The calpain inhibitor MDL-28170 and the AMPA/KA receptor antagonist CNQX inhibit neurofilament degradation and enhance neuronal survival in kainic acid-treated hippocampal slice cultures
Abstract
The cytoskeleton controls the architecture and survival of the central nervous system neurons by maintaining the stability of axons, dendrites and cellular architecture, and any disturbance in this genuine structure could compromise cell survival. The developmentally regulated intracellular intermediate filament protein neurofilament (NF), composed of the light (NF-L), medium (NF-M) and high (NF-H) molecular weight isoforms, is expressed abundantly in nerve cells but its significance in nerve cell survival in stress situations in the brain is unknown. We have used Western blotting, immunocytochemistry, and Fluoro-Jade B and thionin stainings to clarify the effect of kainic acid (KA) treatment on NF protein stability, and its importance for neuronal survival in hippocampal slice cultures. The contribution of N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/KA glutamate receptor subtypes, calpain proteases and l-type Ca2+-channels to these processes were also assessed. Our results indicated that KA-induced degradation of NF was a fast process, similarly affecting all three NF proteins. It was effectively inhibited by the AMPA/KA receptor antagonist CNQX and the calpain inhibitor MDL-28170, whereas the Ca2+-channel blocker nifedipine and the NMDA receptor antagonist MK-801 had no significant effect. Moreover, KA-induced neuronal damage was effectively decreased in cultures treated with CNQX and MDL-28170. Our results suggest that the stability of NF proteins is an important factor contributing to neuronal survival after excitotoxic injury, and that both AMPA/KA receptor antagonists and calpain inhibitors might serve as neuroprotectants against this type of insult in the immature hippocampus.
Introduction
The three neurofilament proteins, the low (NF-L, 68 kDa), medium (NF-M, 160 kDa) and high molecular weight (NF-H, 200 kDa), constitute the major component of the cytoskeleton in axons, cell bodies and dendrites (Trojanowski et al., 1986; Lee & Cleveland, 1996). In the hippocampus, the expression of NF proteins is strictly developmentally regulated, and the proteins are also distributed heterogeneously within different hippocampal neurons, pyramidal CA1 and CA3 neurons, and dentate gyrus (DG) granule cells in adult and immature rats (Shetty & Turner, 1995a; Lopez-Picon et al., 2003). However, the reason for this heterogeneity and its functional importance is unknown.
The structural stability of NF proteins is suggested to be essential for neuronal survival, and deterioration of NF proteins could contribute to nerve cell death (Siman et al., 1989; Rami et al., 1997; Minger et al., 1998; Lankiewicz et al., 2000; Stys & Jiang, 2002). One of the most susceptible brain regions for damage in various pathological conditions, e.g. ischaemia and epileptic seizures, is the hippocampus (Ben-Ari, 1985; Schmidt-Kastner & Freund, 1991; Bengzon et al., 2002). Moreover, the principle nerve cell types of the hippocampus are selectively vulnerable in these insults (Buckmaster & Dudek, 1997; Fujikawa et al., 2000; Zhang et al., 2002). For example, kainic acid (KA)-induced seizures in adult rats have resulted in NF degradation with concomitant CA3 and CA1 cell damage within days of the insult (Wang et al., 1994; Shetty & Turner, 1995b; Yang et al., 1996), whereas neither NF degradation nor neuronal death has occurred after a similar insult in the developing rat hippocampus (Lopez-Picon et al., 2004).
Calpain proteases, expressed ubiquitously in the brain, are activated by increased intracellular calcium levels, and are suggested to contribute to excitotoxic neuronal death by targeting components essential for cell survival, e.g. enzymes, transcription factors and cytoskeletal proteins, including NF proteins (Pant, 1988; Siman et al., 1989; Greenwood et al., 1993; Brana et al., 1999; Rami, 2003). Moreover, calpain inhibition protects NF from degradation, resulting in nerve cell rescue from ischaemic (Chen et al., 1997; Li et al., 1998; Kunz et al., 2004), and excitotoxic damage (Rami et al., 1997; Lankiewicz et al., 2000; Wu et al., 2004).
Although calpains seem to be involved in the process of nerve cell death, the intracellular pathways mediating this are not understood fully. Organotypical hippocampal slice cultures (OHCs), in which the maturation of cells, synapses and connectivity occurs as in their in vivo counterparts (Frotscher et al., 1995; Holopainen & Lauren, 2003; Holopainen, 2005), are an ideal tool to study the molecular determinants of nerve cell survival.
Using this model, we studied the contribution of NF proteins to neuronal survival by using Western blotting and immunocytochemistry in OHCs with KA-induced excitotoxic nerve cell damage (Holopainen et al., 2004). In addition, we hypothesized that the highly permeable, novel calpain inhibitor MDL-28170, the glutamate receptor antagonists (CNQX and MK-801), and the l-type Ca2+-channel blocker nifedipine could protect neurons from death.
Materials and methods
Organotypical slice cultures
Hippocampal slice cultures were prepared from 6-7-day-old [postnatal day (P)6-7] Sprague-Dawley rats using the modified method of Stoppini et al. (1991), and as described recently in detail by Holopainen et al. (2001). After decapitating the rat, the brain was placed immediately in cold Gey's balanced salt solution (Gibco, Invitrogen, Paisley, UK) supplemented with glucose (6.5 mg/mL). Hippocampal slices (400 µm) were cut perpendicular to the septotemporal axis using a McIlwain tissue chopper, and placed on top of semipermeable membrane inserts (Millipore Corporation, Bedford, MA, USA) in a six-well plate containing culture medium (50% minimum essential medium, 25% Hanks's balanced salt solution, 25% heat-inactivated horse serum, 25 mm HEPES, supplemented with GlutaMaxII and 6.5 mg/mL glucose; pH adjusted to 7.2]. Slices were cultured in an incubator (37 °C, 5% CO2) for 7 days in vitro (DIV) with a change of medium carried out twice a week. No antibiotics were used. All treatments of animals were carried out according to the European Community Council directives 86/609/EEC and had the approval of the Animal Use and Care Committee of the University of Turku.
Pharmacological treatments
To determine the time course of the KA-induced degradation of NF proteins, hippocampal slices (7 DIV) were incubated with KA (5 µm; OPIKA-1, Ocean Produce International, Shelburne, NS, Canada) for 3, 6, 12, 24 and 48 h, and thereafter prepared for Western blotting. In addition, the 7-DIV cultures were treated with the following compounds for 24 h: KA (5 µm); the AMPA/KA selective antagonist CNQX (10 µm; Tocris Cookson Ltd, Avonmouth, UK); the l-type Ca2+-channel blocker nifedipine (10 µm; Tocris), the calpain protease inhibitor MDL-28170 (0.5, 5, 25 and 50 µm; Biomol, Plymouth Meeting, PA, USA); and the NMDA-receptor antagonist MK-801 (0.5 µm; Tocris). Treated and control slices were then used for Western blotting, immunocytochemistry and neuronal death studies. In all experiments, 7-DIV cultures were used as KA treatment at this in vitro age induces selective CA3 nerve cell damage while sparing the other main hippocampal nerve cell types (Holopainen et al., 2004).
Antibodies
Three different monoclonal antibodies were used for the Western blotting and immunocytochemical studies. Clones N52, NN18, and NR4 (all from Sigma, St. Louis, MO, USA) were used to detect phosphorylation-independent epitopes of NF-H, NF-M, and NF-L, respectively. All three antibodies were mouse IgG isotypes. As secondary antibodies, an HRP-conjugated goat anti-mouse IgG antibody (Sigma) was used in Western blots, and a biotin SP-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) was used in the immunocytochemical studies.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) immunoblotting
Western blot analysis was performed to determine the changes in the NF protein levels in response to different treatments as indicated above. Immunoblotting was carried out as described in detail for NF proteins in OHCs (Holopainen et al., 2001) with some modifications. Briefly, control and treated groups of slices from two different culture batches (24 slices for each time point and treatment group in both batches) were collected in ice-cold homogenization buffer containing 50 mm TRIS-HCl (pH 7.4), 1% SDS, 2 mm EDTA, 1 mm phenyl methyl sulphonyl chloride and 0.7 mm dithiothreitol, homogenized using an Ultra-Turrax T25 homogenizer (Janke and Kunkel, Staufen, Germany), homogenates immediately boiled, and then centrifuged at 10000 g for 30 min at 4 °C. Supernatants were collected, frozen and stored at −80 °C until required for use. The protein concentration of the samples was measured using the Lowry-based Biorad DC Protein assay (Biorad, Hercules, CA, USA). Equal amounts of protein were applied to each lane for SDS-PAGE, separated by electrophoresis with a 7.5% acrylamide minigel using Mini-protean III (Biorad), and then transferred to a polyvinylidene fluoride Immobilon-P (Millipore) membrane using the Mini Trans-blot Cell system (Transblot SD; Biorad). Membranes were incubated at 4 °C overnight with the monoclonal primary antibodies for NF-L (1 : 3500), NF-M (1 : 4000), and NF-H (1 : 3000). Thereafter, samples were incubated at room temperature (RT) with the horseradish peroxidase-conjugated secondary antibody for 1 h. The signal was obtained using the chemiluminiscence ECL system (Amersham, Bucks, UK) and Hyperfilm ECL (Amersham). The optical signals were quantified with Image J 1.33 (NIH, USA). Immunoblotting studies were repeated 4–6 times for each batch of slices and experimental conditions. Because of the rapid developmental changes in the expression of the NF protein (Lopez-Picon et al., 2004), a separate control group was used for the short (3, 6 and 24 h) and for the long (48 h) treatment groups.
Immunocytochemistry
Immunocytochemistry was used to detect possible regional changes in the expression and localization of NF proteins after the treatments. After 7 DIV, slices were transferred to fresh culture medium containing KA alone (5 µm), or combined with CNQX (10 µm), MDL-28170 (0.5 µm), nifedipine (10 µm) and MK-801 (0.5 µm), and cultured for a further 24 h. The immunocytochemistry was carried out as described recently in detail for NF proteins in OHCs (Holopainen et al., 2001), with some modifications. Briefly, slices were first washed with 0.1 m phosphate-buffered saline (PBS; pH 7.4), fixed with 4% paraformaldehyde (PFA) for 1 h at RT, and then processed with an antigen retrieval to enhance epitope detection and to reduce the background, as recently published by Fritschy et al. (1998). Following this, slices were detached mechanically from the insert, and the further staining steps were carried out with free-floating slices. Slices were first incubated in blocking solution (BS) containing 2% bovine serum albumin, 2% goat serum and 0.1% Triton X-100 in Tris-saline (TS-Triton) buffer (pH 7.4) for 1 h at RT, and thereafter with the primary antibodies for 24 h at 4 °C in BS at the following dilutions: NF-L (1 : 1000); NF-M (1 : 1500); and NF-H (1 : 1500). After washing, the slices were incubated with the biotin-conjugated secondary antibody (1 : 4000) in BS, and finally with the avidin-peroxidase conjugate (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA, USA). The staining was detected with 3,3′-diaminobenzidinetetrahydrochloride (DAB; Sigma) as a chromogen, and processed further as described previously (Lopez-Picon et al., 2003). Slices, in which the primary antibody was omitted but that were otherwise treated as above, served as the negative controls for the immunostaining. Slices (n = 12–15) from at least three different culture batches were used in all experimental groups, and for each antibody. The immunoreactivity was scored visually by using the following scheme: -, negative; -/+, weakly positive; +, positive; + +, moderately positive; + + +, strongly positive.
Thionin stainings
Thionin staining was used to determine whether KA treatment (24 h) alone or combined with CNQX (10 µm) and MDL-28170 (0.5, 5, 25 and 50 µm) resulted in neuronal loss in slices. After treatment, the slices were cultured for an additional 48 h in normal medium (recovery phase), as our preliminary time course experiments showed that 24 h in normal medium after the treatment was not long enough to verify the long-term consequence of the treatments. For the staining, hippocampal slices were removed from semipermeable membranes to gelatin-coated glass slides and briefly stained in 0.1% thionin, dehydrated in an alcohol series, cleared in xylene and coverslipped. Slices from at least three different culture batches (n = 7–12 in each treatment) and stained in different experiments were used for each treatment. Neurons in the CA3 regions were scored using the following scheme: 0, no stained neurons in the cell layer (all neurons dead in terms of loss of Nissl staining); 1, some stained neurons; 2, a sparse number of stained neurons; 3, many stained neurons, but with slightly disturbed cell layer integrity; 4, numerous stained neurons with good cell layer integrity (regarded as normal).
Light microscopy
After immunocytochemical and thionin stainings, slices were examined with a Leica DM R microscope (Heerbrugg, Switzerland) under bright field optics. A digital camera (Olympus U-TV1 X; Olympus Optical Co., Ltd, Tokyo, Japan) was used to capture pictures using an Olympus BX60 microscope (Olympus), and images were processed further using Adobe Photoshop (version 6.0; Adobe Systems Inc. San Jose, CA, USA) and Corel Draw (version 11.0).
Fluoro-Jade B
Fluoro-Jade B (FJB) was used to study the KA-induced neuronal degeneration, as described in detail by Holopainen et al. (2004). This dye is an anionic tribasic fluoresce derivative with excitation peaks of 362 nm and 390 nm and an emission peak of 550 nm, and it stains degenerating neurons (Schmued & Hopkins, 2000). The FJB staining was studied in 7-DIV slices treated for 24 h with KA (5 µm) alone, or combined with CNQX (10 µm) and MDL-28170 (0.5, 5, 25 and 50 µm). Slices, attached to the membrane, were first rinsed with cold PBS, fixed with 4% PFA for 1 h at RT, washed twice with PBS and once with water, transferred to 0.06% potassium permanganate (Sigma) for 2 min, washed with water, and then transferred to 0.001% FJB solution for 30 min. Finally, the slices were detached from membranes, transferred to gelatin-coated glass slides, dried, immersed in xylene and coverslipped.
Confocal microscopy and verification of FJB staining
All specimens stained with FJB were examined with a Leica TCS SP confocal microscopy system (Leica, Heidelberg, Germany) equipped with an argon-krypton laser (Omnichrome, Melles Griot, Carlsbad, CA, USA). The laser wavelength used for excitation of FJB was 488 nm, and the emission detection window was 500–600 nm. Occasionally, the emission detection settings were changed slightly for optimal performance. Confocal image stacks were acquired at 2-µm steps by using 10 × objective (HC PL APO 10 ×/0.40), and processed with Leica TCS NT/SP Scanware (version 1.6.587) software. The algorithm used was 2-D maximum intensity projection, which determines the maximum of all intensity values in a stack of sections, and displays them in one single image. Reconstruction of the images of the whole hippocampal slice was accomplished afterwards from 1-mm2 maximum projections using Adobe Photoshop. As our recent study indicated that KA treatment mainly damaged the pyramidal CA3a/b region (Holopainen et al., 2004), we focused our studies on this specific region. For our scoring analyses, the area of stained neurons (i.e. degenerating neurons) was measured blinded (T.-K. and K.-L.) from maximum projections of FJB-stained hippocampi (n = 10–15 in each experiment group) using ImageJ software (NIH, USA), as described recently in detail (Kukko-Lukjanov et al., 2006). The following scoring system, in which the scoring numbers indicate the area (in mm2) of FJB-stained CA3a/b neurons, was used to evaluate the extent of damage: 0, no FJB-stained neurons (regarded as normal); 1, up to 0.100 mm2; 2, 0.101–0.200 mm2; 3, 0.201–0.300 mm2; and 4, > 0.300 mm2.
Statistical analysis
The overall differences in the signal intensity in Western blots of NF-L, NF-M, NF-H after KA treatment of different durations and treatments, and the overall group differences in the score numbers after the FJB and thionin stainings were assessed with one-way analysis of variance (anova) with the Tukey-Kramer multiple comparison test as a post hoc test. The difference between two experimental groups was assessed with Student's two-tailed t-test. The level of significance was set at P < 0.05. The statistical analyses were performed using GraphPad Prism software (version 4.0; GraphPad Program, San Diego, CA, USA).
Results
Time course of NF degradation
Western blotting was used to assess the time course of KA-induced NF protein degradation in hippocampal slices at 7 DIV. Figure 1A shows representative Western blots, and Fig. 1B shows the semiquantitative analysis of the NF-L, NF-M and NF-H proteins in control cultures and in cultures after KA treatment for 3, 6, 12, 24 and 48 h. As early as 3 h after treatment, the signal for all the NF proteins started to decrease, and this decline was significant (P < 0.01) after 6 h. The levels of all the NF proteins were then ∼ 30% lower in KA-treated slices than in control slices. The signals continued to decrease significantly (P < 0.001) with the treatment time, and after the 24-h KA treatment the values were ∼ 40% of those detected in control cultures. By 48 h small, but not further significant, changes compared with the 24 h treatment were detected.
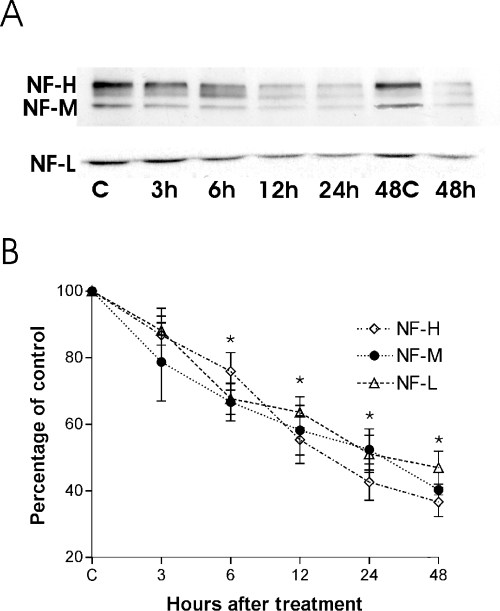
A representative Western blot of the expression of NF-L, NF-M and NF-H after KA treatment (5 µm) for 3, 6, 12, 24 and 48 h in organotypical hippocampal slices (7 DIV). Note that the control groups were different, one for the shorter times (3, 6, 24 h), and the other for 48 h (A). B shows the semiquantitative analysis of the Western blot signals for NF-L, NF-M and NF-H (given as percentage of the corresponding control). Data are given as means ± SEM (n = 8). The significance of differences between controls and KA-treated hippocampal slices was set as *P < 0.05. C, control; KA, kainic acid; NF, neurofilament.
Effect of pharmacological treatments on NF stability
To elucidate the contribution of different glutamate receptor subtypes, the significance of l-type Ca2+-channels and calpains in the regulation of KA-induced NF degradation, additional Western blots were carried out in hippocampal slices (7 DIV) treated for 24 h with 5 µm KA together with the AMPA/KA selective antagonist CNQX (10 µm), the calpain inhibitor MDL-28170 (0.5 µm), the Ca2+-channel blocker nifedipine (10 µm) and the NMDA receptor antagonist MK-801 (0.5 µm). Figure 2A shows representative Western blots of NF-L, NF-M and NF-H in control and treated cultures, and Fig. 2B shows the semiquantitative analysis of the levels of the NF protein after the treatments. In KA-treated cultures, the signal for all the NF proteins decreased by about 60%, whereas CNQX and the calpain inhibitor MDL-28170 effectively blocked the KA-induced degradation, resulting in the NF levels similar to those of control slices (Fig. 2B). Nifedipine and MK-801 did not significantly block the KA-induced NF protein degradation.
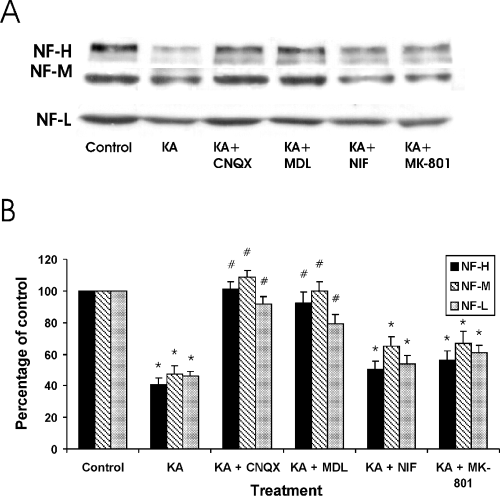
The expression of NF proteins after KA treatment alone or combined with different compounds. (A) A representative Western blot of NF-L, NF-M and NF-H (Fig. 2A), and the semiquantitative analysis of the signals (B) in control slices, and in slices after 24-h treatment with the following compounds: KA (5 µm); KA + CNQX (10 µm); KA + MDL-28170 (0.5 µm); KA + NIF (10 µm); and KA + MK-801 (0.5 µm). Control levels where set as 100%. KA, kainic acid; NF, neurofilament. *P < 0.05, comparing control slices and slices treated with different compounds. #P < 0.05, comparing slices treated with KA alone or combined with different compounds.
Immunocytochemistry
Immunocytochemistry was carried out to detect any changes in the expression of NF-L, NF-M and NF-H at the cellular level in 7-DIV slices treated for 24 h either with KA (5 µm) alone or combined with CNQX (10 µm), MDL-28170 (0.5 µm), nifedipine (10 µm) and MK-801 (0.5 µm). Table 1 shows the visual scoring of NF-L, NF-M and NF-H immunoreactivity in control and treated slices, and representative images of NF-L and NF-H staining are shown in 3, 4, respectively.
| NF-L | NF-M | NF-H | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CA3 | CA1 | DG | CA3 | CA1 | DG | CA3 | CA1 | DG | |
| Control | + + | + | + | + + | + | + | + + | + + | + |
| KA | –/+ | –/+ | –/+ | –/+ | –/+ | –/+ | + | + | + + |
| KA + CNQX | + + + | + | + + | + + | + | + + | + + + | + + | + + |
| KA + MDL-28170 | + + + | –/+ | + + | + + | –/+ | + + | + + + | + + | + + |
| KA + NIF | –/+ | + | –/+ | –/+ | + | –/+ | + | + | –/+ |
| KA + MK-801 | –/+ | –/+ | –/+ | –/+ | –/+ | –/+ | –/+ | + | –/+ |
- Concentrations used (in µm): KA, 5; CNQX, 10; MDL-28170, 0.5; NIF, 10; MK-801, 0.5. Scoring of the staining intensity: -, negative; -/+, weakly positive; +, positive; + +, moderately positive; + + +, strongly positive. The scoring is a mean of 10–12 slices from at least three different culture batches. CA3 and CA1, CA3 and CA1 hippocampal regions including cell bodies and stratum oriens, radiatum and lacunosum; DG, dentate gyrus of the hippocampus (dentate granule cells and molecular layers); NF, neurofilament; NIF, nifedipine.
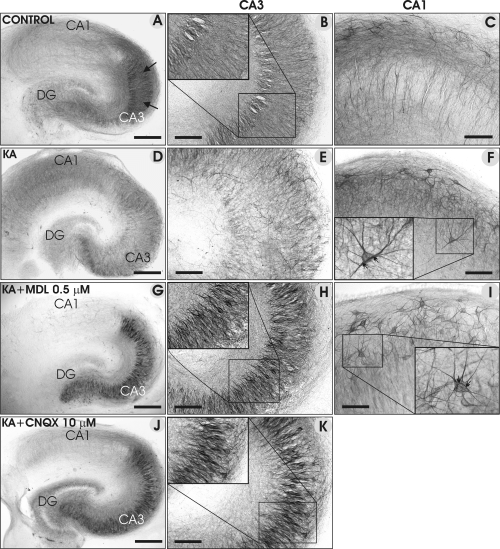
Representative images of NF-L immunostainings in control (A–C) slices, and in slices treated with KA (5 µm; D–F), KA + MDL-28170 (G–I) and KA + CNQX (J and K). Note the pronounced decrease in NF-L immunoreactivity in the CA3 region of KA-treated slices (the CA3 region is marked with black arrows in A), and its increase in the presence of MDL-28170 and CNQX. The inserts in H and K show the staining of CA3a/b pyramidal neurons under a higher magnification, and the inserts in F and I show examples of heavily stained interneurons and their processes. Scale bars, 300 µm (A, D, G and J); 100 µm (B, C, E, F, H, I and K).
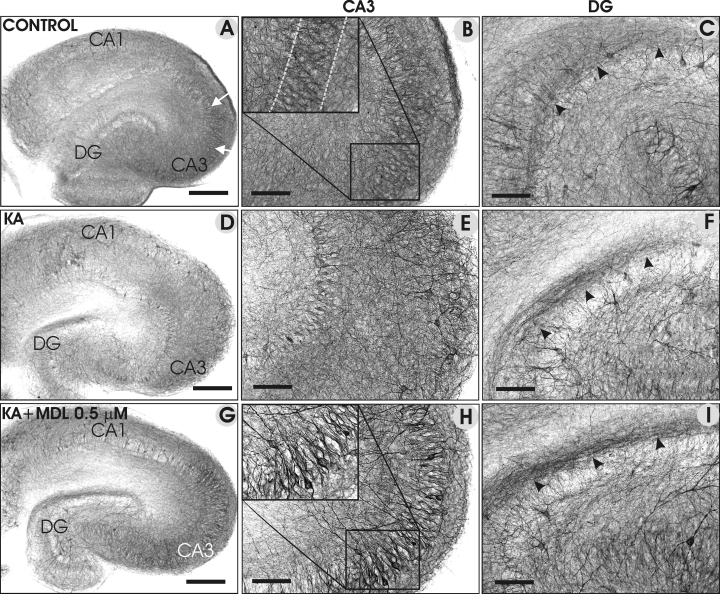
Representative images of NF-H immunostainings of control slices (A–C), in slices treated with KA (5 µm; D–F) and KA + MDL-28170 (G–I). In control slices, the CA3 pyramidal cells were positively stained for NF- H (A and B), whereas the immunoreactivity of NF-H was decreased in KA-treated slices (D and E). In the presence of MDL-28170, the NF-H immunoreactivity (G and H) was enhanced in the CA3 pyramidal cells compared with that in control slices. Increased immunoreactivity in the inner molecular layer of the DG (indicated by black arrowheads) was detected in KA-treated (F), and particularly in the KA + MDL-28170-treated slice (I), compared with a control slice. Scale bars, 300 µm (A, D and G); 100 µm (B, C, E, F, H and I).
In control cultures, the NF-L staining intensity varied region-specifically (Table 1). The CA3 pyramidal cell bodies were moderately NF-L immunoreactive (Fig. 3A and B), whereas mainly neuronal processes were stained in the CA1 region (Fig. 3C). In KA-treated slices, the NF-L immunoreactivity decreased in all hippocampal subregions, but more markedly in the CA3 pyramidal cells (Fig. 3D and E), whereas heavily immunopositive neurons were found in the CA1; on the basis of their morphology, these cells were regarded as interneurons (Fig. 3F). In the presence of KA + MDL-28170 (0.5 µm; Fig. 3G–I), the intensity of staining within the hippocampal subregions either remained at the control level, or even increased (Table 1), specifically in the CA3 pyramidal neurons and in their proximal processes (Fig. 3H). Moreover, the demarcation between immunopositive CA3 neurons and immunonegative/lightly stained CA1 neurons was surprisingly abrupt (Fig. 3G), and numerous heavily stained interneurons appeared in the CA1 region (Fig. 3I). In KA + CNQX-treated slices (Fig. 3J and K), the staining pattern was remarkably similar to that of KA + MDL-28170, being enhanced in the CA3 pyramidal neurons, the DG hilar region and also in the molecular layers. Moreover, heavily stained interneurons appeared in the CA1 region (data not shown) whereas DG granule cell bodies remained lightly stained (Fig. 3J).
In general, the NF-H staining resembled that of NF-L (Table 1). Representative images show that the moderate NF-H immunostaining in control slices (Fig. 4A–C) decreased markedly in KA-treated slices in many hippocampal subregions (Fig. 4D–F), particularly in the CA3 pyramidal cell layer (Fig. 4D and E). In the presence of KA + MDL-28170 (0.5 µm), staining was enhanced in the CA3 cell bodies (Fig. 4G and H) and in their proximal processes. Moreover, the inner molecular layer staining was more pronounced in KA- and KA + MDL-28170-treated slices (Fig. 4G and I) than in control slices (Fig. 4C), whereas granule cell bodies remained lightly stained.
The NF-M immunoreactivity resembled that of NF-L and NF-H in control and KA-treated slices, but in the presence of KA + CNQX and KA + MDL-28170, the staining intensity of the CA3 cell bodies and interneurons remained at the control level (Table 1). After KA + nifedipine and KA + MK-801 treatments, the immunoreactivity of all the NF proteins remained unaltered or even slightly decreased (Table 1).
Neuronal death
Neuronal death was studied by using FJB staining (Fig. 5A–D) in 7-DIV cultures treated for 24 h with KA (5 µm) alone, or combined with CNQX (10 µm), and MDL-28170 (0.5, 5, 25 and 50 µm). In control cultures, no FJB-stained neurons were detected in any of the hippocampal subregions (Fig. 5A), indicating a good survival of neurons. In contrast, the CA3a/b region was stained massively with FJB in KA-treated slices, indicating pronounced neuronal damage (Fig. 5B). The area of FJB-stained neurons was decreased significantly in the presence of KA + CNQX (Fig. 5C and I), and decreased concentration-dependently in slices treated with KA + MDL-28170 (50 µm) (Fig. 5D and I), whereas nifedipine and MK-801 (data not shown) had no effect. As FJB-staining indicated neuronal damage after the 24-h treatment with KA, we were interested to know whether or not stained neurons were permanently damaged and would later disappear, and the conventional thionin staining was carried out after the 48-h culture period in normal medium. The staining confirmed that the main hippocampal cell layers were well-preserved in control cultures (Fig. E), whereas KA treatment resulted in a massive loss of CA3 neurons (Fig. 5F and J), which was alleviated significantly by CNQX (Fig. 5G and J), and dose-dependently by MDL-28170 (Fig. 5H and J). It is noteworthy that CA1 pyramidal neurons and DG granule cells were not affected by KA treatment.
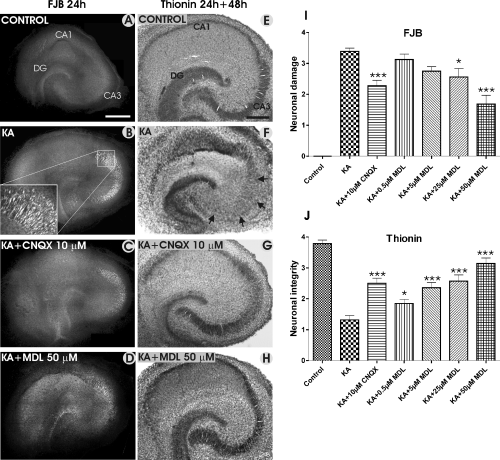
Representative images of neuronal damage, which was studied by using Fluoro-Jade B (FJB) and thionin staining in control slices, and in slices treated with KA, KA + CNQX, and KA + MDL-28170. FJB staining (A–D) was carried out after the 24-h treatment, and thionin staining was performed 48 h after finishing the 24-h treatment (E–H). Note the extensive FJB staining in the CA3 region in the KA-treated slice (B), which was decreased significantly in the presence of CNQX (C and I) and MDL-28170 (D). Thionin staining reveals well-preserved main neuronal cell layers in the control slice (E) and the extensive CA3a/b and CA3c pyramidal cell loss in the KA-treated slice, as indicated by arrows (F). This extensive damage was not detected in the slices treated either with KA + CNQX (G) or KA + MDL-28170 (H). I shows the FJB staining results in the CA3a/b region, and J shows the results of the visual scoring of the thionin staining in the CA3a/b region, indicating the neuronal integrity. *P < 0.05 and ***P < 0.001, comparing KA alone and KA in combination with CNQX and MDL-28170. Scale bars, 400 µm (A–D); 300 µm (E–H).
Discussion
There were three main findings from our present study in OHCs. First, KA-induced degradation of NF proteins was a rapid process, which affected all three NF proteins similarly, and resulted in greatly weaker immunoreactivity in KA-treated slices than in control slices, particularly in the CA3 pyramidal cell region. Second, KA-induced NF degradation was effectively inhibited with the specific AMPA/KA receptor antagonist CNQX, and the calpain inhibitor MDL-28170, which also preserved the immunoreactivity or even enhanced it, particularly in the CA3 pyramidal neurons and interneurons. Third, CNQX and MDL-28170 effectively protected neurons from KA-induced death in OHCs.
Calpain- and KA-induced neurofilament degradation
Our present results showed degradation of all three NF proteins in response to KA treatment in OHCs, a finding in accordance with recent studies in various models of brain insults. For example, NF-M and NF-H degradation has been detected within 1 h of hypoxic insult in the optic nerve (Stys & Jiang, 2002), and shortly after oxygen and glucose deprivation in acute brain slices prepared from adult rats (Tekkok et al., 2005). Moreover, NF-H degradation occurs within 1–2 h of traumatic spinal cord injury (Schumacher et al., 1999), and within hours of a glutamate-induced insult in cultured cortical neurons (Chung et al., 2005). Also, our earlier study indicated that KA-treatment (5 µm, 48 h) induced a pronounced decrease in the expression of all NF proteins, the older cultures (21 DIV) being affected more severely than the young ones (7 DIV; Holopainen et al., 2001).
Calpains are known to be directly involved in glutamate-induced hippocampal damage, and the activation of these proteases is suggested to be an early, requisite step in the cascade of events initiated by excitotoxic and hypoxic injuries which, through various intracellular signalling pathways, contributes to cytoskeletal degradation, and finally leads to nerve cell death both in vivo and in vitro (Siman et al., 1989; Minger et al., 1998; Lankiewicz et al., 2000; Stys & Jiang, 2002; Rami, 2003; Araujo et al., 2004; Wu et al., 2004). Moreover, spectrin breakdown products, indicators of the Ca2+-activated calpain activity, have been detected in the adult rat hippocampus 3 h after the intracerebroventricular injection of KA (Siman et al., 1989), and as little as 1 h after KA-induced seizures in the DG of the adult rat hippocampus (Bi et al., 1996). In addition, increased calpain activity occurs within 20 min of the ischaemic insult in the CA1 region (Rami, 2003). The fast NF degradation shown in our present study, together with earlier studies by other groups, suggests that the degradation of cytoskeleton might be an early step and not a late consequence in the process of neuronal death. Moreover, these studies further suggest that calpain activation could be of major importance in many pathological conditions affecting the human brain, including excitotoxic nerve cell damage.
AMPA/KA receptor antagonist and calpain inhibitors promote NF integrity
The marked KA-induced degradation of all three NF proteins in our OHCs was effectively inhibited with the calpain inhibitor MDL-28170 and with the potent AMPA/KA receptor antagonist CNQX, whereas the blockade of Ca2+ entrance with the l-type Ca2+-channel blocker nifedipine and the use of the NMDA receptor antagonist MK-801 had no protective effect. The observed beneficial effect of calpain inhibitors in preserving cytoskeletal integrity is in accordance with earlier studies, which have shown different calpain inhibitors to be effective in ameliorating excitotoxic and hypoxic injuries, and decreasing spectrin, calcineurin and NF breakdown (Li et al., 1998; Kunz et al., 2004; Wu et al., 2004). Moreover, calpain inhibition has been effective in protecting neurons from excitotoxic insults both in vitro (Chen et al., 1997; Lankiewicz et al., 2000; Araujo et al., 2004; Wu et al., 2004) and in vivo conditions (Wu et al., 2004; Higuchi et al., 2005). The fact that the calpain inhibitor was now added simultaneously with KA, and not before KA, mimics the clinical situation in a more relevant way, and further corroborates the idea that calpain activation at the early stage of the insult might contribute to NF degradation. This renders neurons more vulnerable to insults, which were effectively inhibited by the calpain inhibitor.
The low KA concentration (5 µm) used in our study is within the range (3–8 µm), which activates mainly KA receptors, although AMPA receptors are also activated to some extent (Kristensen et al., 2001), allowing calcium influx. In the presence of CNQX, calcium entry via AMPA/KA receptors is reduced, and results in lower calpain activation. This could explain the observation that MDL-28170 prevented NF degradation to the same extent as did CNQX. This speculation is in accordance with the recent study by Araujo et al. (2004), in which they showed that the specific AMPA receptor antagonist NBQX inhibited the cleavage of calpain substrate in cultured hippocampal neurons in a manner similar to that of the calpain inhibitor MDL-28170. Moreover, our hypothesis of the CNQX mechanism of action is in agreement with a recent study, in which AMPA receptor-positive modulators activated calpain proteases and resulted in a breakdown of spectrin, and this has effectively been inhibited by the calpain inhibitor (Jourdi et al., 2005).
Neuronal damage and neuroprotection
The current FJB and thionin staining results showed that the neuronal death process (FJB staining), and the ultimate death (thionin staining) in OHCs were effectively attenuated by CNQX, and attenuated in a concentration-dependent manner by MDL-28170. Moreover, these treatments not only protected NF proteins from degradation, but also enhanced their immunoreactivity in specific hippocampal subregions, i.e. in the CA3 and hilar regions and in the inner molecular layers of the DG. Although the molecular basis for this is currently unknown, it is tempting to speculate that it as a sign of an intracellular protection process, as recently suggested in a mouse model of amyotrophic lateral sclerosis (Lariviere & Julien, 2004). As caspase-3 and poly(ADP)ribose polymerase, the key mediators of the apoptotic pathway, are not activated in our experimental conditions (Holopainen et al., 2004), calpain activation could be the key mediator of NF degradation and contribute to neuronal death, and, as suggested earlier, might convert excitotoxic nerve cell death into a caspase-independent process (Lankiewicz et al., 2000).
In conclusion, our present study in KA-treated OHCs, as a model system for excitotoxic nerve cell damage, suggests that there exist a link between the degradation of NF proteins by calpain proteases and neuronal damage after the insult. As the NF proteins are crucial for many basic cellular functions, e.g. radial growth, dendritic arborization, maintenance of myelinated axons and axonal transport (Lee & Cleveland, 1996; Fuchs & Cleveland, 1998; Julien & Mushynski, 1998; Julien, 1999), their stability is essential for nerve cell integrity, intracellular architecture and survival. Consequently, the inhibition of their degradation could be an important step in a cascade of events promoting nerve cell viability after brain insults. However, the relationship between inhibition of calpains, cytoskeletal degradation and neuronal survival is complex, and further research is required to better understand the intracellular signalling pathways involved in calpain- and AMPA receptor-mediated effects on the cytoskeleton, and nerve cell survival in the immature hippocampus.
Acknowledgements
The financial support of the Special State Grant for Clinical Research (EVO), and the Arvo and Lea Ylppö Foundation to I.E.H., the Finnish Graduate School for Neurosciences to F.R.L.-P., and the Finnish Cultural Foundation to T.-K.K.-L. are gratefully acknowledged.
Abbreviations
-
- AMPA
-
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
-
- BS
-
- blocking solution
-
- CNQX
-
- 6-cyano-7-nitroquinoxaline-2,3-dione
-
- DG
-
- dentate gyrus
-
- DIV
-
- days in vitro
-
- FJB
-
- Fluoro-Jade B
-
- KA
-
- kainic acid
-
- MK-801
-
- (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate
-
- NBQX
-
- 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulphonamide
-
- NMDA
-
- N-methyl-D-aspartic acid
-
- OHC
-
- organotypical hippocampal culture
-
- PBS
-
- phosphate-buffered saline
-
- PFA
-
- paraformaldehyde
-
- RT
-
- room temperature
-
- SDS-PAGE
-
- sodium dodecyl sulphate polyacrylamide gel electrophoresis




