Impaired behavioural flexibility and memory in mice lacking GDNF family receptor α2
Abstract
The glial cell line-derived neurotrophic factor (GDNF) family receptor GFRα2 is the binding receptor for neurturin (NRTN). The main biological responses of GFRα2 are mediated via the Ret receptor tyrosine kinase, although it may also signal independently of Ret via the neural cell adhesion molecule NCAM. GFRα2 is expressed in many neurons of both the central and peripheral nervous system. Mice lacking GFRα2 receptors do not exhibit any gross defects in the central nervous system structure. However, they display profound deficits in the parasympathetic and enteric nervous system, accompanied by significant reduction in body weight after weaning. Here we present the results of behavioural analysis of the GFRα2-knockout mice. The knockout mice did not differ from wild-type mice in basic tests of motor and exploratory activity. However, differences were established in several memory tasks. The knockout mice were not impaired in the acquisition of spatial escape strategy. However, the deficit in flexibility in establishing a new strategy was revealed during reversal learning with the platform in the opposite quadrant of the pool. Furthermore, the knockout mice displayed significant impairment in contextual fear conditioning and conditioned taste aversion tests of memory. The results suggest that GFRα2 signalling plays a role in the development or maintenance of cognitive abilities that help in solving complex learning tasks.
Abbreviations
-
- BDNF
-
- brain-derived neurotrophic factor
-
- CREB
-
- cAMP responsive element-binding protein
-
- CS
-
- conditioned stimulus
-
- GDNF
-
- glial cell line-derived neurotrophic factor
-
- GFL
-
- GDNF family ligands
-
- GFRα2
-
- GDNF family receptor α2
-
- LTP
-
- long-term potentiation
-
- NCAM
-
- neural cell adhesion molecule
-
- NRTN
-
- neurturin
-
- MAPK
-
- mitogen-activated protein kinase
-
- PLCγ
-
- phospholipase-Cγ
Introduction
Neurotrophic factors comprise several groups of proteins that have roles in the growth and differentiation of neurons during development. One family consists of glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs), which are crucial for the development and maintenance of several populations of central and peripheral neurons (reviewed in Baloh et al., 2000; Airaksinen & Saarma, 2002). GFLs bind to GDNF family α-receptors (GFRα), and signal through the receptor tyrosine kinase Ret (reviewed by Airaksinen & Saarma, 2002). Neurturin (NRTN) activates Ret preferentially via GFRα2 (Fig. 1), whereas GFRα1 is the preferred coreceptor for GDNF. Several intracellular signalling cascades including Akt, mitogen-activated protein kinase (MAPK), Src and phospholipase-Cγ (PLCγ) pathways are activated downstream of Ret, similar to those used by the Trk receptors for the neurotrophin family (reviewed by Chao, 2003). Recent studies indicate that GFLs may also signal independently of Ret. In particular, the neuronal cell adhesion molecule (NCAM) was shown to bind to GFL/GFRα complexes and mediate Src-family kinase and subsequent downstream signalling activation (Paratcha et al., 2003). Although most, if not all, biological functions of GFLs are mediated via Ret during embryonic development, signalling via NCAM has been proposed as taking place during postnatal development and in adult neuronal plasticity.
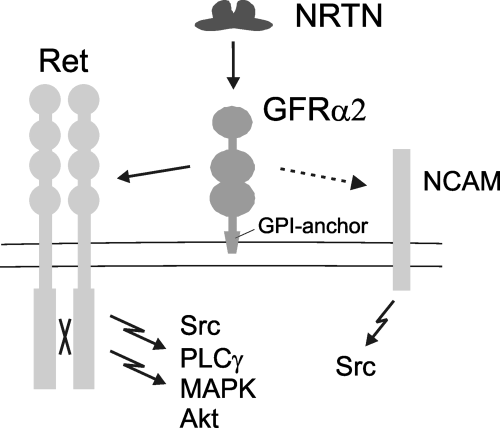
Proposed ligand–receptor interactions and intracellular signalling pathways linked to GFRα2. Ret, Ret receptor tyrosine kinase; NRTN, neurturin; GFRα2, GDNF-family receptor α2; GPI-anchor, glycosyl-phosphatidylinositol anchor; NCAM, neural cell adhesion molecule; Src, Src-type kinases; MAPK, mitogen-activated protein kinase; PLCγ, phospholipase-Cγ.
In contrast to the neurotrophins (particularly the brain-derived neurotrophic factor; BDNF) that are critically involved in plasticity, memory and other cognitive processes (Tyler et al., 2002), the role of GFLs in such functions is less studied. Intracerebroventricular administration of GDNF has been shown to improve spatial learning in aged rats (Pelleymounter et al., 1999), whereas heterozygous GDNF-deficient mice have been shown to exhibit impaired spatial learning in the water maze (Gerlai et al., 2001). In addition, Messer et al. (2000) have suggested a role for GDNF in the adaptations of mesolimbic dopaminergic neurons to drugs of abuse.
We have previously shown that mice lacking GFRα2 are viable and fertile, although their growth is significantly retarded after weaning (Rossi et al., 1999). However, soon thereafter the GFRα2-knockout mice start to catch up in growth, and by 4 months of age they weigh only 10–20% less than their wild-type littermates. The GFRα2-knockout mice have severely reduced parasympathetic cholinergic innervation of lacrimal and salivary glands and pancreas. The innervation of the myenteric plexus and gastrointestinal motility are reduced as well (Rossi et al., 2003). The mice lacking NRTN show apparently a similar phenotype regarding defects in the parasympathetic and enteric nervous system, although they do not show growth retardation (Heuckeroth et al., 1999). The GFRα2-knockout mice display suppressed epileptogenesis and reduced maintenance of hyperexcitabilty in the hippocampal kindling model (Nanobashvili et al., 2000). Although the morphology of the hippocampus in the knockout mice appears normal, at least at the light microscopy level, basal hippocampal synaptic transmission shows subtle deficits (Nanobashvili et al., 2000). The present study was aimed at establishing a general behavioural profile and memory functions in the GFRα2-knockout mice.
Materials and methods
Animals
GFRα2-knockout mice and wild-type littermates in (C57BL/6 × 129Sv) F1 background were obtained by mating congenic GFRα2 heterozygous mice. The congenic mice were produced by backcrossing the heterozygote mice separately with mice of the two background strains (C57BL/6JOlaHsd and 129SvHsd) for at least nine generations. Altogether 59 male mice (29 wild-type and 30 knockout) at the age of 3–4 months were used for the experiments. At this age, the average body weight was 27.5 g for the knockout mice and 32.5 g for the wild-type littermates. The animals were maintained under a 12-h light–dark cycle (lights on at 07.00 h) at relative humidity 50–60% and 21 ± 1 °C, with food and water available ad libitum. All animal experiments were approved by the local ethical committee for animal research at the University of Helsinki.
Behavioural tests were conducted essentially as described elsewhere (Voikar et al., 2004).
Spontaneous activity
The fear conditioning system (TSE, Bad Homburg, Germany) was used for automatic measurement of distance travelled (cm) and number of rearings during a 5-min test. Testing was performed in a well illuminated (≈ 700 lx) transparent acrylic cage (35 × 20 × 20 cm) with a flat black floor.
Y-maze
Exploratory activity was further assessed in the Y-maze under reduced light conditions (≈ 100 lx). Each arm was 30 cm long and 7 cm wide with transparent walls (15 cm high). The number and the sequence of the arm entries were recorded during 5 min. The alternation percentage was calculated as the number of alternations (entries into three different arms consecutively) divided by the total possible alternations (i.e. the number of arms entered minus 2) and multiplied by 100. In addition, the number of rearings and number of faecal boli were counted.
Fear conditioning
The experiment was carried out using a commercially available system (TSE, Germany). Training was performed in an acrylic cage (35 × 20 × 20 cm) within a constantly illuminated (≈ 550 lx) sound-attenuating box. The animals explored the cage freely for 120 s. Thereafter a conditioned stimulus (CS; 10 kHz tone, 75 dB, pulsed 5 Hz) was given for 30 s. The tone was terminated by a footshock [unconditioned stimulus (US), 0.7 mA, 2 s, constant current]. Two CS–US pairings were separated by 30 s.
Contextual memory was tested 24 h after the training. The freezing (absence of any movements for > 3 s) was measured during 3 min in the conditioning cage. Cued memory was tested 2 h later in a novel context. After 120 s of free exploration in the new context the CS was applied for an additional 120 s and freezing was measured as above. Freezing was corrected for the baseline measures (preconditioning for context test and new context for cue test) and expressed as a change from the respective values.
Water maze
The system consisted of a black circular swimming pool (Ø 120 cm), escape platform (Ø 10 cm) submerged 0.5 cm under the water (temperature 22–23 °C) surface, and a computer-interfaced video tracking system (Noldus, the Netherlands). The animals were released to swim in random positions facing the wall and the time to reach the platform was measured. Two training sessions consisting of three trials each were conducted daily. The intertrial interval was ≈ 3 min and the intersession interval was 5 h. The platform remained in a constant location for 3 days (six sessions) and was thereafter moved to the opposite quadrant for 2 days (four sessions). The platform was removed from the pool for transfer tests that were conducted ≈ 18 h after the fourth, sixth and tenth sessions. Spatial memory was estimated from the time spent in the circular zone around the platform location (Ø 30 cm) and in corresponding zones of the three remaining quadrants. Because the mutants tended to float more than their wild-type littermates, we applied the preference score rather than direct comparison of time in zones. The preference score was calculated by subtracting the average time spent in the three zones where the platform was not located from the time in the target zone. In addition, the distance swum and the thigmotaxis were measured. Thigmotaxis was defined as the time spent swimming within the outermost ring (20 cm from the wall) of the water maze. After completing the spatial version of the water maze the platform was made visible in the new quadrant. The mice were trained for 3 days with three trials per day (2 min between trials).
Conditioned taste aversion
The mice were singly housed, water-deprived and adapted to a specific drinking schedule (two sessions per day for 2 days). During each session (25 min), two bottles filled with water were available. The amount of liquid consumed was determined by weighing the bottles before and after the drinking session. On day 3, one bottle with 0.5% saccharin solution was presented for 25 min. One hour later the mice were injected intraperitoneally with lithium chloride (LiCl; 0.14 m) at a dose of 2% of body weight. Test trials where the animals had access to saccharin and water simultaneously were carried out 24 and 48 h later. The percentage of saccharin intake was used as an aversion index.
Histology
GFRα2 immunohistochemistry was performed as described (Nanobashvili et al., 2000). Animals were deeply anaesthetized with chloral hydrate and perfused intracardially with 4% paraformaldehyde in phosphate-buffered saline, pH 7.5, and post-fixed for 2 h at room temperature. Frontal free-floating cryosections from the brains were stained with polyclonal goat antibodies against GFRα2 (from R & D) and detected with Cy3-labelled secondary antibodies (Jackson).
Statistics
Data were analysed by means of one-way or repeated-measures (for water maze escape latency, distance and thigmotaxis in the transfer tests) analysis variance (anova) with genotype as an independent variable.
Results
Locomotor and exploratory activity
Both groups displayed similar locomotor activity in the open-field test, expressed as distance travelled (F1,44 = 0.01, P = 0.91). Furthermore, the number of faecal boli left in the test apparatus (F1,44 = 0.1, P = 0.72) and vertical activity (number of rearings, F1,44 = 3.3, P = 0.08) did not differ between the groups. There was no difference between the genotypes in the time spent in the centre vs. corners of the test cage (data not shown). The Y-maze was applied for further investigation of exploratory activity. There was no difference in the number of arm entries (F1,57 = 0.5, P = 0.49), in the number of faecal boli (F1,57 = 0.7, P = 0.43), rearing behaviour (F1,57 = 2.9, P = 0.09) or percentage of spontaneous alternations (F1,57 = 1.4, P = 0.23). Both wild-type and knockout mice were able to walk on the rotating rod for similar times (data not shown).
Water maze
The groups started with similar escape latencies (Fig. 2A and B) and were able to improve significantly over the first four training sessions although the knockout mice were slower (genotype, F1,57 = 8.7, P < 0.01; session, F3,171 = 58.8, P < 0.01; genotype × session, F3,171 = 0.2, P = 0.88). Both groups displayed similar preference for the target zone in the first transfer test (F1,57 = 0.01, P = 0.91). The difference between the groups regarding escape latency remained significant in the additional two sessions. However, despite the longer latencies the knockout mice displayed good spatial preference equal to the wild-type group in the second transfer test (F1,57 = 0.1, P = 0.75). Furthermore, the preference was increased significantly when compared to the first transfer test (P < 0.01). Thereafter the platform was moved to the new quadrant, opposite to the previously used one. The knockout mice were significantly impaired during reversal training (genotype effect, F1,57 = 11.6, P < 0.01; session effect, F3,171 = 24.8, P < 0.01; interaction of genotype and session, F3,171 = 3.4, P < 0.05) and this was confirmed by the third transfer test. They exhibited significantly lower preference to the new target zone than the wild-type mice (F1,57 = 4.8, P < 0.05). The knockout mice displayed significantly reduced swimming activity in all three transfer tests as measured by the distance travelled (genotype, F1,57 = 13.3, P < 0.01; test, F2,114 = 3.8, P < 0.05; genotype × test, F2,114 = 0.8, P = 0.43). However, the time spent in thigmotaxis was not different in any test (genotype, F1,57 = 1.2, P = 0.28; test, F2,114 = 1.0, P = 0.37; genotype × test, F2,114 = 0.6, P = 0.54). The cued training suggests that the knockout mice were able to improve over 3 days although they still remained slower in swimming to the visible escape platform (effect of genotype, F1,44 = 19.4, P < 0.01; effect of trial, F8,352 = 25.2, P < 0.01; interaction of genotype and trial, not significant).
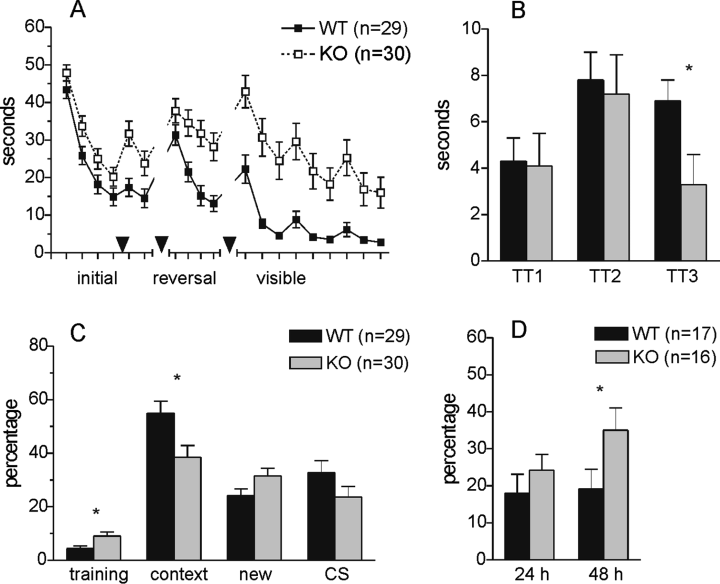
Impaired learning and memory in GFRα2-knockout mice. (A) Escape latency in the water maze. The arrowheads indicate when the transfer tests were performed. (B) Preference score for the target zone in the transfer tests. (C) Percentage of freezing in different phases of fear conditioning. (D) Saccharin aversion index (percentage of consumed saccharin solution) during choice tests 24 and 48 h after conditioning with intraperitoneal injection of LiCl. *P < 0.05 between the genotypes.
Fear conditioning
Although (Fig. 2C) the knockout mice displayed slightly enhanced freezing before the conditioning occurred (F1,57 = 6.2, P < 0.05), they appeared to freeze significantly less than the wild-type mice when returned to the training context 24 h later (F1,57 = 6.9, P = 0.01). There was no difference in the freezing behaviour in the novel context before application of the conditioned stimulus (F1,57 = 3.6, P = 0.06). However, repeated-measures anova revealed significant interaction between the group and the context (F1,57 = 14.6, P < 0.01). The wild-type mice appeared to discriminate well between the two contexts, because they froze significantly less in the novel environment than in the shock context (P < 0.01). In contrast, the knockout mice displayed similar freezing in both environments (P = 0.11). The application of the CS in a novel context increased freezing in both groups (F1,57 = 2.4, P = 0.13).
Conditioned taste aversion
Both groups (Fig. 2D) displayed similar aversion to saccharin 24 h after conditioning (F1,31 = 0.9, P = 0.36). However, the difference between the groups became evident 48 h after conditioning, when the wild-type mice had retained the previous avoidance pattern but it was decreased in the knockout mice (F1,31 = 4.0, P = 0.05).
Expression of GFRα2 protein in mouse brain
Gfra2 mRNA (Fig. 3) is abundantly expressed in neurons in different regions of adult mouse brain (Golden et al., 1998). Our previous study (Nanobashvili et al., 2000) indicated that, in the hippocampus, GFRα2 protein is specifically localized in the axons. We used specific antibodies to reveal the expression of GFRα2 protein in adult mouse brain. GFRα2 protein was widely expressed in the neuropil of adult mouse brain in regions relevant to cognitive function (Fig. 3). No specific expression above background was detected in similar sections from the knockout mice (data not shown).
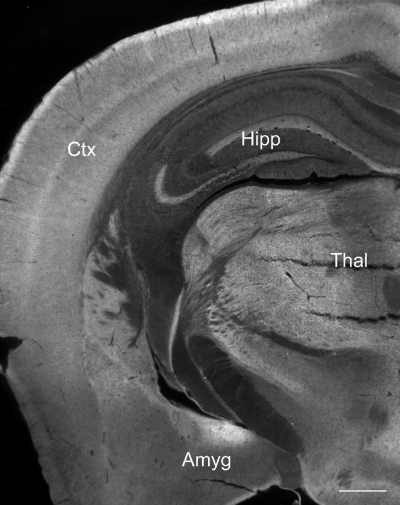
GFRα2 protein is widely expressed in adult mouse brain. Shown is a frontal section immunostained with GFRα2-specific antibodies. Amyg, amygdala; Ctx (neo)cortex; Thal, thalamus; Hipp, hippocampus. Scale bar, 0.5 mm.
Discussion
In the present study we have characterized the performance of adult male GFRα2-knockout mice in three commonly used learning and memory models. The most obvious phenotypic change in these mice is substantial growth retardation, which begins around weaning and is maximal at the age of 1 month, apparently due to alimentary tract defects (Rossi et al., 2003). The weight difference of the adult mice used in the current study was ≈ 15%. Despite that, the knockout mice do not show any gross defects in the central nervous system structure (Rossi et al., 1999; J. Rossi and M. S. Airaksinen, unpublished data). At the behavioural level this was confirmed by the normal locomotor and exploratory activity and intact coordination displayed by the knockout mice. However, despite intact general appearance, the GFRα2-deficient mice were significantly impaired in three different tests of learning and memory. The mutant mice displayed reduced contextual memory and context discrimination in the fear-conditioning paradigm, impaired behavioural flexibility in spatial learning and decreased retention of conditioned taste aversion. Thus, we consider it unlikely that the observed behavioural changes are secondary to the peripheral nervous system defects described earlier.
There is little previous evidence regarding the role of GFLs in cognitive processes. In one behavioural study, GDNF heterozygous mice (in two genetic backgrounds) were shown to be impaired in spatial learning which, it has been suggested, depends on hippocampal function (Gerlai et al., 2001). The GDNF mutant mice could improve their performance in the water maze test as the training progressed, although they remained less precise than the wild-type mice. The reversal learning, which was clearly affected in GFRα2-deficient mice, was not studied by Gerlai et al. (2001).
It has been suggested that the phenomenon of long-term potentiation (LTP) is suitable model of learning and memory at the cellular and molecular levels (Bliss & Collingridge, 1993). Mouse knockout studies have added a substantial piece of evidence regarding the mechanisms underlying both LTP and memory. However, the changes observed are not always uni-directional and unaffected LTP certainly does not preclude memory impairments (Sweatt, 2003). The lack of GFRα2 has been shown to reduce epileptogenesis and maintenance of hyperexcitabilty in the hippocampal kindling model. However, the GFRα2-knockout mice did not exhibit impairment in hippocampal LTP in the CA1 region, although mossy fibre LTP was not studied (Nanobashvili et al., 2000). Notably, GFRα2 expression in the hippocampus is most prominent in the mossy fibers. As natural variations in infrapyramidal mossy fibers have shown correlations with reversal learning yet not with acquisition (Schopke et al., 1991), further studies on mossy fibre structure and function in GFRα2 knockouts are warranted.
Neurotrophin BDNF and its receptor TrkB are critically involved in the mechanisms of synaptic plasticity and memory (Tyler et al., 2002). It is known that the neurotrophins and GFLs share similar intracellular signalling pathways (Airaksinen & Saarma, 2002). Namely, GFLs activate Src-family kinases and subsequently trigger phosphorylation of MAPK, PLC-γ and cAMP responsive element-binding protein (CREB). All these factors have been shown to be critically involved in complex cognitive processes. Most strikingly, conditional knockout mice lacking TrkB receptors in the forebrain have been shown to display a behavioural phenotype remarkably similar to that of GFRα2-knockout mice, characterized by inappropriate coping responses when faced with complex or stressful learning paradigms (Minichiello et al., 1999), and decreased behavioural flexibility (Vyssotski et al., 2002). It has recently been shown that TrkB mediates hippocampal plasticity via recruitment of PLC-γ (Minichiello et al., 2002). In addition, inhibition of MAPK (Selcher et al., 1999) or Src-kinase (Bevilaqua et al., 2003) leads to impaired memory. The role of CREB in learning and memory has been more difficult to prove with genetically modified mice. The reason for the inconsistent results might be due to the different genetic background of the mice employed or to compensatory up-regulation of other transcription factors (Balschun et al., 2003). Altogether, these findings indicate that genetic alteration of GFL signalling may lead to impairment of cognitive functions in mice.
Consistent with previous mRNA localization in mouse brain (Golden et al., 1998), we show that GFRα2 protein is abundantly expressed in several brain regions (neocortex, amygdala, hippocampus and thalamus) that are important for learning complex tasks. Therefore, it is tempting to speculate that the lack of GFRα2 affects the development or function of neuronal circuits responsible for learning and memory. Interestingly, although highly expressed in thalamus and brainstem (including monoaminergic nuclei), the level of Ret mRNA expression in many cortical regions is very low or absent (Golden et al., 1998; Burazin & Gundlach, 1999). This discrepancy in GFRα2 and Ret expression in the neocortex suggests possible involvement of other signalling mechanisms or receptors such as NCAM (Paratcha et al., 2003). It is noteworthy that the loss of NCAM in mice is associated with impairments in spatial learning (Cremer et al., 1994). It will be important to find out whether similar behavioural deficits as in GFRα2-knockout mice are observed in mice lacking NRTN (Heuckeroth et al., 1999). Different experimental designs (e.g. conditional knockout) are needed in order to exclude developmental effects and to better localize the site of action of GFRα2. However, the present study indicates that endogenous GFRα2 signalling is involved in cognitive processes.
Acknowledgements
This study was supported by grants from the Academy of Finland and the Sigrid Juselius Foundation (to M.S.A.)




