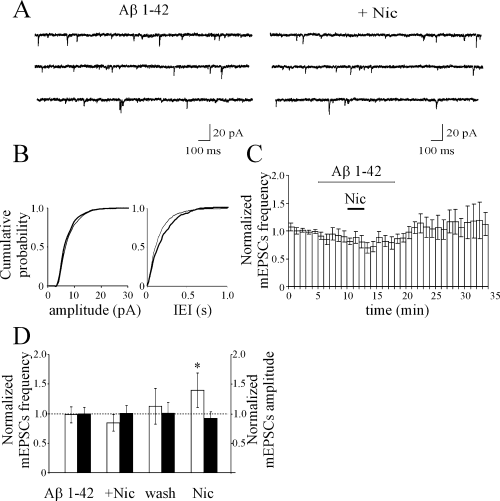
In AD11 mice, nicotine-induced potentiation of miniature excitatory postsynaptic currents (mEPSCs) recorded in CA3 principal cells is prevented by Aβ 1–42 peptide. (A) Representative traces of mEPSCs recorded from a CA3 pyramidal cell (from AD11 mice) during bath application of Aβ 1–42 (100 nm, left) and Aβ 1–42 plus nicotine. (B) Cumulative distribution amplitude and interevent intervals (IEIs) during application of Aβ 1–42 (thin line) and Aβ 1–42 plus nicotine (thick line) for the cell shown in A (P > 0.05 for both amplitude and IEI distributions, Kolmogorov-Smirnov test). (C) Average time course of mEPSC frequency normalized to control values (dashed lines) obtained from six cells in control, during Aβ 1–42 (open horizontal bar) and Aβ 1–42 plus nicotine (filled horizontal bar). (D) mEPSC frequency (open bars) and amplitude (filled bars) normalized to controls (dashed line) obtained in the presence of Aβ 1–42, Aβ 1–42 plus nicotine (+ Nic) after washing out Aβ 1–42 and nicotine (wash) and after application of nicotine alone. Note that nicotine was still able to enhance the frequency of mEPSCs after the peptide was washed out (n = 4). *P < 0.05.
Discussion
In view of the importance of the nicotinic receptors system in high cognitive functions and their postulated role in AD, this work was undertaken to explore the ability of nicotinic receptors to modulate glutamatergic synaptic transmission in a comprehensive mouse model of neurodegeneration.
The present experiments clearly showed that in the CA1 region of the hippocampus of 5- to 6-month-old AD11 mice, which at this age starts to be selectively enriched in Aβ expression, nicotine failed to increase synaptic efficacy at Schaffer collateral–CA1 synapses. The lack of nicotine response occurred in the absence of any detectable change in the passive or active membrane properties of the recorded neurones and in the presence of an unaltered synaptic transmission. In contrast, in the CA3 hippocampal region of 6-month-old AD11 mice, where Aβ expression was notably absent, nicotine was still able to transiently enhance the frequency of miniature glutamatergic events. This effect was, however, prevented by adding Aβ 1–42 to the extracellular solution.
Nicotine persistently enhances synaptic efficacy at Schaffer collateral–CA1 synapses of wild-type mice
In WT mice, the potentiating effect of nicotine at low probability Schaffer collateral–CA1 synapses was similar to that observed in the neonatal rat hippocampus (Maggi et al., 2003). As in the neonatal hippocampus, a single exposure to nicotine at 1 µm, a concentration close to that present in the smoker's blood immediately after smoking a cigarette (Dani & Heinemann, 1996), was able to increase the probability of glutamate release and to induce a persistent change in synaptic efficacy. Therefore, it appears that the ability of nicotine to enhance glutamate release at low probability synapses is not restricted to a critical period of postnatal development but also persists in adulthood. However, it should be stressed that, in contrast to immature neurones, in the present work we failed to detect synapses whose probability of release was so low that they appeared silent. As in immature neurones, the action of nicotine was mediated by α7 nAChRs as it was prevented by the selective α7 nAChR antagonist α-BGT. The presence of α7 nAChRs has been well documented on glutamatergic nerve terminals (Gray et al., 1996; Fabian-Fine et al., 2001), on the soma of GABAergic interneurones (Alkondon et al., 1998) and on the dendrites of CA1 pyramidal cells (Ji et al., 2001). We can exclude an indirect effect of nicotine on α7 receptors located on GABAergic interneurones as nicotine-induced enhancement of synaptic transmission persisted in the presence of bicuculline or picrotoxin, which block GABAA receptors. The possibility of a direct effect on the dendrites of CA1 principal cells is unlikely as nicotine application was never associated with changes in the holding current or input conductance. In previous studies, attempts to elicit nicotinic responses from CA1 pyramidal neurones, either by locally applying acetylcholine from a puff pipette (Frazier et al., 1998) or by locally uncaging carbachol (Khiroug et al., 2003), failed to induce any measurable current in these neurones, even on dendrites, suggesting that, if present, α7 receptors should be poorly expressed. Therefore, α7 nAChRs localized on glutamatergic terminals should be responsible for the observed effects. The nicotine-induced potentiation of synaptic strength relied completely on presynaptic mechanisms as it was associated with: (i) an increase in the frequency but not in the amplitude of mEPSCs; (ii) an increase in the probability of successes and a reduction in the number of transmitter failures; and (iii) a decrease in the PPR and in the CV, considered traditional indices of presynaptic modifications (Zucker, 1989; Clements, 1990).
A calcium rise via presynaptic nAChRs and voltage-dependent calcium channels would increase the release probability. This would be sufficient to drive postsynaptic cells above the threshold for firing action potentials. Moreover, the calcium rise may activate second messengers and different transduction pathways further leading to an increase in glutamate release. Thus, nicotine acting on nAChRs has been shown to activate protein kinases including protein kinase A (Dajas-Bailador et al., 2002), protein kinase C (Cox & Parsons, 1997; Soliakov & Wonnacott, 2001), the phosphatidyl-inositol 3-kinase signalling cascade (Kihara et al., 2001), mitogen-activated protein kinase (Dineley et al., 2001) and janus kinase 2. Interestingly, janus kinase 2 has been shown to be neuroprotective against Aβ 1–42 (Shaw et al., 2002).
Nicotine fails to up-regulate glutamate release at Schaffer collateral–CA1 but not at mossy fibres–CA3 synapses of AD11 mice
In striking contrast with WT mice, in AD11 mice nicotine was unable to change the synaptic efficacy in the vast majority of CA1 pyramidal neurones. Similarly to the present experiments, nicotine failed to potentiate any response at CA3–CA1 synapses of mice lacking the gene coding for α7 nAChRs (Le Magueresse, Cherubini and Changeux, unpublished observations). This suggests that in AD11 mice a reduced expression and/or activity of α7 nAChRs on glutamatergic terminals can be responsible for the observed effects. It is unlikely that α7 nAChRs have a reduced affinity as a 100-fold increase in nicotine concentration was still unable to produce any response in AD11 mice. Previous work from rat hippocampal neurones in culture has demonstrated that α7 nAChRs can be blocked in a non-competitive way by nanomolar concentrations of Aβ (Liu et al., 2001). Thus, the impairment of the cholinergic function found in AD11 mice could be attributed to the presence of Aβ expression in the stratum radiatum. Locally produced Aβ may interact with presynaptic nAChRs, hampering their function (Liu et al., 2001; Pettit et al., 2001). Aβ may directly or indirectly interfere with nAChRs by altering the gating properties of the receptors or the transduction mechanisms downstream of receptor activation. In support of this view are the observations that in AD11 mice, at CA3 mossy fibres terminals which at this age are devoid of Aβ, nicotine was still able to enhance the frequency of spontaneous mEPSCs and that addition of exogenous Aβ 1–42 to the external solution blocked the potentiating effect of nicotine.
According to recent reports (Dineley et al., 2001; Dougherty et al., 2003), Aβ interacting with nAChRs can activate different signal transduction cascades. In particular, Aβ has been shown to directly evoke a sustained increase in calcium in presynaptic terminals via nAChRs. This would further prevent the entry of calcium following activation of presynaptic nAChRs by nicotine through an occlusion mechanism. Therefore, the apparent inhibitory action of Aβ on the nicotine-induced increase in glutamate release observed in AD11 mice could be due to the failure of nicotine to further stimulate receptors already exposed to Aβ-evoked elevation of intracellular calcium (Dougherty et al., 2003). It should be stressed that α7 nAChRs are highly permeable to calcium (Tsuneki et al., 2000; Fucile, 2004). In control conditions the entry of calcium through presynaptic α7 nAChRs (McGehee et al., 1995; Gray et al., 1996; Coggan et al., 1997; Lena & Changeux, 1997; Mansvelder & McGehee, 2000) can be amplified by mobilization of calcium stores through calcium-induced calcium release mechanisms (Sharma & Vijayaraghavan, 2003). The Aβ-induced calcium rise in presynaptic nerve terminals may occlude nAChRs activated by ‘ambient’ acetylcholine thus leading to changes in calcium dynamics and to a down-regulation of basal synaptic transmission. This may account for the reduced probability observed in hippocampal slices of AD11 but not WT mice both in control and during exposure to a high calcium-containing solution. This is also consistent with previous data showing a reduction in the PPR of field potentials evoked in the cerebral cortex of anti-NGF mice by paired stimulation of the white matter (Pesavento et al., 2002). It is worth noting that mice engineered to express different levels of Aβ exhibit a different degree of synaptic loss and cognitive deficits which correlates well with the cortical levels of the peptide (Selkoe, 2002).
In conclusion, the present experiments show that, at an early stage of the neurodegeneration in anti-NGF AD11 mice, the loss of cholinergic function correlates with the distribution of Aβ-positive dystrophic neurites in the CA1 area. The deficit in cholinergic signalling and calcium homeostasis in the hippocampus may represent the first functional signs of synaptic modulation deficits, which might parallel early cognitive impairment. This set of observations in a mouse model of Alzheimer's-like neurodegeneration may have interesting correlates with human AD, where impaired nicotinic functions and perturbed calcium homeostasis appear to be widespread abnormalities in both the familial and sporadic forms of AD and certainly contribute to the progress of the disease (Mattson, 2004).
Acknowledgements
We are grateful to Daniela Avossa for her useful comments during the preparation of this manuscript. This work was supported by Telethon grant GGP 030416 to E.C. and A.C.
Abbreviations
-
- Aβ
-
- β-amyloid
-
- AD
-
- Alzheimer's disease
-
- α-BGT
-
- α-bungarotoxin
-
- CV
-
- coefficient of variation
-
- EPSC
-
- excitatory postsynaptic current
-
- mEPSC
-
- miniature excitatory postsynaptic current
-
- nAChR
-
- nicotine acetylcholine receptor
-
- NGF
-
- nerve growth factor
-
- PPR
-
- paired-pulse ratio
-
- P success
-
- probability of success
-
- WT
-
- wild-type