T-type Cav3.3 calcium channels produce spontaneous low-threshold action potentials and intracellular calcium oscillations
Abstract
The precise contribution of T-type Ca2+ channels in generating action potentials (APs), burst firing and intracellular Ca2+ signals needs further elucidation. Here, we show that Cav3.3 channels can trigger repetitive APs, generating spontaneous membrane potential oscillations (MPOs), and a concomitant increase in the intracellular Ca2+ concentration ([Ca2+]i) when overexpressed in NG108-15 cells. MPOs were dependent on Cav3.3 channel activity given that they were recorded from a potential range of −55 to −70 mV, blocked by nickel and mibefradil, as well as by low external Ca2+ concentration. APs of distinct duration were recorded: short APs (sAP) or prolonged APs (pAP) with a plateau potential near −40 mV. The voltage-dependent properties of the Cav3.3 channels constrained the AP duration and the plateau potential was supported by sustained calcium current through Cav3.3 channels. The sustained current amplitude decreased when the resting holding potential was depolarized, thereby inducing a switch of AP shape from pAP to sAP. Duration of the [Ca2+]i oscillations was also closely related to the shape of APs. The Cav3.3 window current was the oscillation trigger as shown by shifting the Cav3.3 window current potential range as a result of increasing the external Ca2+ concentration, which resulted in a corresponding shift of the AP threshold. Overall, the data demonstrate that the Cav3.3 window current is critical in triggering intrinsic electrical and [Ca2+]i oscillations. The functional expression of Cav3.3 channels can generate spontaneous low-threshold calcium APs through its window current, indicating that Cav3.3 channels can play a primary role in pacemaker activity.
Introduction
Calcium influx through voltage-gated calcium channels actively participates in cellular depolarization and simultaneously acts as an intracellular messenger. Calcium channels are involved in a variety of processes from cellular excitability to gene or protein regulation. Although high-voltage-gated calcium channels have been widely studied, little is known about the role of low-voltage- T-type calcium channels in the control of the membrane potential and intracellular Ca2+ signalling that they generate (Perez-Reyes, 2003). Studies have suggested that T-type Ca2+ channels induce action potential (AP) burst firing activity and low-threshold Ca2+ spikes triggered by hyperpolarization-activated cationic channels, Ih (Wilcox et al., 1988; Bal & McCormick, 1993; Huguenard, 1996). T-type Ca2+ channels are encoded by three genes (Cav3.1, Cav3.2 and Cav3.3; for reviews see Lacinova et al., 2000; Perez-Reyes, 2003). Each Cav3 isotype has distinct electrophysiological characteristics and, among them, Cav3.3 channels display unique features. Its current displays slow activation and inactivation kinetics that generate a sustained Ca2+ current under a prolonged cellular depolarization (Monteil et al., 2000; Frazier et al., 2001). Cav3.3 channels also produce a large window current in the membrane potential's range of −60 to −40 mV, in which a fraction of the channels are always open and do not inactivate (Lee et al., 1999). This property would enable Cav3.3 channels to induce a sustained increase in the intracellular Ca2+ concentration [Ca2+]i, as reported for skeletal muscle Cav3.2 channels (Bijlenga et al., 2000). Cav3.3 window current could contribute to depolarization of the cell membrane and to regulation of neuron excitability (Hughes et al., 1999; Pignatelli et al., 2005). Although the electrophysiological properties of Cav3.3 channels suggest that they have a prominent role in cellular excitability (Chemin et al., 2002a), it is unknown whether Cav3.3 channels can be a primary trigger of burst firing activities. Indeed, in native cells expressing Cav3.3 channels, their intrinsic role in cellular excitability and Ca2+ homeostasis is occluded by several other ionic conductances that also contribute to neuronal firing activity (Crunelli et al., 2005).
In this study, we have investigated the ability of Cav3.3 channels intrinsically to initiate AP firing and [Ca2+]i oscillations in NG108-15 neuroblastoma cells transfected with a cDNA coding for human Cav3.3 channels. Our hypothesis was that a Cav3.3 window current would be capable of triggering spontaneous membrane potential oscillations (MPOs) and possibly APs. We report that spontaneous MPOs and [Ca2+]i oscillations were correlated with the functional expression of Cav3.3 channels and occurred independently of any other depolarizing channel activity. The MPO properties, AP waveforms and [Ca2+]i oscillations are shown to be dependent on the electrophysiological properties of Cav3.3 channels. Together, the results show that functional expression of Cav3.3 channels may produce pacemaker activity and [Ca2+]i oscillations.
Materials and methods
Cell preparation
The clonal NG108-15 cell line, originally formed by Sendai virus-induced fusion of the mouse neuroblastoma clone N18TGG-2 and the rat glioma clone C6 BV-1, was a generous gift of IdRS (Suresnes, France). Cells were grown in plastic dishes with Dubelcco's modified Eagle's medium supplemented with 7.5% fetal bovine serum (v/v), 2 mm glutamine, hypoxantine-aminopterin-thymidine complement and 10 000 U/mL penicillin/streptomycin in an humidified environment of 5% CO2, 95% air at 37 °C. At confluence, cells were mechanically resuspended, diluted three-fold and plated in a new plastic dish. For patch-clamp experiments, cells were seeded at 60 000 cells/mL on a glass slide and used within the next 3 days.
Transfection
Four hours after seeding at 60 000 cells/mL, NG108-15 cells were transiently cotransfected with pBK-CMV plasmid constructs that encode for Cav3.3a subunit (AF211189, Chemin et al., 2001) and pIRES plasmid constructs that encode for reporter gene CD8 in a ratio of 5 : 1 with JET-PEI reagent according to the manufacturer's recommendations. Twenty-four hours after transfection, positive transfected cells where identified with anti-CD8 antibody-coated beads and further analysed by using voltage-clamp experiments.
Membrane current recording
Voltage-clamp and membrane current recordings were made with a standard patch-clamp technique using a List EPC-7 patch-clamp amplifier (Darmstadt-Eberstadt, Germany). Whole-cell recordings were performed with patch pipettes having resistances of 2–5 MΩ. Membrane potential and current records were stored and analysed on a PC (Pclamp system, Axon Instruments, Foster City, CA, USA). Junction potential was electronically compensated for before acquisition with EPC-7 patch-clamp amplifier (7.1 ± 1 mV, n = 5, for K solution and 4.5 ± 0.8 mV, n = 4 for Cs solution). Cell capacitance was determined in each cell tested by imposing 10-mV hyperpolarizing steps from the holding potential (−70 mV) and analysing the amplitude and the time course of the recorded currents. Current density was expressed as the maximum amplitude of the current per capacitance unit (pA/pF). The currents were measured by a test pulse from −70 mV to 0 mV, a representative value for peak AP. The kinetics of inactivation of the current was calculated with a mono-exponential function [f(t) = A exp(–t/τ) + C) according to the Pclamp set-up. Steady-state inactivation experiments were performed by a double pulse protocol. The holding potential was −70 mV, and a test pulse to 0 mV was preceded by a prepulse of 5-s duration and of variable amplitude (from −100 mV to 10 mV). A steady-state activation curve was constructed from the current–voltage relationship, which was fitted using a combined Boltzmann and linear Ohmic relationship as described by Chemin et al., 2002a, b). Activation and inactivation curves were fitted with a Boltzmann equation, which gives the potential for half-activation and half-inactivation. Recovery from inactivation experiments was performed by a double pulse protocol. The holding potential was −70 mV, and a test pulse to 0 mV was preceded by a prepulse of 500 ms at 0 mV. The interpulse interval was of variable duration (0–3 s).
Cells were superfused with an extracellular pseudo-physiological solution (Ca solution) containing (mm): 130 NaCl, 5.6 KCl, 1 MgCl2, 2 CaCl2, 11 glucose, 10 HEPES and 0.2 µm tetrodotoxine (TTX), adjusted to pH 7.4 with NaOH. In some experiments, CaCl2 was omitted, increased to 8 mm or substituted by BaCl2 or SrCl2. The basic pipette solution (K solution) contained (mm): 5 NaCl, 50 KCl, 65 K2SO4, 2 MgCl2, 2 ATP and 10 HEPES, adjusted to pH 7.3 with KOH. To record T-type current, without contamination from the potassium channel, the following intracellular solution (Cs solution) was used: (mm): 130 CsCl, 10 EGTA, 5 ATPNa2, 2 MgCl2 10 HEPES, adjusted to pH 7.3 with CsOH. Drugs were applied on the cells via pressure ejection from a glass pipette. All experiments were performed at 32 ± 2 °C.
Intracellular calcium concentration measurement
Measurements of [Ca2+]i were performed with an indo-1 set-up as described by Quignard et al. (2001). Briefly, cells were preloaded with the membrane-permeable indo-1 AM (1 µm) for 30 min; 50 µm indo-1 was added to the pipette solution and entered the cells after establishment of the whole-cell recording mode. [Ca2+]i was estimated from the 405/480-nm fluorescence ratio using a calibration determined within cells.
Chemicals
Antibodies raised against CD8 were purchased from Dynal (Compiegne, France). Mibefradil was a generous gift of Roche (Basel, Switzerland). All other chemicals were purchased from Sigma (St Louis, MO, USA).
Statistics
Values are given as means ± SEM. A Student's t-test was performed to estimate the significance of the differences between two mean values. A value of P < 0.05 was considered significant.
Results
Cav3.3 channel activity induces MPOs
Figure 1 shows that in the current-clamp configuration (K/Ca solutions), NG108-15 cells expressing Cav3.3 channels produced MPOs. In ∼50% cells, an artificial current was injected to hyperpolarize the membrane potential below −55 mV in order to allow de-inactivation of T-type current and to trigger MPOs. These MPOs were considered to be of low threshold as they were recorded from −70 to −55 mV. They exhibited different waveforms that we have classified in three classes according to the shape and the length of the so-called ‘action potentials’. Short APs (sAP, Fig. 1A) displayed a rather fast repolarization phase shorter than 300 ms. Prolonged APs (pAP, Fig. 1B) presented a delayed repolarization with an inflexion point leading to a short plateau phase (duration of less than 1500 ms). Long pAP (Fig. 1C) showed a long plateau phase of several seconds around −40 mV. These different types of oscillations were also recorded in the cell-attached mode (data not shown, n = 15).
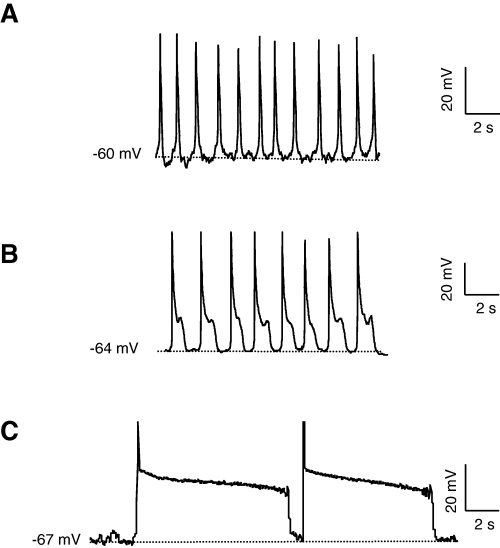
Spontaneous membrane potential oscillations in Cav3.3-transfected NG108-15 cells. (A–C) Typical membrane potential oscillations in transfected NG108-15 cells (current-clamp, records obtained in different cells). Note the different shape of spontaneous activity (A) sAP, (B) pAP and (C) long pAP. Hatched lines indicate the level of potential noted on the left. Measurements were performed in Ca/K solution.
Several experimental findings indicated further that these MPOs could be attributed to the activity of Cav3.3 T-type Ca2+ channels. Indeed, such MPOs were never observed on nontransfected cells, at any potential tested (from −30 to −90 mV, n = 29, Fig. 2A). Only transfected cells displaying a T-type current density higher than ∼ 11 pA/pF (75% of transfected cells) showed MPOs (Fig. 2B). Indeed, mean current density of oscillating cells was around 20 pA/pF, whereas nonoscillating cells had a current density of about 7.5 pA/pF. Furthermore, MPOs were inhibited by T-type Ca2+ channel inhibitors. Nickel at 300 µm, a concentration that inhibits > 50% of the Cav3.3 T-type current, occluded oscillations (Fig. 2C) whereas 30 µm nickel, a concentration which weakly inhibits Cav3.3 current (data not shown; see also Perez-Reyes, 2003), did not alter the oscillations (Fig. 2D). Oscillations were also inhibited by mibefradil (1 µm) and pimozide (1 µm) and by lowering the extracellular Ca2+ concentration (0.1 mm) (n = 3 for each condition, data not shown). An involvement of other conductances in generation of MPOs was unlikely as they were inhibited neither by a (1) Na+ channel inhibitor, TTX (1 µm); (2) nor by an L-type Ca2+ channel inhibitor, oxodipine (1 µm, data not shown, n = 3); (3) nor by substitution of Cl– and Na+ ions by the nonpermeable agents methane sulphonate or NMDG, respectively, (4) nor by the nonselective cationic channel inhibitor flufenamic acid (1 µm) (data not shown, n = 3). Additional experiments showed that MPOs could also be recorded in the presence of barium as charge carrier, and in the presence of extracellular CsCl (10 mm), which is known to block Ih (Pape, 1996) (Fig. 2E). In all cases, sAP and pAP were recorded. The Cav3.3-induced MPOs were not dependent on cell type, given that HEK-293 cells transfected with the Cav3.3 cDNA also showed sAP or pAP oscillations with similar properties (n = 4).
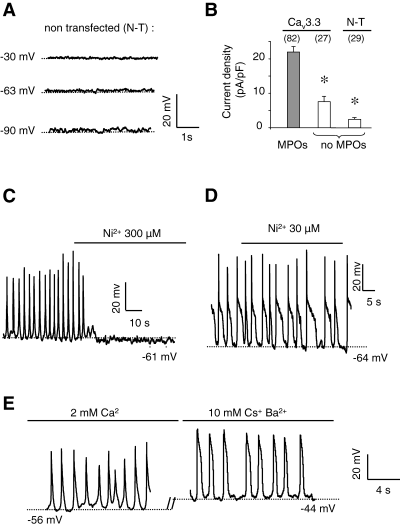
Cav3.3 T-type current and spontaneous membrane potential oscillations. (A) Typical current-clamp recording of nontransfected (N-T) NG108-15 cells, set at three different potentials (−90, −63 and −30 mV). Note the absence of oscillations. (B) Histogram of T-type current density of oscillating (grey bar) or nonoscillating (white bars) transfected cells (Cav3.3) and nontransfected cells (N-T). Current densities were first mesured in the voltage clamp mode (HP −70 mV, step depolarization 0 mV), then cells were switched to the current clamp mode. (C) Typical inhibitory effect of NiCl2 (300 µm) on MPOs recorded on Cav3.3-transfected cells. (D) Absence of inhibitory effect of NiCl2 (30 µm) on MPOs recorded on Cav3.3-transfected cells. (E) Typical oscillations recorded in the presence of 2 mm CaCl2 and 10 mm BaCl2, 10 mm CsCl and 0.1 µm TTX. Note the high membrane potential between APs (due to the high concentration of BaCl2, which induced a shift to more positive values of the steady-state inactivation and activation curve). Measurements were performed in Ca/K solution (current-clamp, records obtained in different cells). *P < 0.05, compared with MPOs.
Figure 3 shows that [Ca2+]i oscillations were associated with the Cav3.3-dependent MPOs. The waveform of [Ca2+]i oscillations was controlled by the shape of the APs. As illustrated in Fig. 3A, long pAP induced concomitant and maintained [Ca2+]i increases of 130 ± 20 nm over the basal value (115 ± 10 nm, n = 20), whereas more rapid APs induced Ca2+ oscillations (293 ± 40 nm, n = 10) with peaks of shorter duration, generating a maintained rise of the basal [Ca2+]i (Fig. 3B). Similar to that obtained for the MPOs, the [Ca2+]i oscillations were inhibited by lowering extracellular Ca2+ concentration as well as by reducing the membrane potential beyond −70 mV (Fig. 3B). These Ca2+ oscillations were also inhibited by nickel and mibefradil, further supporting that Cav3.3 T-type channels were the pathway of Ca2+ entry. Together, these data indicated that Cav3.3 channel expression may be responsible for significant changes in both membrane potential and [Ca2+]i.
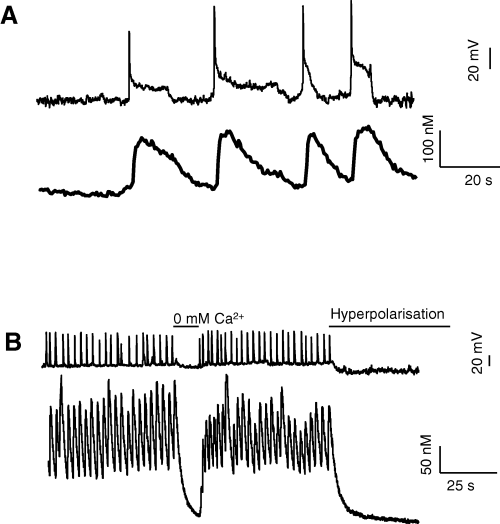
[Ca2+]i oscillations triggered by spontaneous Cav3.3 activity. (A) Effects of spontaneous long pAP on intracellular calcium concentration measured in indo-1-loaded NG108-15 cells. Each pAP induced a sustained increase of [Ca2+]i. (B) Oscillations of intracellular calcium concentration induced by shorter APs. Oscillations were inhibited when extracellular Ca2+ concentration was reduced or when membrane potential was hyperpolarized to −80 mV. Current-clamp, records obtained in different cells. Measurements were performed in Ca/K solution.
MPOs are related to the electrophysiological properties of the Cav3.3 channel
As the different types of MPOs could be observed in the same cell, we hypothesized that the membrane potential could influence the MPO waveforms. In a given Cav3.3-transfected NG108-15 cell, variations in membrane potential switched the MPO waveform from sAP to pAP and long pAP, as shown in Fig. 4A. Variations of a few millivolts in the membrane potential could considerably modify the MPO waveforms (Fig. 4A). The sAP, pAP and long pAP could be defined by the average membrane potentials at which they were recorded (Fig. 4B), by their rate of firing (Fig. 4C) and by their amplitude (48.6 ± 1.3 mV, n = 145; 57.6 ± 1.5 mV, n = 160; 76.1 ± 1.9 mV, n = 101; for sAP, pAP and long pAP, respectively). Consequently, the frequency of APs decreased whereas their amplitude increased, when hyperpolarizing the cell and shifting AP waveforms from sAP to pAP. Nevertheless, the rising kinetics of the three AP types were similar (25.5 ± 0.5 ms, n = 406). The Ca2+ currents, supporting MPOs, were then characterized in a Cs/Ca solution. A step depolarization from −70 to 0 mV revealed typical characteristics to that of the Cav3.3 T-type current described earlier (Chemin et al., 2001): kinetics of inactivation, 67.25 ± 9.01 ms (n = 10); potential for half-inactivation (V0.5 inact), −59.96 ± 0.20 mV (n = 5); half-life recovery from inactivation, 125.10 ± 7.29 ms (n = 4).
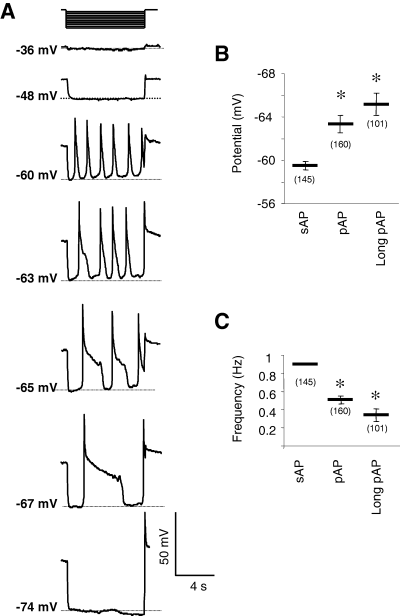
AP waveform dependence on resting membrane potential in NG108-15 cells. Measurements were performed in Ca/K solution, in the current-clamp mode. Hatched lines indicate the level of potential noted on the left. (A) Switch from sAP to pAP waveform obtained by increasing hyperpolarizing injected current in the same NG108-15 cell. Note the short voltage range where APs were recorded and the lack of APs for membrane potential before −50 mV and below −70 mV. (B) Mean resting membrane potential value before sAP, pAP and long pAP with a duration over 1.5 s (C) Mean instantaneous frequency of sAP, pAP and long pAP. Data are means ± SEM, with the number of experiments indicated in parentheses. *P < 0.05 compared with value obtained in sAP conditions.
To study the properties of the Cav3.3 current during APs, we then performed AP clamp experiments using digitized (sAP, pAP) and mock APs that mimicked pAP as waveform stimuli, in Cs/Ca solution. As shown in Fig. 5A, sAP induced only a transient inward current (Fig. 5A, a3) and no such current in nontransfected cells (Fig. 5A, a2). By contrast, a pAP or mock APs with a plateau phase at −40 mV induced a two-component inward current: a transient inward current followed by a sustained inward current that occurs during the plateau phase (Fig. 5A, b3 and c3). By contrast, no such inward current could be observed in nontransfected cells but rather a small residual outward current (Fig. 5A, b2 and c2). The transient inward current was carried out by T-type channels, as it was inhibited by nickel (500 µm, n = 4). The amplitude of the sustained current was dependent on the plateau potential value and the maximum current amplitude was obtained for a plateau potential of between −50 and −30 mV, which correspond to the value of the plateau potential of pAP. Indeed, this current could not be recorded at −70 mV (where only a rapid deactivating current was recorded, Fig. 5A, c3). Moreover, the amplitude of the sustained current depended on the resting membrane potential. Detectable at a holding potential of −70 mV, the sustained current was inactivated at a holding potential of −50 mV (Fig. 5B and C). The lack of plateau potential displayed by sAP could be explained by the depolarized resting membrane potentials from which they are triggered and at which the sustained current was inhibited. In the next set of experiments, the sustained Cav3.3 current was characterized further. It was of similar amplitude after substitution of Ca2+ by Ba2+ or Sr2+ ions, and it was significantly enhanced when the extracellular Ca2+ or Ba2+ concentration was increased to 10 mm. It was inhibited by mibefradil (data not shown) and nickel (Fig. 5D) in a dose-dependent manner. The effect of Cl– or Na+ ions was not tested as the sustained current was not significantly altered after substitution of these ions by the nonpermeable agents methane sulphonate or NMDG, respectively. It was not a Ca2+-activated current, as it could be recorded after substitution of Ca2+ by Ba2+ (Fig. 5D) or chelation of intracellular Ca2+ by EGTA (10 mm) (data not shown). Furthermore, it was not inhibited by the nonselective cationic channel inhibitor flufenamic acid (1 µm). Additional experiments confirmed that this sustained Ca2+ current was specific for the Cav3.3 channels given that nontransfected cells or cells transfected with Cav3.1 channels did not display any sustained inward current in this experimental situation (n = 5, data not shown). Overall, these results indicated that the pAP plateau was supported by Cav3.3 T-type Ca2+ channels.
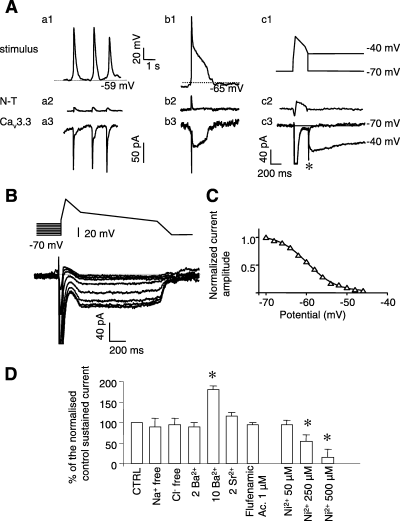
Properties of Cav3.3 sustained currents. (A) sAP (a1), pAP (b1) and mock depolarization that mimicked pAP (c1) were used as stimuli on nontransfected cells (a2, b2, c2; N-T) and Cav3.3-transfected cells (a3, b3, c3; Cav3.3). Peak of the stimuli induced concomitant inward peak current (a3, b3, c3), related to the activation of Cav3.3 current (for clarity, large transient inward currents over 100 pA were omitted), absent in nontransfected cells. Sustained current could be observed only with pAP stimuli (b3) or depolarization that mimicked pAP during the plateau phase at −40 mV. Note the absence of this sustained current in nontransfected cells (a2, b2, c2) or with cells stimulated with an sAP stimuli (a2). The sustained current was also absent for a plateau potential value of −70 mV (only a fast deactivating current could be recorded (*). (B) Voltage dependence of the sustained current. Increasing holding potential from −70 to −50 mV (4-mV amplitude step) induced a drastic reduction of the sustained current, indicating that this current was voltage-dependent. (C) Steady-state inactivation curve of the sustained current derived from B. The potential for half-inactivation was −61 ± 3 mV (n = 5). Note that this curve was very similar to the steady-state inactivation curve of T-type Ca2+ current (see Fig. 6). Maximum current amplitude was normalized to 1. (D) Normalized current amplitude of the sustained inward current, challenged with pAP waveforms as stimuli (as in Fig. 5A), in the absence of extracellular sodium (Na+ free), chloride (Cl– free), after substitution of CaCl2 by BaCl2 (2 or 10 mm) or SrCl2, flufenamic acid and in the presence of different concentrations of NiCl2. In Na+-free conditions, sodium ions were substituted by N-methyl-d-glucamine (NMDG). In Cl– free solution, chloride was substituted by methane sulphonic acid. Data are means ± SEM (n = 6). *Value significantly different from that obtained in control conditions (P < 0.05). Experiments were performed in the presence of Cs/Ca solution to highlight the inward current and, for clarity, large transient inward currents over 150 pA were omitted.
Role of Cav3.3 window current in triggering MPOs
We measured [Ca2+]i and steady-state Ca2+ entry using K/Ca solution, in transfected NG108-15 cells loaded with indo-1. A 100-ms step depolarization induced a large inward T-type current concomitant with a large [Ca2+]i increase (195 ± 17 nm, n = 12, from a basal level of 120 ± 12 nm, Fig. 6A). The variation in [Ca2+]i amplitude was voltage-dependent and the voltage/[Ca2+]i relationship was similar to the voltage/current relationship (Fig. 6B). Analysis of the steady-state activation and inactivation properties of the Cav3.3 T-type Ca2+ channels in NG108-15 cells revealed that a steady-state Ca2+ current, referred to as a ‘window current’, could exist in the membrane potential range where the MPOs were elicited (Fig. 6C). Such a T-type Ca2+ channel window current could control the resting membrane potential as it corresponds to a continuous depolarizing current (Crunelli et al., 2005). Such an effect on [Ca2+]i was assessed by using a ramp potential from −85 to 0 mV in voltage-clamp conditions at a slow depolarization speed (0.5 mV/s; Fig. 6D). In Cav3.3-transfected NG108-15 cells, this ramp protocol induced a significant increase in the [Ca2+]i of 104 ± 25 nm over the basal level (118 ± 15, n = 6, Fig. 6D). This bell-shaped Ca2+ response exhibited large [Ca2+]i increase in the potential range of the window current. A [Ca2+]i increase of similar characteristics was also observed when the ramp protocol was reversed (from 0 to −85 mV; hyperpolarization speed, 0.5 mV/s; [Ca2+]i increase, 98 ± 12, n = 5, Fig. 6E). The [Ca2+]i increase was inhibited by nickel (500 µm, 20 nm ± 10 nm, n = 3) and absent in nontransfected cells (6.1 ± 1 nm, n = 6) stimulated with a depolarizing or hyperpolarizing ramp. These results suggested that the window current mediated a [Ca2+]i increase between −70 and −40 mV that could explain the triggering of MPOs.

Intracellular calcium concentration and window current. (A) Simultaneous recording of the Ca2+ current and variation of [Ca2+]i in indo-1-loaded Cav3.3-transfected cells. A rapid square pulse depolarization (100 ms, from −70 to 0 mV) induced only a slow Ca2+ spike (Ca/K solution). (B) Correlation between the current–voltage relationship of the Cav3.3 current and [Ca2+]i recorded simultaneously (n = 5, holding potential −70 mV). (C) Normalized activation (○) and inactivation (•) curves, which show window current potential range (Ca/Cs solution). The activation curves were constructed from B. Steady-state inactivation curves were constructed from the current amplitude of a step depolarization to 0 mV from a prepulse of 5 s (from −100 to +10 mV). All curves were fitted with a Boltzmann equation. (D) Long-lasting variation of the intracellular Ca2+ concentration in response to very slow ramp depolarization from −85 to 0 mV or (E) in response to very slow ramp hyperpolarization from 0 to 85 mV on Cav3.3-transfected cells and nontransfected cells (N-T). Experiments performed in voltage-clamp, whole-cell configuration in Ca/K solution.
To explore further the relationships between the Cav3.3 window current and the MPOs, the voltage range of the Cav3.3 window current was shifted by modifying the external Ca2+ concentration. Increasing extracellular Ca2+ concentration from 2 mm to 8 mm induced a significant +9.1 ± 1.9 mV (n = 6) shift of the potential of half-activation (V0.5 act) (Fig. 7A and B) and consequently of the window current potential range. The threshold potential required to obtain similar spontaneous oscillations as at 8 mm compared with 2 mm (see Fig. 7C) was shifted significantly toward depolarized potentials by +8.1 ± 2.4 mV (n = 5). These data are in good agreement with a role of the Cav3.3 window current in controlling MPOs. Similar results were obtained in the presence of Ba2+ as charge carrier when the Ba2+ concentration was increased from 2 to 10 mm (data not shown). Overall, these data indicate that the spontaneous MPOs described are directly triggered by the activity of Cav3.3 T-type Ca2+ channels and that the MPO waveform reflects the specific electrophysiological properties of the Cav3.3 T-type Ca2+ channels, including those of its window current.
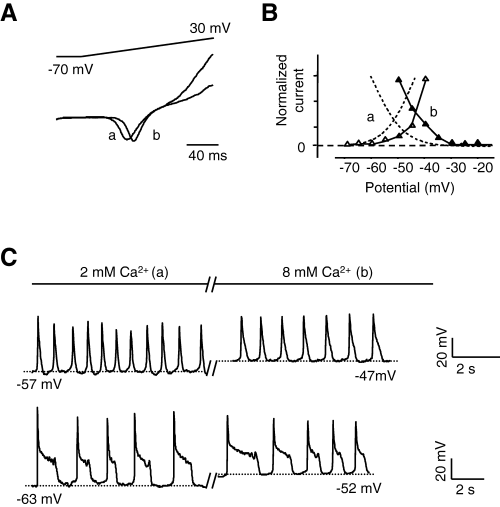
Cav3.3 window current controls membrane potential oscillations. (A) Typical recording of Cav3.3 current stimulated with a ramp depolarization from a holding potential of −70 mV in the presence of 2 mm (a) or 8 mm (b) extracellular CaCl2. Current amplitudes were normalized to illustrate the shift to more positive potential of the threshold voltage for activation. (B) Window current potential range in different external CaCl2 concentrations (a, 2 mm; b, 8 mm). Overlaps between activation () and inactivation () curves in the presence of 8 mm CaCl2 (window current, data obtained as in Fig. 6C) were shifted to more positive value than same curves (hatched lines) in the presence of 2 mm CaCl2. Only the window current of a representative experiment was shown to illustrate the potential shift in comparison with the steady-state activation and inactivation curves obtained in Fig. 6C. (C) Typical oscillations with sAP or pAP in 2 mm (a) extracellular CaCl2 concentration and after application of 8 mm CaCl2 (b); note the shift of the membrane potential between APs. Measurements were performed in Ca/K solution in the whole-cell current-clamp mode. Hatched lines indicate the level of potential noted below.
Discussion
The main finding of this study is that Cav3.3 channels can generate spontaneous and repetitive low-threshold Ca2+ APs of various shape associated with intracellular Ca2+ oscillations. These membrane potential and [Ca2+]i oscillations are closely related to the channel's electrophysiological properties and do not involve other pacemaker currents. The Cav3.3 window current is sufficient to provide the depolarizing drive triggering APs during each cycle of firing.
Functional expression of the Cav3.3 channels in proliferative NG108-15 neuroblastoma cells results in a large T-type Ca2+ current, as reported earlier (Chemin et al., 2001), without contamination by high-threshold Ca2+ currents (Lukyanetz, 1998). This Cav3.3 current displays slow inactivation kinetics and a large window current component in the range of the resting membrane potential as in HEK-293 cells (Lee et al., 1999; Monteil et al., 2000). We document here that expression of Cav3.3 channels induces spontaneous low-threshold MPOs. MPOs result from the activation of T-type channels because (1) they are present only when an inward Ca2+ current is present, (2) these oscillations become apparent in the hyperpolarized potential range where Cav3.3 channels are not inactivated (i.e. below −50 mV) and (3) they are inhibited by nickel and mibefradil. Furthermore, MPOs can be recorded on intact cells in the cell-attached mode (Fenwick et al., 1982). In nontransfected NG108-15 cells, a small endogenous T-type current is recorded (Lukyanetz, 1998; Chemin et al., 2002a, b). Cav3.2 channels support this current because it inactivates rapidly and it is blocked by low nickel concentration (30 µm, Chemin et al., 2002a, b; and our unpublished observations). We show that this endogenous T-type current does not contribute to oscillation as 30 µm nickel is unable to inhibit oscillations. Moreover, oscillations are not inhibited by Na+ and high threshold-activated Ca2+ channel blockers (TTX, oxodipine) and the shape, frequency and threshold of these MPOs are significantly different from those reported for the firing activity induced by high-threshold-activated Ca2+ channels (Takahashi et al., 1999).
In several cell types, low-threshold MPOs are associated with Ih activity (Pape, 1996). Other studies, however, have shown that Ih cannot promote oscillations unless a depolarizing current is present between APs (Feigenspan et al., 1998). In NG108-15 cells, no functional Ih current could be recorded and the application of extracellular CsCl, an inhibitor of Ih, did not inhibit oscillations. ZD7288, a well-known inhibitor of Ih, could not be used as it is also described as an inhibitor of T-type channels (Felix et al., 2003). We then provide several arguments supporting that, in transfected cells, pacemaker activity relies on the T-type window current. Sodium or chloride ions are not involved in pacemaker activities as their substitution by NMDG and methane sulphonate, respectively, does not impede the oscillations. The potential range of spontaneous APs is similar to that of the T-type window current. When the window current potential range is shifted to more depolarized values, the potential between APs is similarly shifted (for both sAP and pAP). These data strongly indicate that the depolarizing current between APs is carried out by Ca2+ through Cav3.3 channels. More importantly, we were able to visualize the steady-state Ca2+ entry through the T-type window current by direct [Ca2+]i measurements. Using slow ramp protocols in voltage-clamp experiments, a significant [Ca2+]i increase occurs between −70 and −40 mV, which corresponds to the potential range of the T-type window current. Nonvoltage-gated conductance could not account for the shape of this signal because in such a case the [Ca2+]i increase at −80 mV should be more important than at −50 mV due to the Ca2+ driving force. Nevertheless, the small voltage range difference between Ca2+ and the window current signal could be due to accumulation of intracellular Ca2+ and the slow kinetics of Ca2+ extrusion. MPOs are not specific to NG108-15 cells; they can also be recorded in transfected HEK-293 cells, which lack endogenous pacemaker currents (Ludwig et al., 1999). Together, these results demonstrate that Cav3.3 T-type Ca2+ channels can play a primary role in pacemaker activity. These observations comply well with a mathematical model, which further indicates that MPOs are present only if there is an appropriate ratio of Cav3 current to leak current (Williams et al., 1997; Hughes et al., 1999). A small window current may not be able to overcome background hyperpolarizing currents and to induce oscillations. Indeed, the MPOs were recorded only in cells that showed large T-current density. Together, our results demonstrate that the activity of Cav3.3 channels is important in inducing and maintaining oscillations. The ability of T-type current to induce MPOs could be enhanced when there is an increase in its density as a result of their specific subcellular localization as in dendrites of reticularis thalami neurons (Joksovic et al., 2005).
The shape of APs within MPOs correlates well with the electrophysiological properties of Cav3.3 channels. The activation kinetics of the Cav3.3 current (around 7 ms, Perez-Reyes, 2003) can easily support the rising phase of the APs (which last up to 25 ms). Each AP peak correlates well with Cav3.3 peak current, as demonstrated in AP clamp experiments (Fig. 5). The transient repolarization phase of pAP, before the plateau phase, could be primary due to voltage-dependent inactivation of Cav3.3 current. Indeed, inactivation kinetics is strongly voltage-dependent and the more the cell is depolarized, the faster is the inactivation of the current. Concomitant activation of outward current(s) could also possibly contribute to this transient repolarization phase. The amplitude of the sAP is smaller than the amplitude of the pAP, in agreement with the lower number of available channels at depolarized potential (as observed on the steady-state inactivation curve). The plateau phase of pAP involves a sustained inward current through Cav3.3 channels. The sustained current was recorded only in hyperpolarized cells (below −62 mV). At a more depolarized holding potential, sustained Cav3.3 current was drastically reduced. These data provide a likely explanation for the lack of a plateau in more depolarized cells. The nature of the sustained Cav3.3 current is rather complex, involving deactivation, inactivation and activation properties (Frazier et al., 2001). At the plateau potential (−40 mV), the kinetics of channel closing is faster than inactivation kinetics, which enables Cav3.3 channels to re-open (Frazier et al., 2001). In addition, participation of the Cav3.3 window current should be considered at this potential. Finally, although it is known that in subthalamic nucleus or thalamic neurons Ca2+-activated currents contribute to the plateau phase (Beurrier et al., 1999; Hughes et al., 2002), we report that in our experimental conditions the plateau phase of pAP does not involve activation of other depolarizing conductances such as sodium-, chloride- or Ca2+-activated currents. Furthermore, pAP could also be recorded in HEK-293 cells, indicating further that endogenous background channels are poorly involved in the plateau phase of cells or cellular domains expressing large Cav3.3 current density.
We document here that Cav3.3 channels participate in the control of [Ca2+]i and Ca2+ signalling at different levels. Cav3.3 channels can mediate a voltage-dependent increase of [Ca2+]i, as well as a maintained [Ca2+]i increase, in relation to the Cav3.3 window current. These data confirm that window current controls not only membrane potential (Crunelli et al., 2005; this study) but also [Ca2+]i. This Cav3.3-mediated [Ca2+]i increase is likely to activate various Ca2+-dependent mechanisms, as shown for the Cav3.2 window current-dependent [Ca2+]i increase in fusion of human myoblasts (Bijlenga et al., 2000). The [Ca2+]i increase-related window current observed in NG108-15 cells (∼100 nm) is greater than that reported for myoblasts, possibly due to the larger amplitude of the Cav3.3 window current (Lee et al., 1999). During MPOs, Cav3.3 channel induces a maintained increase of [Ca2+]i that is larger than the Ca2+ increase induced by a single depolarization. The maintained increase in [Ca2+]i may result from the summation of sequential activation of Cav3.3 channels. Overall, these data indicate that Cav3.3 channels can generate a variety of Ca2+ signals that may differentially impact neuron physiology.
The physiological relevance of these results may be of importance. Cav3.3 channels are mainly expressed in the central nervous system, such as in lateral habenula, reticular and latero-dorsal thalamic nuclei. In these nuclei, slowly inactivating T-type currents have been recorded (Wilcox et al., 1988; Bal & McCormick, 1993) that could further be associated with Cav3.3 channels (Perez-Reyes, 2003). In those neurons, oscillatory activity has been linked to T-type channel de-activation (for a review see Huguenard, 1996). Similar to our data, the frequency of rebound oscillation was shown to depend on membrane potential, and the duration of each AP was inversely related to its amplitude (Wilcox et al., 1988). In reticular thalamic nuclei, a slow inactivating T-type current, likely to be related to Cav3.3 channel, is essential for generation of burst firing activity (Joksovic et al., 2005), confirming that Cav3.3 channels can act as a pacemaker current. The spike bursts are prolonged and less self-limiting in these neurons than in neurons that express other types of T-type channels (Mulle et al., 1986; Huguenard & Prince, 1992; Huguenard et al., 1993). Our description of the Cav3.3 sustained current may explain the long duration of the burst.
Together, our data demonstrate that Cav3.3 channels can generate a pacemaker current triggering low-threshold Ca2+ APs and that Cav3.3 sustained current regulates intrinsic electrical and [Ca2+]i oscillations. It is therefore tempting to suggest that in cells that express Cav3.3 channels, as in reticular thalamic nuclei neurons, Cav3.3 currents can directly contribute to the fine tuning of burst duration, as evidenced in NG108-15 cells over-expressing Cav3.3 channels.
Acknowledgements
This work was supported by grants from the Centre National de la Recherche Scientifique, the Centre National des Etudes Spatiales and the Association Française contre les Myopathies, France. We thank N. Biendon for secretarial assistance and J. Mironneau for his support, constructive remarks and corrections of the manuscript.
Abbreviations
-
- APs
-
- action potentials, [Ca2+]i, intracellular Ca2+ concentration
-
- MPOs
-
- membrane potential oscillations
-
- TTX
-
- tetrodotoxine
-
- sAP
-
- short APs
-
- pAP
-
- prolonged APs




