An in vitro and in vivo study of early deficits in associative learning in transgenic mice that over-express a mutant form of human APP associated with Alzheimer's disease
Abstract
Transgenic mice over-expressing a mutated form of the human amyloid precursor protein (APP, 695 isoform) bearing a mutation associated with Alzheimer's disease (V642I, so-called London mutation, hereafter APPLd2) and wild-type controls were studied at age periods (3 and 10 months) prior to the overt development of neuritic amyloid plaques. Both 3- and 10-month-old APPLd2 mice had reflex eyelid responses like those of controls, but only younger mice were able to acquire a classical conditioning of eyelid responses in a trace paradigm. In vitro studies on hippocampal slices showed that 10-month-old APPLd2 mice also presented deficits in paired-pulse facilitation and long-term potentiation, but presented a normal synaptic activation of CA1 pyramidal cells by the stimulation of Schaffer collaterals. It is proposed that definite functional changes may appear well in advance of noticeable structural alterations in this animal model of Alzheimer's disease, and that specific learning tasks could have a relevant diagnostic value.
Abbreviations
-
- ACSF
-
- artificial cerebrospinal fluid
-
- APP
-
- amyloid precursor protein
-
- CS
-
- conditioned stimulus
-
- EMG
-
- electromyographic
-
- EPSP
-
- excitatory postsynaptic potential
-
- LTP
-
- long-term potentiation
-
- PBS
-
- phosphate-buffered saline
-
- TBS
-
- theta-burst stimuli
-
- US
-
- unconditioned stimulus
Introduction
An important question regarding animal models of Alzheimer's disease is whether functional learning and memory deficits are a direct consequence of neuritic amyloid deposits in hippocampal and neocortical structures. It has been proposed that some functional changes precede amyloid β plaque deposits (Moechars et al., 1999), and that early phenomena such as synaptic depression, evoked by excessive production of amyloid β peptides, can underlie the initial cognitive deficits (Kamenetz et al., 2003; Ohno et al., 2004). The proteolytic pathways related to the synthesis of amyloid β peptides from the amyloid precursor protein (APP) have been described recently (Selkoe, 2002). Furthermore, some early cognitive manifestations of Alzheimer's disease have been related to functional alteration of cholinergic axon terminals projecting to the hippocampal and associative cortices (Wong et al., 1999). It has been shown in vitro that cholinergic neurons play an important role in the regulation of hippocampal glutamatergic synapses related to long-term potentiation (LTP) processes (Fernández de Sevilla et al., 2002), and that cholinergic drugs disrupt the acquisition of associative learning tasks (Múnera et al., 2000, 2001).
The availability of transgenic mice that mimic human Alzheimer's disease (Moechars et al., 1999; Selkoe, 2002) has made it of interest to develop learning tasks applicable in those small mammals (Vogel et al., 2002). Classical conditioning of nictitating membrane/eyelid responses appears to be a more suitable (associative) learning procedure for mice than are the usual T- and water-maze tests (Chen et al., 2000; Bao et al., 2002; Koekkoek et al., 2002, 2003; Liu et al., 2002; Takatsuki et al., 2003; Ohno et al., 2004), mainly because it can be compared with similar studies carried out in patients with Alzheimer's disease (Weiss et al., 2002; Woodruff-Pak et al., 1990; Woodruff-Pak & Santos, 2000). Moreover, this associative learning procedure has been widely used in other species of mammals, such as rats (Weiss et al., 1999), rabbits (Thompson & Krupa, 1994) and cats (Gruart et al., 1995). We have developed here an ‘electrical shock/SHOCK’ conditioning procedure corresponding to a trace paradigm; that is, a learning task involving the hippocampus (see Múnera et al., 2000, 2001 for references). The ‘electrical shock/SHOCK’ is a modification of a method developed previously − the ‘air-puff/AIR-PUFF’ conditioning (Gruart et al., 1995, 2000).
The aim of this study was to determine the learning capabilities of 3- and 10-month-old wild-type mice using the classical conditioning of eyelid responses, and to compare the results with those obtained from 3- and 10-month-old transgenic mice over-expressing a mutant form of the human APP before amyloid plaque formation. The transgenic mice examined here over-express the so-called ‘London’ V642I mutated form of the 695 isoform of human APP driven by the Thy-1 promoter in a C57B1/6 background. These mice do not develop plaques up to 13 months of age, as previously described (Moechars et al., 1999; Blanchard et al., 2003) and controlled in the present study. In a parallel in vitro study, we also assessed synaptic transmission, paired-pulse facilitation and LTP at the synapses of Schaffer collaterals on CA1 pyramidal cells, in hippocampal slices of 10-month-old wild-type and APPLd2 mice.
The current results support an early derangement of conditioned eyelid responses and hippocampal synaptic plasticity in a transgenic model of Alzheimer's disease, in advance to the appearance of neuritic amyloid plaques. Part of this work has been presented in abstract form (Domínguez del Toro et al., 2003).
Materials and methods
Experimental animals
Male transgenic APPLd2 mice, generated by Moechars et al. (1999) and bred into a C57Bl/6 background for at least six generations at Charles River (Lyon, France), were used in the present study along with wild-type littermate controls. For behavioural studies, animals were divided into four groups: (i) 3-month-old wild-type mice (n = 14); (ii) 3-month-old APPLd2 mice (n = 10); (iii) 10-month-old wild-type mice (n = 10); and (iv) 10-month-old APPLd2 mice (n = 9). Additional animals (n = 4 per group) were used for pseudoconditioned controls. In vitro studies were performed in 10-month-old mice, using > 8 slices per genotype. All studies were carried out following the guidelines of the European Union Council (86/609/EU) and Spanish regulations (BOE 67/8509-12, 1988) for the use of laboratory animals in acute and chronic experiments.
Surgery
Animals were anaesthetized with a mixture of Ketalar (ketamine, 35 mg/kg) and Rompum (xylazine, 2 mg/kg), i.p., and implanted with bipolar stimulating electrodes on the left supraorbitary branch of the trigeminal nerve and with bipolar recording electrodes in the ipsilateral orbicularis oculi muscle. Electrodes were made of 50 µm, Teflon-insulated, annealed stainless steel wire (A-M Systems, Carlsborg, WA-98324, USA), with their tips cleaned of the isolating cover for ≈ 0.5 mm. The electrode tips were bent as a hook to facilitate a stable insertion in the upper eyelid. The wires were connected to a four-pin socket (RS-Amidata, Madrid, Spain). The socket was fixed to the skull with the help of two small screws and dental cement (Gruart et al., 2000).
Classical conditioning training
For recordings the animal was placed in a small (5 × 5 × 10 cm) plastic chamber located inside a Faraday box. Classical conditioning was achieved using a trace paradigm. For this, a short (50 µs), weak (1.5 × threshold, defined as the shock intensity able to evoke a blink in 50% of the cases), square, cathodal pulse was presented as a conditioned stimulus (CS). The unconditioned stimulus (US) consisted of a long (500 µs), strong (2–3 × threshold), square, cathodal pulse. The US started 250 ms after the end of the CS. In total, two habituation, 10 conditioning and five extinction sessions were carried out per animal. A conditioning session consisted of 60 CS–US presentations, and lasted ≈ 30 min. In 10% of the cases, the CS was presented alone. CS–US presentations were separated at random by 30 ± 5 s. For habituation and extinction sessions, only the CS was presented, also for 60 times per session at intervals of 30 ± 5 s. For pseudoconditioning, unpaired CS and US presentations were carried out for 10 sessions (60 times/session).
Recording procedures
The electromyographic (EMG) activity of the orbicularis oculi muscle was recorded using GRASS P511 differential amplifiers with a bandwidth of 1 Hz to 10 kHz (Grass-Telefactor, West Warwick, RI 02893, USA). Basic properties of blink reflex were studied in responses evoked by single 50 µs, 2 × threshold, square, cathodal pulses presented at a rate of 1/30 s. Reflex blinks were studied before the initiation of the conditioning sessions.
For criteria, we considered as a ‘conditioned response’ the presence of EMG activity during the CS–US period that lasted > 10 ms and was initiated > 30 ms after CS onset. In order to obtain a quantitative index of conditioned response evolution, the integrated EMG activity recorded during the CS–US interval was averaged and compared with the activity recorded (for 250 ms) immediately before CS presentation. The integrated EMG activity recorded during the CS–US interval should be at least 2.5 times larger than the averaged activity recorded immediately before CS presentation.
Electrophysiological recordings in vitro
Hippocampal slices (≈ 0.5 mm thick) were prepared from APPLd2 and wild-type controls at 10 months of age. Slices were cut transversally on a McIlwain chopper (Campden Instruments, Loughborough, England) and placed in a submersion-type recording chamber through which artificial cerebrospinal fluid (ACSF (in mm): NaCl, 124; KCl, 3; NaH2PO4, 1.25; MgSO4, 1.3; CaCl2, 2; NaHCO3, 26; glucose, 10) was continuously superfused at 2.5–3 mL/min. The ACSF was bubbled with a mixture of 95% O2/5% CO2 and maintained at 31.5 °C. Test stimulations (0.1 ms in duration) were delivered at constant voltage every 30 s through bipolar stainless steel electrodes placed in the stratum radiatum of the CA1 area. Extracellular field excitatory postsynaptic potentials (EPSPs) were recorded with a monopolar tungsten electrode implanted in the same region. To analyse synaptic excitability, input/output plots were constructed individually for each slice by applying single stimuli in increments of 0.5 V from threshold to maximum. The latter was determined as the last stimulation voltage for which there was no alteration in the shape of the EPSP due to traces of population spike firing. The slope of the input/output plot was calculated by linear regression for each slice. Synaptic plasticity was evaluated as paired-pulse facilitation and LTP at half-maximal stimulation strength according to individual input/output plots. The paired-pulse facilitation was explored by delivering a set of five pairs of stimuli at decremental intervals (400, 200, 100, 50 and 25 ms). To induce LTP, repeated brief trains of high-frequency stimulation were delivered following a protocol comprising three trains of 10 bursts of four pulses, each burst at 100 Hz, with 200 ms between bursts and 15 s between trains (i.e. theta-burst stimulations; Capocchi et al., 1992).
Immunohistochemical study
Brains of APPLd2 mice were checked for amyloid β peptide deposition at two ages (13 and 17 months) beyond the age at which the behavioural and electrophysiological studies were conducted. Animals were killed by cervical elongation and their brains were removed, postfixed for 6 days in 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4 °C, and rinsed in PBS. Hemibrains were then dehydrated and embedded in paraffin for sectioning (6 µm thickness). The amyloid β peptide immunostaining was carried out with biotinylated mouse monoclonal anti-amyloid β antibody (amyloid β17−24, 4G8, Senetek, St. Louis, MO, USA) following procedures described previously (Blanchard et al., 2003).
Data analysis
EMG data were stored directly on a computer with the help of the Signal Average Program of Cambridge Instruments (Cambridge, England) and analysed off-line for quantification of reflex and conditioned responses. Data illustrated in Fig. 2 represent the percentage of conditioned responses per session. The acquisition, representation and quantification programmes used for data illustrated in Fig. 3 were developed by one of us (R.S.-C.) with the help of MATLAB (Natick, MA 01760-2098, USA). Mean values and their standard errors (SEM) are indicated. Statistical significance was determined by two-way anova using age and genotype as the two factors. An extra combination with session-by-session factor was also carried out. The difference was considered significant for P < 0.05. For the slice electrophysiology, signal was acquired by a computer through an analogue/digital converter and analysed offline for EPSP amplitudes (DataWave Technologies, Longmont, Colorado, USA). Data were analysed by two-way anova followed by pair-wise comparisons between control and APPLd2 mice at each time.
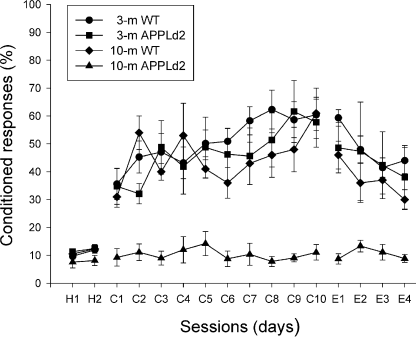
Learning curves for wild-type and APPLd2 mice. H1, H2 represent data from habituation sessions, C1–C10 represent data from conditioning sessions and E1–E5 represent data from extinction sessions. The percentage of conditioned responses (± SEM) is shown as a function of session progression. Differences between 3- and 10-month-old wild-type and 3-month-old APPLd2 mice compared with 10-month-old APPLd2 mice were statistically significant (P < 0.05, two-way anova) for conditioning (C1–C10) and extinction (E1–E4) sessions, but not for habituation (H1, H2) and the fifth extinction sessions.
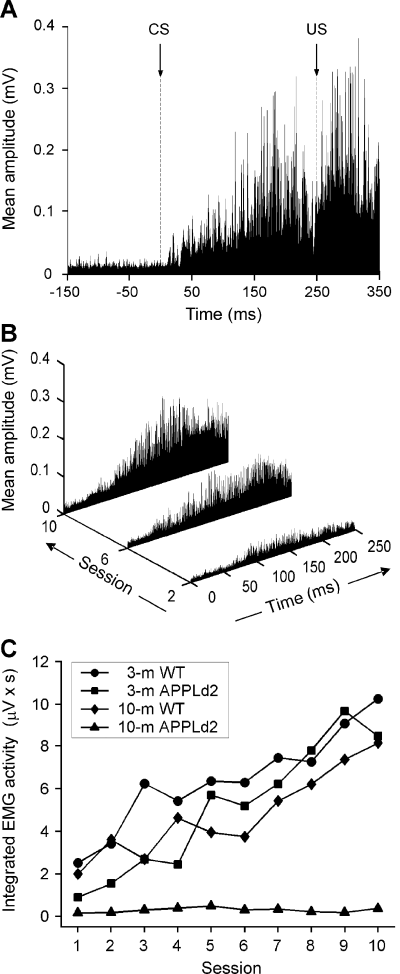
Quantitative analysis of conditioned responses collected from the four experimental groups. (A) Rectified and averaged (n = 60 trials) electromyographic (EMG) record, from a 3-month-old APPLd2 mouse, in the ninth conditioning session. The arrow indicates CS presentation. The illustrated EMG record ranges from 150 ms before to 350 ms after CS initiation. (B) Evolution of rectified and averaged EMG recordings from the second, sixth and 10th conditioning sessions for another 3-month-old APPLd2 mouse. Note the increase in EMG area across conditioning. (C) Evolution of the integrated EMG activity (in µV × s) across conditioning sessions for the four groups of mice.
Results
Reflexively evoked eyelid responses are normal in 3- and 10-month-old APPLd2 mice
In a first series of experiments, we checked the functionality of neural circuits involved in reflex eyelid responses in both wild-type and transgenic animals. The electrical stimulation of the supraorbitary branch of the trigeminal nerve in 3-month-old wild-type mice evoked an early EMG activation of the orbicularis oculi muscle at a latency of 7.2 ± 0.7 ms (R1 component), followed by a second (R2) EMG activation, with a latency from the stimulus of 16.8 ± 1.1 ms (Fig. 1A). The mean amplitudes of R1 (1.11 ± 0.52 mV) and R2 (0.86 ± 0.53 mV) responses in controls were not significantly different. The 10-month-old wild-type animals, and 3- and 10-month-old APPLd2 mice presented reflex eyelid responses of similar latency and amplitude values for both (R1, R2) EMG components to those obtained in 3-month-old controls (Fig. 1B). Accordingly, 3- and 10-month-old APPLd2 mice presented blink neural circuits with similar functional properties to those of controls.
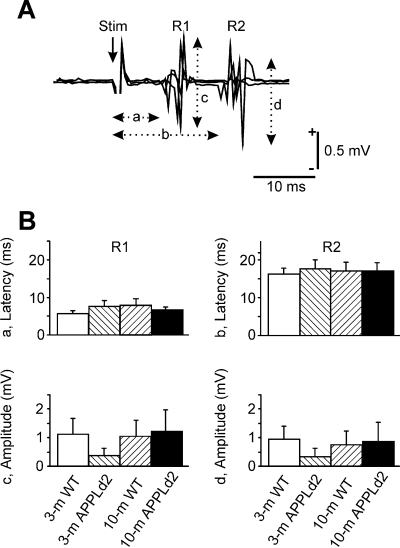
Reflexively evoked blinks in wild-type and APPLd2 mice. (A) Three superimposed EMG traces recorded in a wild-type animal following a single electrical stimulation (50 µs, 2 × threshold) of the supraorbitary branch of the trigeminal nerve. Note the two characteristic R1 and R2 components. The latencies of R1 (a) and R2 (b) components as well as their respective amplitudes (c and d) were quantified. (B) Mean (± SEM) values collected for latency and amplitude of both R1 and R2 components of electrically evoked blinks in the four groups of mice. No significant difference (two-way anova) was observed between groups for any of the four (a–d) parameters.
Classical conditioning of eyelid responses is impaired in 10-month-old, but not in 3-month-old, APPLd2 mice
We compared the learning capabilities of 3- and 10-month-old wild-type mice with those of 3- and 10-month-old APPLd2 mice, using a trace paradigm. As illustrated in Fig. 2, 3-month-old wild-type animals presented a normal learning curve, when compared with previous descriptions in rabbits and cats, using similar trace conditioning procedures (Gruart et al., 1995, 2000). However, applying the criteria indicated in Materials and methods, the learning curve of 3-month-old wild-type mice presented an abrupt initiation. These animals presented a mean percentage of 35.5 ± 5.5 responses during the first conditioning day, and reached asymptotic values by the seventh conditioning sessions (58.3 ± 5.0 responses, see Fig. 2). The integrated EMG activity (expressed in µV × s) recorded during the CS–US interval for 3-month-old wild-type animals presented a steadier increase across conditioning (Fig. 3C). Ten-month-old wild-type and 3-month-old APPLd2 mice presented learning curves similar to those described for 3-month-old controls (2, 3). However, 10-month-old APPLd2 mice presented a very poor learning performance, never reaching values above those recorded during the habituation sessions (2, 3). Because they presented normal blink reflexes in response to electrical stimulation of the supraorbitary branch of the trigeminal nerve, but were unable to develop noticeable eyelid conditioned responses, the absence of the latter responses was not due to any technical problem with the EMG recording system, nor to any motor impairment in facial motor and premotor pathways. The percentages of conditioned eyelid responses collected from 3- and 10-month-old wild-type and 3-month-old APPLd2 mice were significantly different (P < 0.05, two-way anova) from values in 10-month-old APPLd2 mice (2, 3). These results indicate that the learning deficit was age-related, a fact noticed with the experimental procedures used here, even before the appearance of plaque deposits in cortical structures. Pseudoconditioned animals never reached > 17% of responses, i.e. the same levels reached during habituation sessions (Fig. 2) by the four experimental groups.
The computer programme developed enables precise quantification of the EMG area of the orbicularis oculi muscle during the CS–US interval, and its comparison with the corresponding area for the 250 ms preceding CS presentation. As illustrated in Fig. 3, these data give a better indication of learning evolution across conditioning sessions than does the use of predetermined criteria (see Fig. 2).
Paired-pulse facilitation and LTP are impaired in 10-month-old APPLd2 mice
Synaptic transmission in hippocampal slices was analysed by applying single test stimuli of graded strength to the Schaffer collaterals. The overall shape and size of the EPSP elicited in slices from the 10-month-old APPLd2 mice at the level of the CA1 synapses was similar to those obtained in 10-month-old controls. The linear regression of the input/output data indicated that the synaptic excitability was similar in APPLd2 and wild-type mice (Fig. 4A). Thus, the mean slope factors were 0.27 ± 0.03 for transgenic mice (n = 8) and 0.32 ± 0.04 for controls (n = 9; P > 0.4, unpaired t-test).
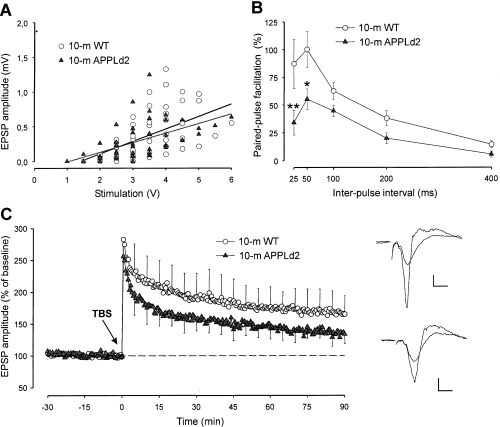
Properties of excitatory synapses in the CA1 hippocampus of 10-month-old wild-type and APPLd2 mice. (A) Excitatory postsynaptic potential (EPSP) amplitudes recorded in the stratum radiatum, plotted as a function of stimulus voltage applied to the Schaffer collaterals for each individual slice. A linear regression analysis of the input/output functions indicated no alteration in synaptic excitability (the dark line corresponds to 10-month-old wild-type mice: slope = 0.179; r = 0.67; the grey line corresponds to APPLd2 mice: slope = 0.137, r = 0.57). (B) Paired-pulse facilitation. The data shown are mean ± SEM amplitude of the second EPSP expressed as a percentage of the first for the five interstimulus intervals. The asterisks denote the significantly lower paired-pulse facilitation in transgenic animals (*P < 0.05; **P < 0.01, post-hoc Bonferroni test following significant two-way anova). (C) LTP. Time-course of changes in EPSP amplitude (mean ± SEM) following tetanic stimulation of the Schaffer collaterals. Theta-burst stimulation (TBS) was applied at the time marked by the arrow. The values in each experiment were normalized with respect to the 30 min baseline recording period. Insets at the right show superimposition of typical traces collected before and after LTP induction for wild-type (upper pair of traces) and APPLd2 (lower pair of traces) mice. Calibration bars: horizontal, 5 ms; vertical, 0.2 mV.
Paired-pulse facilitation is a characteristic presynaptic short-term plastic property of excitatory synapses of the hippocampus that is related to neurotransmitter release processes (Zucker, 1989). Following two stimulations in rapid succession, the amplitude of the second EPSP elicited by the pair of stimuli is increased with respect to the first EPSP at short interpulse interval (< 500 ms). There was a significant decrease in the amount of paired-pulse facilitation in APPLd2 mice over a range of intervals from 25 to 400 ms (Fig. 4B). A two-way anova and post-hoc pair-wise comparisons between control and APPLd2 mice at each time showed that the amount of paired-pulse facilitation was significantly lower at 25 and 50 ms intervals in APPLd2 mice.
LTP at CA1 synapses is an N-methyl-d-aspartate (NMDA)-receptor dependent, protracted form of plasticity thought to underlie learning and memory (Bliss & Collingridge, 1993). The LTP is induced when brief but intense sets of stimuli, such as the theta-burst stimuli (TBS) used in the present study, are applied to the CA1 pathway (Bliss & Collingridge, 1993). The LTP was significantly reduced in the 10-month-old APPLd2 mice (Fig. 4C). A two-way anova comparing EPSP amplitude data, collected at time points 1, 5, 10, 20, 30, 45, 60 and 90 min following TBS, indicated that the APPLd2 mice had significantly lower LTP levels than the 10-month-old controls (F1,135 = 4.47, P < 0.05). Moreover, the area under their LTP curve was reduced by 37% with respect to controls.
Histological study
As already reported (Moechars et al., 1999; Blanchard et al., 2003), the onset of amyloid β peptide deposition in the brain of APPLd2 mice was observed at 13 months of age, but never in 10 month-old mice (Fig. 5A). Moreover, no evidence of cell damage or loss was noticed in 10-month-old APPLd2 animals. In 13-month-old mice, first deposits were predominantly detected in the subiculum, as illustrated in Fig. 5B. With ageing, the deposits were progressively more abundant through the hippocampus and the cortex (see Fig. 5C, at 17 months). More detailed histopathological characterization of amyloid β peptide deposits and the associated neuritic pathology and inflammatory response has been published elsewhere (Blanchard et al., 2003; Moechars et al., 1999).
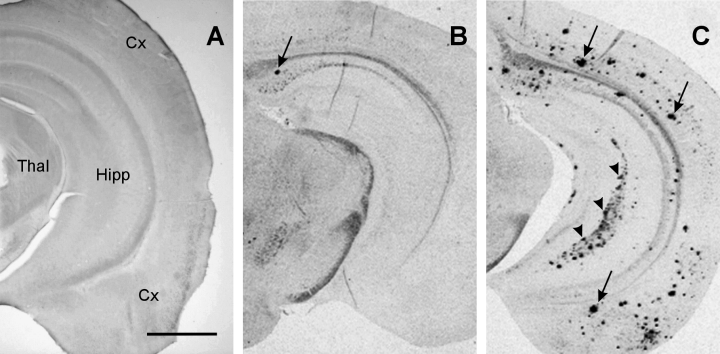
Age-dependent progression of cerebral amyloid β peptide deposition in APPLd2 mice. Photomicrographs illustrating amyloid β peptide immunostaining (with the 4G8 antibody) in a transversal brain tissue section of (A) 10-month-old, (B) 13-month-old and (C) 17-month-old APPLd2 mice. First deposits, at 13 months old, were found in a very limited number predominantly within the subiculum (arrow in B). Amyloid β peptide deposition progressively enlarged with ageing predominantly in the hippocampus and cortex (arrows in C). Original magnification × 25. Calibration bar, 1 mm. Abbreviations: Cx, cortex; Hipp, hippocampus; Thal, thalamus.
Discussion
Learning capabilities of wild-type and APPLd2 mice
The present results demonstrate that 10-month-old APPLd2 mice develop learning and memory impairments that antedate overt amyloid β deposition. This observation suggests that functional alterations could occur before senile plaque formation, a proposition already sustained by others from molecular, cellular and/or functional studies (Moechars et al., 1999; Wong et al., 1999; Buttini et al., 2002; Selkoe, 2002; Terwel et al., 2002; Van Dam et al., 2003). As already pointed out by others (Kelly et al., 2003), this was an age-dependent phenomenon, since 3-month-old APPLd2 mice acquired classical conditioning eyelid responses similar to those of wild-type controls. The learning deficit observed in 10-month-old APPLd2 mice was correlated to a partial, but significant diminution in LTP recorded in vitro from hippocampal slices in the absence of change in basal synaptic excitability, as described before by Moechars et al. (1999). However, in contrast to the latter study, a diminution in the paired-pulse facilitation test was also observed in 10-month-old APPLd2 animals, suggesting that presynaptic short-term plasticity alterations appear at the age studied here (Zucker, 1989). There are some conflicting results regarding LTP in APP mice, but these are related to the APP695SWE mutation (Chapman et al., 1999; Fitzjohn et al., 2001), but not for the APP/London mutation used by us. A diminution of LTP in APPLd2 mice has been consistently shown in preceding studies (Moechars et al., 1999; Dewachter et al., 2000, 2002). Moreover, LTP is also reduced in double transgenic animals expressing both human APP (K670N:M671L) and a mutated form of presenilin-1 (M146L), another protein involved in familial forms of Alzheimer's disease, the expression of which alone does not decrease LTP (Trinchese et al., 2004).
Weiss et al. (2002) have reported a significant impairment for delay eyeblink conditioning in 6- and 10-month-old transgenic mice overexpressing the V717F amyloid precursor protein. But, in this case, those animals have evident signs of neuronal loss at the hippocampus, as demonstrated with magnetic resonance imaging. In the present study, no plaque deposition or neuronal loss were observed in 10-month-old APPLd2 mice.
The analysis of reflex and learned eyelid responses is a useful tool for determining the functional state of animal models of Alzheimer's disease
As described firstly in humans (Kugelberg, 1952), the electrical stimulation of the supraorbital nerve produces an early EMG activation of the orbicularis oculi muscle, denominated R1, corresponding to the activation of the low-threshold mechanoreceptors located in the eyelashes and eyelid skin. This activity is followed a few ms later by a second (R2) EMG response, corresponding to the activation of slow-conducting mechano-, thermo- and nociceptor fibres (Evinger et al., 1991; Gruart et al., 1995). Both R1 and R2 components of reflexively evoked blink responses appeared normally in the four groups of animals tested here. These results suggest that learning deficits observed in 10-month-old APPLd2 mice were not caused by any motor impairment of facial motor and/or premotor pathways.
The classical conditioning of nictitating membrane/eyelid responses is a widely used technique (Marshall-Goodell et al., 1992; Thompson & Krupa, 1994; Gruart et al., 1995; Weiss et al., 1999; Takatsuki et al., 2003) that appears to be a very useful tool for comparative studies in both transgenic models (Weiss et al., 2002) and pateints with Alzheimer's disease (Woodruff-Pak et al., 1990). Moreover, the acquisition of trace conditioning paradigms is mainly related to the proper functioning of hippocampal circuits (Múnera et al., 2000, 2001; Liu et al., 2002; Takatsuki et al., 2003); that is, of the structure most directly affected in the early stages of Alzheimer's disease. When EMG data collected from both wild-type and transgenic animals were analysed using traditional criteria (Gruart et al., 2000; Marshall-Goodell et al., 1992), a learning curve with an abrupt initiation was obtained. The computer programme developed here, however, allows a quantitative representation of the actual amplitude of the evoked conditioned response across the successive conditioning sessions. Indeed, and as shown elsewhere, the integrated EMG activity of the orbicularis oculi muscle is linearly related to eyelid position (Gruart et al., 1995, 2000).
There is presently an active debate regarding the merits and demerits of EMG recordings for a precise measurement of conditioned eyelid responses (Christian et al., 2004; De Zeeuw et al., 2004). It is obvious that for an appropriate determination of eyelid kinematics − referred mainly to the measurement of eyelid position and velocity − it is advisable to use the search coil technique (as shown in cats and rabbits by Gruart et al., 1995, 2000) or the magnetic distance measurement technique (as shown in mice by Koekkoek et al., 2002). Nevertheless, we consider that EMG recordings are suitable for the present study, as we did not deal here with the oscillatory and/or timing properties of the learned eyelid responses. Moreover, it has been shown in cats (Gruart et al., 1995) that EMG recordings from the orbicularis oculi muscle can be linearly related with eyelid position (EMG area) and velocity (EMG peak amplitude).
According to the present results, evident functional alterations are present in APPLd2 mice before plaque deposition. It has been proposed recently that amyloid β peptides can play a direct role in the regulation of synaptic excitability in hippocampal circuits (Kamenetz et al., 2003). At the same time, cholinergic terminals are involved in normal hippocampal functioning (Fernández de Sevilla et al., 2002) and choline acetyltransferase fibres relate better to cognitive deficits than to amyloid β peptide load (Wong et al., 1999; Buttini et al., 2002). In addition, scopolamine affects both the acquisition of classically conditioned eyelid responses in alert behaving cats and pyramidal cell firing (Múnera et al., 2000). Thus, the current results reinforce previous proposals regarding (cholinergic) synaptic failures in advance of amyloid plaque deposits as a possible cause underlying cognitive deficits observed in patients with Alzheimer's disease (Selkoe, 2002; Terry & Buccafusco, 2003; Trinh et al., 2003).
Acknowledgements
This study was supported by MCYT/BFI2002-00936, FISS/01/0194 and JA/CVI-122 grants. E.D.T. was a fellow of the Marie Curie Program. A.R.M. was a Post-Doctoral fellow from the Spanish MECD. We thank Ms María Sutil for help in behavioural studies, and Mr Roger Churchill for help in the edition of the manuscript.




