Galectin-1 in regenerating motoneurons
Abstract
The exogenous application of recombinant galectin-1 has recently been shown to promote the rate of peripheral nerve regeneration. Endogenous neuronal galectin-1 expression has recently been demonstrated to increase after axotomy. Here we demonstrate a significant increase in the endogenous neuronal expression of galectin-1 mRNA in facial motoneurons after either a nerve resection or crush injury in mice. This increase in galectin-1 expression was due in part to the loss of target-derived factor(s) as indicated by both the return of galectin-1 expression to control levels following target re-innervation and the increase in galectin-1 expression after blockade of axonal transport by an interneuronal colchicine injection. Furthermore, interneuronal injections of glial-derived neurotrophic factor into the uninjured nerve also increased galectin-1 mRNA expression within facial motoneurons suggesting that positive signals may also be involved in the regulation of galectin-1 expression. Galectin-1 null mutant mice showed an attenuated rate of functional recovery of whisking movement after a facial nerve crush.
Introduction
Galectins are an evolutionarily conserved family of carbohydrate-binding proteins found in organisms ranging from Caenorhabditis elegans to humans (Barondes et al., 1994). The expression of galectin (Gal)1, one of the earliest-identified members of this family, occurs in a variety of tissues undergoing growth or rejuvenation such as placental tissue, immune cells and tumour cells (Perillo et al., 1998). Within these tissues, Gal1 mediates biological functions such as inflammation, cell adhesion and/or growth by intra or intermolecular cross-linking of carbohydrate residues (Hughes, 1999; Rabinovich et al., 2002).
Neuronal Gal1 expression occurs within primary sensory neurons and motoneurons (Regan et al., 1986). During the period of motor axonal outgrowth in embryonic rats, Gal1 mRNA expression increases within somata of spinal motoneurons until target muscles are reached, following which, expression is maintained at a lower level throughout adult life (Hynes et al., 1990). Although the precise functions of this protein remain uncertain, (Puche et al., 1996) demonstrated Gal1 modifies olfactory neuron adhesion in vitro and target reaching in vivo. Following sciatic nerve injury, recombinant oxidized Gal1, promotes axonal growth both in vitro and in vivo (Horie et al., 1999; Horie & Kadoya, 2000; Fukaya et al., 2003; Horie et al., 2004). Specifically, recombinant oxidized Gal1 increased the rate and success of spinal motor and sensory axonal growth in vivo as indicated by either neurofilament staining or retrograde tracer application (Horie et al., 1999; Fukaya et al., 2003). Conversely, a loss of function approach using Gal1 function-blocking antibodies in vivo to block endogenous Gal1 resulted in a significant reduction of axonal regrowth. Gal1 promotes neuronal regrowth by causing macrophages to release neuronal growth-promoting factors (Horie et al., 2004).
Following axotomy, the change of endogenous neuronal Gal1 mRNA expression depends on the neuron's growth ability. In the rat, nonregenerating rubrospinal neurons decrease their Gal1 mRNA expression following axotomy, whereas regenerating peripherally axotomized spinal motoneurons have increased Gal1 mRNA expression (McGraw et al., 2004). This increase in axotomized rat facial motoneurons occurs within 6 h (Akazawa et al., 2004). Here, we have used a mouse facial motoneuron model of axonal injury. The facial nerve carries almost entirely motor fibers, and functional recovery can be assessed by movement of vibrissae (Paxinos, 1985; Isokawa-Akesson & Komisaruk, 1987). After a facial nerve crush, whisker movement ceases until the injured axons begin to re-innervate their targets (Gilad et al., 1996; Ferri et al., 1998; Serpe et al., 2002; Kamijo et al., 2003). The amount and character of whisking movement (amplitude and frequency) is directly proportional to the success of axonal regeneration (Tomov et al., 2002).
In the present study, we examined the endogenous Gal1 mRNA expression in adult mouse facial motoneurons after axonal injury. We used both a nerve resection and crush injury to examine Gal1 mRNA changes within the motoneuron cell bodies to ascertain whether Gal1 mRNA expression correlates with the regenerative state of the axons. The recovery of whisking movement of Gal1 null mutant (–/–) to wild-type (wt) mice after facial nerve crush was compared to determine the significance of endogenous Gal1 for axonal regeneration.
Materials and methods
Animals
Age-matched adult male 129P3/J wild-type (The Jackson Laboratory, Bar Harbor Ma, USA), 129P3/J GAL1 null mutant mice (Poirier & Robertson, 1993) and CD-1 mice (University of British Columbiaís animal care facility) were used in this study. Surgical procedures were performed in accordance with the Canadian Council for Animal Care and approved by the University of British Columbia Animal Care Committee.
Facial nerve lesion
Adult mice were anaesthetized with an intraperitoneal injection of ketamine hydrochloride (135 mg/kg, Bimeda-MTC, Cambridge, ON, Canada) and xylazine hydrochloride (6.5 mg/kg, Bayer Inc, Etobicoke, ON, Canada). Facial nerve lesions were performed under anaesthesia as previously described (McPhail et al., 2004), the facial nerve was exposed at its exit from the stylomastoid foramen. The buccal branch of the facial nerve was either transected and a 2–3 mm nerve segment removed to prevent nerve regeneration, or the nerve was crushed twice for a period of 5 s with #5 forceps (Fine Science Tools, North Vancouver, BC, Canada), and the wound closed with sutures.
Facial nerve injections
Facial nerves were exposed as described above. Using a Hamilton syringe and a pulled glass micropipette, 1 µL of saline solution, 50 µm colchicine (Sigma, Oakville, ON) or 2 µg/µL of glial cell line-derived neurotrophic factor (GDNF; gift from Regeneron Pharmaceuticals, Tarrytown, NY, USA) was injected into the uninjured nerve at the same site where the nerve was crushed in previous experiments. The skin was sutured closed and the animals were allowed to survive for three days.
Perfusion/cryosectioning
At appropriate times mice were injected with a lethal dose of chloral hydrate (900 mg/kg) and monitored until breathing was arrested. The animals were then perfused intracardially with phosphate buffer saline (PBS), pH 7.4 followed by cold 4% w/v paraformaldehyde in 0.1 m PB. The brain and spinal cord were removed and the tissue postfixed for 24 h in 4% paraformaldehyde at 4 °C. Tissue was cryoprotected in a 22% sucrose solution in PBS. After cyroprotection, tissue was rapidly frozen in dry ice-cooled 2-methylbutane. All tissue was cryosectioned at a thickness of 14 µm and stored at −80 °C until further processing.
In situ hybridization
The mouse Gal1 probe was a 51-mer oligonucleotide complementary to the 3′-untranslated sequence of Gal1 and 5′-TCACTCAAAGGCCACGCACTTAATCTTGAAGTCTCCATCCGCCGCCATGTA-3′ (GenBank accession number BC002063). The Gal1 probe was complementary to bases 424–474. The mouse probes were end-labelled with 33P-dATP (Perkin-Elmer, Woodbridge, On, Canada) by using deoxynucleotide terminal transferase according to a standard protocol (Kobayashi et al., 1996). Perfusion-fixed sections were hybridized to 1.2 × 106 cpm of probe for 16–18 h at 44 °C. The slides were dipped in Kodak NTB-2 emulsion and exposed for 3 days. Slides were then dehydrated in a series of alcohols and stored at room temperature. Spinal cord sections were later re-hydrated in dH20 for 1 h and then a fluorescent Nissl stain (Neurotrace, 1 : 200, Molecular Probes Inc. Eugene, OR, USA) was added to the slides. Slides were then dehydrated in a series of alcohols and coverslipped with Entallen (Fisher Scientific, Nepean, ON, Canada).
Analysis of in situ hybridization signal in facial motor nucleus (FMN)
At least three sections per animal were analysed, and to prevent a neuron being analysed twice each tissue section was at least 100 µm apart. Nissl and darkfield (silver grain) images were taken of both the injured and the contralateral (uninjured) side using a digital camera attached to a fluorescent microscope (Carl Zeiss, Axioskop, Toronto ON, Canada) in combination with Northern Eclipse software (Empix Inc, Mississauga ON, Canada). All images were analysed with SigmaScan Pro 5 software (SPSS Inc., Chicago, IL, USA). The per cent area occupied by silver grains was determined. This was accomplished by outlining the individual neuronal cell bodies using the Nissl image and applying the resulting layer to the darkfield image. Background autoradiographic signal was then subtracted to obtain the corrected area occupied by silver grains. For each animal, the percentage area occupied by ISH signal per soma was determined for both the axotomized and contralateral (uninjured) side. The data were expressed as percentage of the mean ISH signal per soma on the contralateral uninjured side, as described previously by (Fernandes et al.,1999).
Mouse facial nerve functional analysis
The Gal1–/– (n = 4) and wt (n = 4) mice used in this study were born within 24 h of each other and were 4 weeks old when surgery was performed. Prior to surgery and under light anaesthesia, all but two whiskers in the caudal C-row were trimmed from the whisker pad as previously described by (Tomov et al. 2002) and the wound was sutured closed. To analyse changes in whisker movement over time as an indicator regeneration of the crushed facial nerve, before surgery and after the first 3 days following a facial nerve crush, 2–5 min of whisker movement was recorded using a digital video camera (Cannon, XR50MC) as described by (Tomov et al., 2002). Digital images were then transferred to a Macintosh computer (Apple Computer, Cupertino CA, USA) and individual frames were obtained using iMovie 3.03 (Apple Computer). Individual frames of the maximal protraction were obtained. Using Image J (1.30p, NIH, Bethesda, ML, USA) a straight line was drawn between the tear ducts of the right and left orbits of the eyes. This line represented the 0° angle. The angle between this line and the maximum forward sweep of the vibrissae was recorded. Frequency was analysed by counting the number of vibrissae sweeps per second. For each animal on each day until 14 days after surgery, four separate vibrissae movements were analysed and then averaged for each animal.
Statistics
Quantification was performed blind with respect to the treatment groups. All data are represented as mean ± standard error of the mean (SEM), and all tests were carried out using SigmaStat 3.0 (SPSS Inc). Unless otherwise indicated, a one-way anova with Holm-Sidak posthoc test was used to determine significance between groups. Significance was assigned at P < 0.05.
Results
Gal1 mRNA expression in the facial nucleus
In the facial nucleus of Gal1 wt (129P3/J) mice, in situ hybridization (ISH) for Gal1 mRNA followed by autoradiography was used to determine motoneuronal Gal1 mRNA expression (Fig. 1). Silver grain density was at background levels in Gal1–/– tissue sections, even when exposed for 2 days longer (i.e. 5 instead of 3 days) than Gal1 wt sections, confirming that Gal1 mRNA expression was undetectable in the Gal1–/– mice (Fig. 1). Gal1 wt mice are an inbred line and were used for comparison. Motoneurons in these mice show moderate amounts of silver grains (7.23 ± 0.89 times background with 3 days of exposure). In addition, we examined Gal1 expression in an outbred line of mice (CD-1) to ensure that the particular line of 129P3/J wt mice had similar levels of Gal1 mRNA expression and responded similarly to axonal injury. The baseline levels of Gal1 ISH signal in the uninjured facial motoneurons of these CD1 mice were similar to the 129P3/J line (data not shown). Control experiments with a sense probe to Gal1 revealed only background levels of silver grains on the tissue sections in either the Gal1 wt, Gal1–/– or CD-1 facial motor nuclei (data not shown).
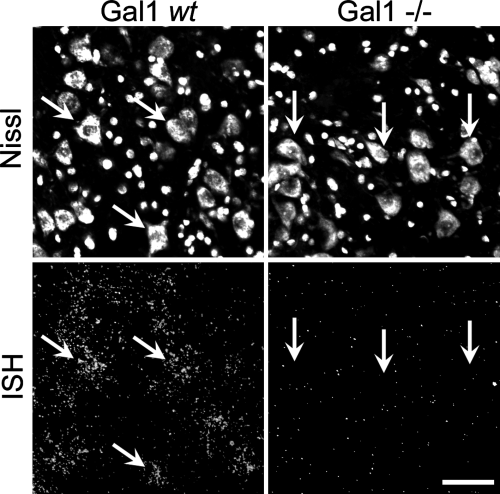
Gal1 is expressed in uninjured motoneurons. Photomicrographs of Gal1 in situ hybridization signal (bottom row) that have been counter stained with the fluorescent nissl stain (top row) within the mouse facial motor nucleus (FMN). Angled arrows on the Gal1 wt photomicrographs (left panels) indicate Gal1 mRNA is neuronally expressed. In Gal1–/– mice (right panels) the photographic emulsion was left on 2 days longer than the Gal1 wt mice sections ensured the absences of mRNA signal was not due to a reduction in signal. Vertical arrows indicate no silver grains over Gal1–/– neuronal cell bodies and that Gal1 mRNA does not show any greater ISH signal than background levels (right panels). Scale bar, 50 microns.
Gal1 expression after resection or crush of the facial nerve
After a facial nerve crush in Gal1 wt mice, ISH signals for Gal1 mRNA significantly increased in the axotomized motoneurons (Fig. 2). The silver grain density after 3 days was 307.3 ± 46.6% of contralateral and 360.9 ± 57.9% after 7 days. Statistically, these increased mRNA levels were significantly different from the levels found in uninjured motoneurons (P < 0.001, P < 0.005, respectively; Fig. 2). By 14 days after a crush injury the nerves have successfully regenerated back to their targets as indicated by nearly complete behavioural recovery (Fig. 6). At this time, silver grain density decreased to 147.8 ± 8.1% of contralateral. This level of expression was no longer significantly different from the uninjured control animals (P > 0.05, Fig. 2 histogram).
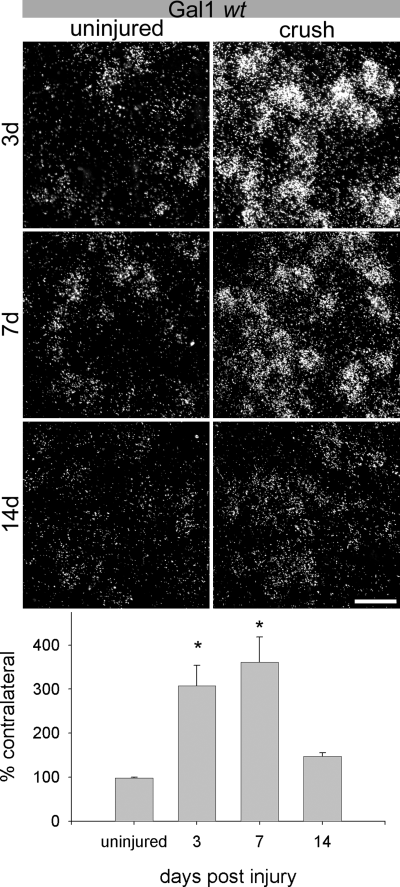
Gal1 mRNA is transiently up-regulated by facial nerve crush in inbred Gal1 wt mice. At 3 days post-lesion (top panel), the silver grain density over facial motoneurons after a crush was 307.3 ± 46.6% (mean ± SEM) of the contralateral and 360.9 ± 57.9% after 7 days (middle panel). These increased mRNA levels were significantly different from the levels found in uninjured motoneurons (P < 0.001, P < 0.005, respectively. By 14 days after a crush (bottom panel), there was an increase of 147.8 ± 8.1% of contralateral, which was not significantly different to uninjured animals (P > 0.05, bottom histogram). *P < 0.05 compared to uninjured animals. Scale bar, 50 microns.

Comparison of the rate of whisking recovery following a facial nerve crush in Gal1 wt and Gal1–/– mice. Using video analysis, the full range of mouse whisker movement (whisking) was recorded (a–b). A horizontal line was drawn between the inner orbits of each eye (b). This line became the 0 degree angle from which the maximum whisking angle and frequency were measured (c). Seven days after a nerve crush, whisking movements were observed. There was a significant difference in the total angle moved on day 9 and 10 between Gal1–/– and wt animals (*P < 0.05). (d) The maximum angle of movement returns to uninjured levels one day later in Gal1–/– mice (+ indicates when maximum angle movement is not significantly different prior to crush lesion). No significant difference in the frequency of movement was observed between Gal1–/– or wt animal prior to or after a nerve crush except 10 days after the lesion (*P < 0.05, e).
Numerous studies have demonstrated strain specific responses in mice to a variety of neurological insults (reviewed in Steward et al., 1999). In the present study, in addition to the inbred 129P3/J mice we also examined Gal1 expression in an outbred strain of mice (CD-1) to ensure that the axonal injury-induced increase in Gal mRNA was not a strain specific response.
We observed a similar Gal1 mRNA expression after axonal injury in both mouse strains; compare the Gal1 wt mouse in Fig. 2 to the CD-1 mouse in Fig. 3. After a facial nerve crush in CD-1 mice, we observed an increase in grain density to 254.8 ± 27.9% of the contralateral side after 3 days and 346.6 ± 30.4% after 7 days. When compared to the Gal1 expression in uninjured motoneurons these increases were significant (P < 0.001, P < 0.005, respectively (Fig. 3). At 14 days after crush we observed a concomitant decline in grain density to 191.1 ± 46.6%. Similar to the Gal1 wt strain this level of expression was no longer significantly different to the uninjured control animal (P = 0.05, Fig. 3 histogram).

Gal1 mRNA is transiently up-regulated by facial nerve crush in outbred CD-1 mice. At 3 days post-lesion (top panel), the silver grain density over facial motoneurons after a crush was 254.8 ± 27.9% (mean ± SEM) of contralateral and 346.6 ± 30.4% after 7 days (middle panel). When compared to the Gal1 expression in uninjured motoneurons these increases were significant (P < 0.001, P < 0.005, respectively). At 14 days post-axotomy (bottom panel), there was not a significant increase of 191.1 ± 46.6% of contralateral (bottom histogram) when compared to the uninjured control animals (P = 0.05, bottom histogram). *P < 0.05 when compared to uninjured animals. Scale bar, 50 microns.
After a facial nerve resection, an injury preventing axonal reconnection with the targets, a significant and rapid increase of Gal1 mRNA occurred within 3 days and persisted until at least 14 days after the injury (Fig. 4), the longest time point examined. Quantification of these results at 3 and 7 days post-injury revealed elevated levels in silver grain density to 257.3 ± 26.6% and 299.6 ± 29.1% of contralateral (Fig. 4). Compared to the ISH signals in uninjured motoneurons these ISH signals were significantly different with P-values smaller than 0.01. At 14 days after a resection injury the Gal1 mRNA expression remained significantly elevated (309.8 ± 83.2% of contralateral), which contrasts with the reduced Gal1 expression at 14 days after a nerve crush (2, 3).
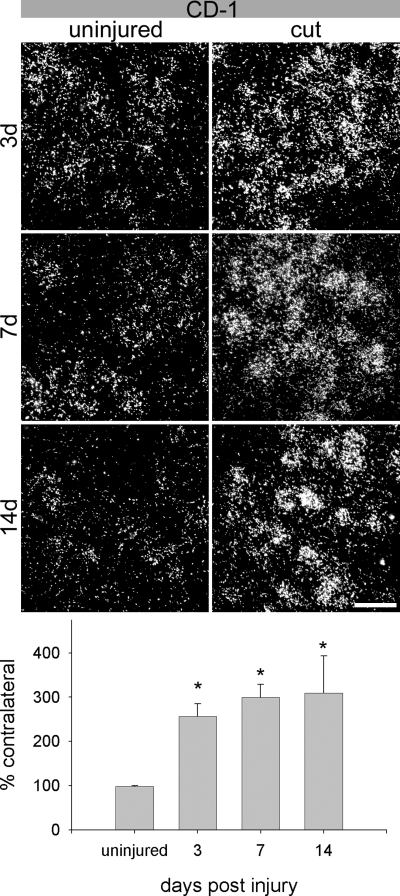
Gal1 mRNA is persistently up-regulated by facial nerve transection in outbred CD-1 mice. At 3 days post-lesion (top panel), the average area occupied by silver grains over axotomized facial motoneurons was 257.3 ± 26.6% (mean ± SEM) of the contralateral side and 299.6 ± 29.1% at 7 days (middle panel). Compared to the levels in uninjured motoneurons these ISH signals were significantly different with P < 0.01. At 14 days following axotomy (bottom panel), there was an increase ISH signal of 309.8 ± 83.2% of contralateral, which was significantly greater than uninjured control animals (P < 0.015, bottom histogram). *P < 0.05 compared to uninjured animals. Scale bar, 50 microns.
Signals increasing Gal1 mRNA expression
As the experiments above suggested a regulation of Gal1 by target-derived factors we investigated the potential role of retrograde transport on Gal1 mRNA regulation. The cessation of axonal transport was achieved through the application of the plant alkaloid colchicine, which halts axonal transport by disassembling neuronal microtubules. We injected colchicine or vehicle solution (n = 3 per group) into the facial nerve of CD-1 mice. Colchicine increased the Gal1 ISH signal to 243.9 ± 45.2% of contralateral by 3 days, rendering it significantly different from uninjured (P < 0.01) or saline (P < 0.02) injected motoneurons (Fig. 5). Saline injection alone increased the average ISH signal to 147.3 ± 7.0% of the untreated contralateral side (Fig. 5) but this level of expression was not significantly different from untreated animals.
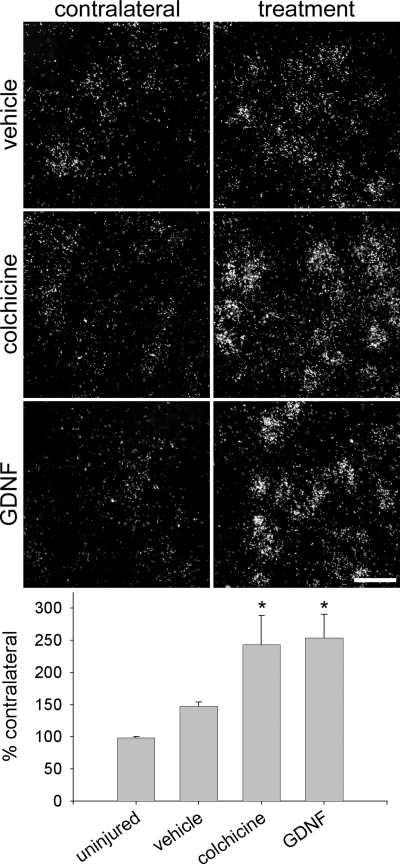
Positive and negative signals regulate Gal1 mRNA. Saline treatment (top right panel) increases the average area occupied by silver grains on the treated side to 147.3 ± 7.0% (mean ± SEM) of the untreated contralateral side. This level of expression was not significantly increased above the untreated group alone (P > 0.05, top left panel and bottom histogram). Colchicine injection (middle right panel) results in a 243.9 ± 45.2% increase (middle left panel) that was significantly greater than the uninjured animal (P < 0.01) or vehicle injection (P < 0.02). GDNF injection (bottom right panel) also leads to a 253.5 ± 36.8% increase (bottom left panel and bottom histogram) compared to the uninjured animal (P < 0.01) or saline injected animal (P < 0.02). *P < 0.05 compared to uninjured and saline treated animals. Scale bar, 50 microns.
In addition to the depletion of target derived factors playing a role in regulating gene expression after axonal injury, the release of trophic factors and cytokines at the site of injury may serve as a positive signal driving the neuronal gene expression response (Fu & Gordon, 1997). Activated peri-lesion Schwann cells increase the expression of glial cell line-derived neurotrophic factor (GDNF) (Hoke et al., 2000) and here we injected GDNF into the intact nerve of CD-1 mice (n = 3 per group). Like colchicine GDNF significantly increased the ISH signal to 253.5 ± 36.8% of the contralateral side, a level significantly higher than the level in uninjured (P < 0.01) or saline injected (P < 0.02) motoneurons (Fig. 5).
Functional analysis of FMN injury
Previous reports have demonstrated that exogenously applied recombinant oxidized Gal1 increases the rate of transected sensory and motor axonal regeneration (Horie et al., 1999; Horie & Kadoya, 2000; Fukaya et al., 2003). Conversely, function-blocking Gal1 antibodies applied to the transection site reduce the rate of axonal regrowth (Horie et al., 1999; Horie & Kadoya, 2000; Fukaya et al., 2003). Here we wanted to examine the whether the endogenous expression affects functional recovery. To accomplish this we crushed the facial nerve in Gal1 wt and Gal1–/– and then assessed functional recovery by recording and measuring whisking movements. Except for two whiskers in the C-row of the whisker pad all whiskers were trimmed close to the skin to allow better visualization (Fig. 6a). Using video analysis (see Materials and methods) we measured the maximum angle and frequency of whisker movement before and after a nerve crush. When compared to the maximum angle achieved before injury, Gal1–/– mice required 12 days to return to preinjury levels (Fig. 6d, grey +) as compared to the 11 days required for the Gal1 wt mice (Fig. 6d, black +). The duration of 11 days in the wt mice is very similar to other mouse strains (Ferri et al., 1998; Serpe et al., 2002; Kamijo et al., 2003). Also, Gal1 wt had achieved a significantly larger maximum angle when compared to Gal1–/– mice at 9 and 10 days post-injury (Fig. 6d, P < 0.05, Student's t-test). When the frequency of movement was measured, there was no difference between Gal1–/– or wt animal prior to or after a nerve crush except at 10 days post-lesion (Fig. 6e). At this time point Gal1 wt mice had a significantly higher whisking frequency than Gal1–/– mice. These data show that both Gal1–/– and wt motor axons successfully regenerate to their targets after a nerve crush but that the rate of full functional recovery is slower in Gal1–/– mice.
Discussion
Both crush and resection of the mouse facial nerve resulted in a significant increase in Gal1 mRNA expression by 7 days post-injury. Furthermore, we demonstrated that both interneural injections of the axonal transport inhibitor colchicine or the trophic factor GDNF induced increased Gal1 mRNA expression in uninjured facial motoneurons. The absence of Gal1 in Gal–/– mice attenuated the rate of recovery of whisker movement after a facial nerve crush.
Gal1 mRNA expression may be regulated by target-derived as well as injury-derived signals
In the crush model, Gal1 mRNA expression was not significantly different from control levels by 14 days after injury. By this time complete functional whisker movement was restored indicating target reinnervation. This observation indicates that the increase of Gal1 mRNA after axonal injury is in part due to the loss of target-derived factor(s). A number of putative signals exist to regulate gene expression after axotomy, but to date they are poorly understood. These are postulated to be either positive signals, such factors originating at the injury site to initiate neuronal changes, or negative signals, such as the interruption of target-derived factors (Cragg, 1970; Fernandes & Tetzlaff, 2000; McGraw et al., 2002). Colchicine application to the uninjured nerve halts axonal transport through microtubule disassembly, resulting in the loss of retrograde transport. The loss of a retrograde signal derived from a target has long been thought of as one of the injury-signalling mechanisms (Cragg, 1970). For example, in the uninjured nerve, when transport is interrupted through colchicine or cold block, gene expression increases significantly for specific genes such as Tα1 tubulin and GAP-43 (Woolf et al., 1990; Wu et al., 1993; Bormann et al., 1998). In the present study, the increased Gal1 expression observed after axonal transport inhibition suggests that Gal1 expression is partly suppressed by (a) target derived factor(s). After axotomy or transport blockade, the putative repressor(s) would be absent, thus increasing Gal1 mRNA expression.
Neuronal gene expression changes also occur due to positive injury signals produced and/or released at the injury site. For example, the neurotrophin GDNF has potent effects on motoneuronal regeneration (Henderson et al., 1994; Blesch & Tuszynski, 2001; Boyd & Gordon, 2003). After a nerve injury, GDNF expression increases in Schwann cells distal to the lesion site where it is taken up by injured axons and retrogradely transported to promote axonal regrowth (Yan et al., 1995; Naveilhan et al., 1997; Burazin & Gundlach, 1998; Leitner et al., 1999; Hoke et al., 2000; Blesch & Tuszynski, 2001). Intrathecal GDNF application also results in an increase of neuronal proteins such as calcitonin gene-related peptide in uninjured motoneurons (Ramer et al., 2003). Here, we report that intraneural GDNF injection increases Gal1 mRNA expression within uninjured motoneurons and suggests that endogenous GDNF is a positive regulator of Gal1.
Taken together, these data indicate that both injury site- and target-derived factors may regulate Gal1 mRNA expression after axotomy.
Endogenous Gal1 facilitates functional recovery
Through the use of Gal1–/– mice we have attempted to ascertain Gal1's putative role in neuronal injury and repair. This null mutant mouse does not have any known compensatory changes in the expression of other galectins or in immune cell numbers (Poirier & Robertson, 1993). During development olfactory neurons do not reach their appropriate targets in Gal1 null mutant mice (Puche et al., 1996). Injured Gal1–/– muscle tissue does not regenerate as readily as injured wild-type muscle, in which Gal1 expression is elevated (Watt et al., 2004). To determine differences in motoneuronal regenerative responses between animals we performed a facial crush on both Gal1 wt and Gal1–/– mice. In the adult mouse, a facial nerve crush results in regeneration and functionally complete whisking behaviour within 11 days in both CD-1 and ICR mice, which is similar to our Gal wt mice (Ferri et al., 1998; Serpe et al., 2002). Using video analysis, we quantified the rate of facial nerve functional recovery by recording the angle and frequency of vibrissae movement (Guntinas-Lichius et al., 2002). In agreement with other whisker movement data (Guntinas-Lichius et al., 2002), we observed that the measured average maximum angle was 49.9 ± 3.6 with a frequency of 7.2 ± 0.6 Hz (Fig. 6). After a facial nerve crush, the motoneuronal regenerative response, as indicated by whisker movement, proceeded through three distinct phases. The first stage, occurring 0–7 days post-injury, resulted in a complete paralysis of movement (Fig. 6). During this time high levels of Gal1 mRNA were expressed within the injured facial somata (3, 4). The second stage, characterized as minimal whisker movement, occurs from 8 to 10 days post-injury (Fig. 6). Here Gal1 mRNA expression presumably remained elevated. The third stage is characterized by complete functional recovery of whisker movement, which is correlated with a decrease in Gal1 mRNA expression in the injured cell bodies. The behavioural recovery usually occurs after the 10th day post-injury. Although little difference in the frequency movement of whiskers was detected, we did observe that Gal1 wt whisking returned to its preinjury state one day prior to the Gal1–/– mice. This one-day delay period in Gal1 null mutant mice is commensurate with other transgenic mouse studies using whisker analysis. For example, p75 null mutant mice recover whisking function one day earlier than wild-type mice (Ferri et al., 1998). As recovery of whisking involves both axonal regeneration rate and synaptogenesis (which have not been separated in this analysis), it is unclear as to which of these processes involves Gal1. However, both gain and loss of function experiments have ascribed Gal1 with a role in both regeneration rate and synaptogenesis (see below).
Gal1's role in axonal repair
These data support other studies demonstrating that Gal1 increases after a peripheral nerve injury and application of Gal1 increases the rate of regeneration through its involvement in the initiation of the regeneration process (Horie et al., 1999; Horie & Kadoya, 2000; Fukaya et al., 2003; Akazawa et al., 2004; McGraw et al., 2004). Exogenous Gal1 infusion into an acellular bridge at the site of a sciatic nerve injury increased both the rate of regeneration and Schwann cell migration (Horie et al., 1999; Fukaya et al., 2003). Conversely, in this model, application of Gal1 function-blocking antibodies significantly reduced the rate of axonal regeneration compared to vehicle-treated animals (Horie et al., 1999; Fukaya et al., 2003). The Gal1–/– mouse has complete functional recovery, albeit slower than in wild-type mice. This indicates Gal1 is not critical for neuronal repair. These data do indicate that Gal1 is one of the myriad of factors contributing to functional recovery following peripheral nerve injury (reviewed in Fernandes & Tetzlaff, 2000). The precise mechanism by which Gal1 influences axonal repair remains elusive but recent evidence suggests macrophage involvement (Horie et al., 2004). Once Gal1 is released into the extracellular space, Gal1 can become oxidized and exhibits neuronal growth-promoting abilities (Horie & Kadoya, 2000; Horie et al., 2004). In the oxidized form, Gal1 acts as a cytokine by binding to and activating macrophages, which then release an unidentified growth-promoting factor that is larger than 10 kDa (Horie et al., 2004). Interestingly, the macrophage-stimulating factor zymosan also causes the release of a 14 kDa factor from macrophages that increases neuronal regrowth of axotomized retinal ganglion cells (Yin et al., 2003). Therefore after injury the neuronal-expressed Gal1 is likely to be secreted into the extracellular space at the injury site thereby inducing macrophages to release both growth-promoting and Schwann cell-activating factors (Cooper & Barondes, 1990; Horie et al., 1999; Fukaya et al., 2003; Horie et al., 2004; Sango et al., 2004). Both macrophages and Schwann cells are known to promote successful regeneration through a variety of mechanisms such as removing myelin debris and providing trophic support (reviewed in Fu & Gordon, 1997). Experiments that alter the responses of macrophages and Schwann cells demonstrate their importance in the regenerative process. For example, acellular nerve grafts do not support peripheral nerve growth when Schwann cell migration or proliferation is inhibited (Chong et al., 1994; Enver & Hall, 1994). In addition, inhibiting the macrophage response also reduces regenerative success (Calcutt et al., 1994; Dailey et al., 1998). In the C57BL/Wlds mutant mouse, delayed Wallerian degeneration occurs due to mutation in an ubiquitination factor (Conforti et al., 2000). In these mutant mice, functional recovery after a facial nerve crush is also delayed due to the failure of macrophages to clear axonal and myelin debris and inhibitory proteins (Perry et al., 1990; Chen & Bisby, 1993; Glass et al., 1993). Conversely, activating macrophages promotes sensory neuronal outgrowth (Lu & Richardson, 1991; Luk et al., 2003). In the present study the absence of Gal1 in Gal1–/– mice may have acted to delay the initial degenerative response following a facial nerve crush, thus reducing the rate of functional recovery.
The results presented in this study demonstrate that endogenous neuronal Gal1 mRNA expression increases after nerve injury. This elevated expression may be the result of both neuronal target loss as well as expression of factors at the injury site. Furthermore we demonstrate that the endogenous neuronal Gal1 expression is associated with the rate of functional recovery after a nerve crush.
Acknowledgements
We would like to thank M. Hampong and C.F.W. Mak for their excellent technical assistance. The Canadian Institute of Health Research and the BC Neurotrauma Fund provided funding for this research. M.S.R. is a Michael Smith Foundation for Health Research Scholar. W.T. is the Rick Hansen Man in Motion Chair in Spinal Cord Research at the University of British Columbia.
Abbreviations
-
- –/–
-
- homozygous null mutation
-
- anova
-
- analysis of variance
-
- Gal1
-
- galectin-1
-
- GDNF
-
- glial cell line-derived neurotrophic factor
-
- ISH
-
- in situ hybridization
-
- wt
-
- wild-type




