JUA clinical guidelines for benign prostatic hyperplasia
This is a translated summary of a full guideline first published as a book in Japanese by The Japanese Urological Association, Clinical guidelines for benign prostatic hyperplasia, 2011. RichHill Medical, Tokyo.
Abstract
The Japanese Urological Association has developed Clinical Guidelines for Benign Prostatic Hyperplasia (BPH) for men with suspected BPH. This article is an English translation of the abridged version of the Guidelines. The Guidelines were formulated on the basis of evidence retrieved from the PubMed database between 1995 and 2009, as well as other relevant sources. The target patients of these Guidelines are men with suspected BPH, and the target users are urologists. A mandatory assessment should include a medical history, a physical examination, the completion of symptom and quality of life questionnaires, a urinalysis, a prostate ultrasonography, measurement of serum prostate specific antigen and postvoid residual urine, and uroflowmetry. Optional tests include keeping a bladder diary, the measurement of serum creatinine, and upper urinary tract ultrasonography. Care should be taken to not overlook coexisting diseases such as infections or malignancies that may obscure the diagnosis. Treatment should consist of conservative therapy or the use of medications such as α1-adrenoceptor antagonists, or both. The use of 5α-reductase inhibitors or anticholinergic agents should be considered in patients with an enlarged prostate (>30 mL) or overactive bladder symptoms (Overactive Bladder Symptom Score ≥6), respectively. Surgical intervention is indicated when non-surgical treatments fail to provide sufficient symptomatic relief, and when bladder outlet obstruction is highly suspected.
Introduction
A number of clinical guidelines have been formulated in Japan for lower urinary tract disorders such as benign prostatic hyperplasia (BPH),1 urinary incontinence,2 overactive bladder (OAB),3 male lower urinary tract symptoms (LUTS),4 interstitial cystitis and hypersensitive bladder syndrome,5 nocturia,6 and neuropathic conditions. The clinical guidelines for BPH have recently been revised extensively, and this article is an English translation of the abridged version of the revised guidelines. The target patients and users of these Guidelines are men suspected of having BPH and urologists, respectively. In the case of the male LUTS Guidelines,4 the target patients and users are men with LUTS and non-urologist physicians, respectively. Readers should be aware of the earlier guidelines, particularly for male LUTS,4 when adopting the BPH Guidelines to clinical practice.
Methodology
The Guidelines were developed by committee members recommended by the Japanese Urological Association (JUA). The members meticulously reviewed relevant references, retrieved via the PubMed and Japana Centra Revuo Medicina databases, and published between 1995 and 2009. Other sources of information included Japanese guidelines for other conditions, the BPH guidelines published by the American Urological Association (AUA)7 and the European Association of Urology (EAU),8 and the meeting reports of the International Consultation on Urological Diseases on male lower urinary tract disorders.9 A draft of the revised guidelines was peer-reviewed by JUA executive members before the guidelines were finalized. Funding was provided by the JUA.
Algorithm
- •
The algorithm (Fig. 1) is applicable to men who are suspected of having BPH. Factors suggestive of BPH include age >50 years, LUTS (increased urinary frequency, voiding difficulties, urgency), and associated complications, such as urinary retention and urinary tract infection (UTI), or both.
- •
The basic assessment of these men, mandatory in all cases, comprises their present and past history, the completion of symptom and quality of life (QOL) questionnaires, a physical examination, urinalysis, uroflowmetry, postvoid residual urine measurement, a prostate ultrasonography, and the determination of prostate-specific antigen (PSA) serum concentrations. Optional assessments, selected on an individual basis, include keeping a bladder diary, advanced urodynamic studies, the measurement of serum creatinine levels, and an upper urinary tract ultrasonography. Additional tests, such as urine cytology, urine culture, endoscopy, and radiological examinations may be indicated when other disorders are suspected. Other disorders include prostatic cancer, prostatitis, OAB, underactive bladder, bacterial cystitis, interstitial cystitis, bladder cancer, bladder stones, urethritis, urethral stricture, neurogenic bladder, hydronephrosis, polyuria, and nocturnal polyuria.
- •
When the patient's history, symptoms, or test results suggest the presence of other disorders, further assessments need to be performed. Findings suggestive of other disorders are a history of urinary retention, urinary tract infection, macroscopic hematuria, pelvic surgery or radiotherapy, and neuropathic diseases; symptoms of bladder pain, perineal pain, monosymptomatic nocturia, and overt OAB symptoms; and test results revealing abnormal findings on rectal examinations, urinalysis, and ultrasonography, elevated PSA levels, positive urinary cytology, increased residual urine, bladder stones, renal dysfunction, polyuria, and nocturnal polyuria.
- •
When lower urinary tract dysfunctions, including symptoms, can be attributed to BPH, both the patient's desire for treatment and the medical need for treatment should be assessed. Medical need includes the event of severe symptoms, a highly enlarged prostate, or complications, such as urinary retention, hematuria, bladder stones, renal insufficiency, and UTI.
- •
When there is no desire on the part of the patient for treatment and no medical need for treatment, watchful waiting is indicated.
- •
In most cases, conservative or medical therapy, or both, such as the use of α1-adrenoceptor antagonists, are first indicated. When there is an indication for surgery, further assessments prior to surgery should be undertaken. Surgery is the preferred treatment in cases in which there are BPH-related complications, when it is anticipated that medical treatment will be insufficient, or if the patient prefers to undergo surgical therapy.
- •
The baseline for medical treatment is the use of α1-adrenoceptor antagonists. When the prostate is enlarged (>30 mL) and when OAB symptoms are evident (overactive bladder symptom score [OABSS]≥6), consideration should be given to changing the medical treatment to 5α-reducatase inhibitors or anticholinergics or both (either instead of or in addition to α1-adrenoceptor antagonists), respectively. If these measures are not fully successful, surgical indications should be evaluated.
- •
Evaluation of surgical indications includes confirming the patient's desire for surgery, making a systemic review of the surgical risks, and an urodynamic evaluation of bladder outlet obstruction (BOO). BOO is to be assessed by pressure-flow studies or it may be feasible by other studies, such as uroflowmetry or bladder wall thickness, or both.
- •
If a patient's condition does not improve or worsens, the patient should be reassessed, as described under the basic assessment section, for the presence of other disorders. If a patient's condition improves with treatment, regular reassessments should be undertaken to detect any possible changes in the patient's status, with therapeutic measures adjusted accordingly.
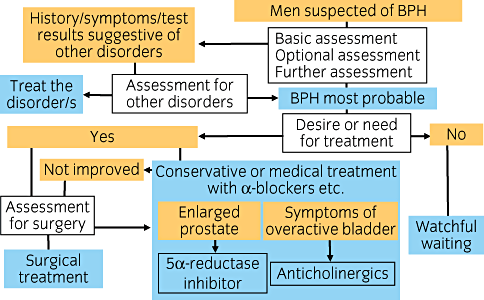
Algorithm for benign prostatic hyperplasia (BPH).
Definition of BPH
Summary
BPH is defined as a disease that manifests as a lower urinary tract dysfunction due to benign hyperplasia of the prostate, usually associated with enlargement of the prostate and LUTS suggestive of lower urinary tract obstruction.
There is no uniform definition for BPH.9,10 The International Continence Society proposed the use of “BPH” exclusively as a histopathological term to refer to non-malignant hyperplasia of prostatic tissue, and coined the terms “benign prostatic enlargement” (BPE) for an enlarged prostate and “benign prostatic obstruction” (BPO) for lower urinary tract obstruction.11 This strict terminology results in the redundant expression “LUTS suggestive of BOO” being used for a condition that is commonly referred to as BPH in clinical practice. In the present Guidelines, BPH needs to be defined in a way that is suitable for use in clinical practice.
Thus, in the present Guidelines, the term “BPH” is used to refer to conditions in which there is benign hyperplasia of the prostate. There are three characteristic pathophysiological features: (i) BPE; (ii) BPO; and (iii) LUTS.12 However, in a clinical setting, it is not necessarily feasible to make an accurate assessment of all three factors. Specifically, the pressure-flow studies that are mandatory for the accurate evaluation of BPO are invasive and are thus not performed routinely, even during urological consultations. Less invasive tests, such as uroflowmetry, the measurement of postvoid residual urine (PVR), and an ultrasonographic assessment of bladder wall thickness, cannot fully replace pressure-flow studies. However, in reality, men with LUTS and lower urinary tract dysfunctions are diagnosed as having BPH when BPE is present, BPO is suggested, and other pathologies are ruled out. Thus, in the present Guidelines, BPH is regarded as a disease with lower urinary tract dysfunction due to benign hyperplasia of the prostate, which is usually associated with enlargement of the prostate and LUTS that are suggestive of lower urinary tract obstruction.
Epidemiology and natural history of BPH
Summary
BPH is a progressive disease that is common in elderly men. Although prevalence varies based on the definition of BPH used, 6 and 12% of Japanese men in their sixties and seventies, respectively, meet all three of the following criteria for BPH: (i) an international prostate symptom score (IPSS) >7; (ii) prostate volume (PV) >20 mL; and (iii) peak urinary flow rate (Qmax) <10 mL/s. Risk factors for the clinical progression of BPH are aging, prostate enlargement, elevated PSA, LUTS, impaired QOL, and decreased urinary flow rate. Lethal comorbidities related to BPH are rare.
Risk factors for BPH
The principal risk factors for BPH are aging and normally functioning testicles. Although no definitive genes responsible for BPH have been identified, a family history of BPH and molecular abnormalities may increase the likelihood of its development. Dietary factors, such as isoflavonoids and lignans in vegetables, grains, and soy, may have a negative impact on the development of BPH.13 In addition, a recent study has reported a relationship between BPH and metabolic syndrome.14 The correlation between erectile dysfunction (ED) and LUTS suggests a common etiology for these conditions, such as changes in sympathetic nerve tone induced by insulin resistance.15
Natural history
BPH is a physiological process that occurs with aging, regardless of race, ethnicity or region.16,17 Longitudinal studies have confirmed age-related increases in PV,18,19 although in a small proportion of men PV has been noted to decrease with aging.20 Recent studies indicate that PV is likely to increase in men in whom the prostate has a visible transition zone with a clear border21,22 and with a large transition zone volume on transrectal ultrasound at baseline.23 The prevalence of LUTS in the general population is age-related.24–27 Longitudinal studies have shown an increase in IPSS with advancing age as a whole,19,28,29 but with simultaneous decreases in IPSS in certain subgroups.28,30 Qmax decrease with aging31,32 and this may be attributable to BPO as well as detrusor underactivity (DU). The relationship between PV, LUTS, and urinary flow rate is generally poor in men presenting at hospital, but it is modest among men in the general population. Prostate enlargement is likely to be involved in the progression of symptoms.19,33
The prevalence of BPH, which varies depending on the definition of BPH used,34 has been estimated on the basis of results of community-based studies in Japan (Table 1).25,31 Specifically, only 2% of men in their forties met the criteria of IPSS >7, PV >20 mL, and Qmax <10 mL/s. However, the proportion of men in their sixties and seventies who meet these criteria increases to 6 and 12%, respectively.
| Age (years) | IPSS >7 (%) | PV >20 mL (%) | Qmax <10 mL/s (%) | Prevalence (%) |
|---|---|---|---|---|
| (1) | (2) | (3) | [(1) + (2) + (3)] | |
| 40–49 | 47 | 20 | 4 | 2 |
| 50–59 | 44 | 35 | 6 | 2 |
| 60–69 | 52 | 39 | 19 | 6 |
| 70–79 | 63 | 37 | 42 | 12 |
- IPSS, international prostate symptom score; Qmax, peak urinary flow rate; PVR, postvoid residual urine.
Factors affecting health-care seeking behavior
A population-based study in Olmsted County revealed that health-care seeking behavior was influenced by the severity of symptoms, particularly if they were bothersome and interfered with an individual's daily activities.35 In Japan there is a marginal overlap in the distribution of QOL scores between the general population and patients with BPH.36
Prediction of disease progression
When acute urinary retention is regarded as an indicator of disease progression, the incidence in a US population has been calculated to be 6.8 per 1000 person-years.37 Older age, a higher IPSS, Qmax <12 mL/s, and PV >30 mL are all risk factors for urinary retention. Surgical treatment would be another indicator of disease progression, and the risk is related to age, the severity of the symptoms, an impaired QOL, decreased urinary flow rate, degree of prostate enlargement, and increased serum PSA levels.30,38
Natural history of BPH after diagnosis
The natural history of BPH can be monitored in men in whom the “watchful waiting” approach is being taken or in those on a placebo in Phase III clinical trials of medical treatments. The baseline PV and the rate of growth of the prostate during follow-up are greater in men with BPH than in men in the general population.39,40 The Medical Therapy of Prostatic Symptoms study,41 which observed men on placebo or doxazosin for 4.5 years, reported that prostate growth could be predicted by baseline PV and serum PSA levels. Although LUTS worsened progressively in men with BPH as a whole, in some patients improved or stable LUTS was noted.42 Symptomatic deterioration, development of acute urinary retention, and conversion to BPH-related surgery tended to occur in men with severe LUTS, a large PV, and high PSA levels at baseline.43–45 Fortunately, a recent study has found that BPH-related deaths or comorbidities, such as hydronephrosis, renal failure, UTI, and bladder stones, are rare. In a study of 737 patients treated with placebo, over 4.5 years of follow-up only one patient developed recurrent UTI and none developed renal insufficiency.46
Pathophysiology
Summary
BPH is composed of stromal elements made up of smooth muscle and connective tissue, as well as glandular and luminal epithelial cells, and arises in the periurethral region of the prostrate. An interaction is seen between stromal and epithelial cells, mediated by proliferative factors, including sex hormones, inflammation, and stimulation of adrenergic nerves. Urethral compression associated with prostatic enlargement causes voiding symptoms. However, age-related DU is also an important cause of voiding symptoms. Urethral compression causes distension, ischemia, inflammation, and oxidative stress to the bladder, followed by changes to the bladder nerves and smooth muscle and the release of urothelial-derived mediators, causing storage symptoms. Even without compression, stimulation of the urethral sensory nerves can cause storage symptoms. Important complications of BPH include recurrent urinary retention, macroscopic hematuria, bladder calculi, recurrent UTI, and postrenal renal failure.
Prostate growth factors
Histologically, prostatic hypertrophic nodules (adenomas) consist of increased numbers of cells, so the term “hyperplasia” is more appropriate than “hypertrophy”. Prostatic adenomas arise in the transitional zone and the periurethral region of the prostate,47 with nodules initially consisting of stromal elements only.48
An interaction is seen between prostatic stromal and epithelial cells, mediated by various growth factors, and the hyperplasia is thought to be caused by an imbalance between cellular proliferation and cell death. Although the importance of male sex hormones to the development of prostatic hyperplasia is undisputed,49 female sex hormones also contribute to its formation. Furthermore, inflammation plays an important role in the onset of hyperplasia and urinary retention.50 A clinical trial of 5α-reductase inhibitor therapy found that prostatic volumes were bigger, and symptom scores higher, in patients with pathological evidence of inflammation.51 Cytokines derived from inflammatory cells also induce epithelial growth factors.52 In addition, the promotion of glandular proliferation in spontaneously hypertensive rats53 and the induction of apoptosis by α1-adrenoceptor antagonists54 suggest the involvement of adrenergic nerves in prostatic hyperplasia.
Prostatic adrenergic receptors
α1-adrenergic receptors (AR) are the main AR in prostatic smooth muscle, and three subtypes have been identified: α1A, α1B, and α1D. In the normal human prostate, the mRNA content of each of the α1A-, α1D-, and α1B-AR subtypes has been reported to be 63, 31, and 6%, respectively, compared with 85, 14, and 1%, respectively, in prostatic hyperplasia.55 In both cases, α1A-AR is the predominant subtype. Conversely, there have been reports of patients in whom there are similar levels of α1A- and α1D-AR (41 and 49%, respectively), and in whom α1A-AR is no longer the predominant subtype.56 A recent study found that the α1A-AR gene expresses two subtypes, α1A and α1 L, with the α1 L subtype being mainly involved in the contraction of the prostate and urethra.57 Noradrenaline-induced contraction of the prostate is nearly absent in α1D-AR knockout mice,57 but observations of reduced urinary frequency and increased bladder volumes58 indicate that α1D-AR are involved in bladder function, not prostatic contractions. Detrusor overactivity (DO) induced by stimulation of the rat urethra is inhibited by α1-adrenoceptor antagonists with a high specificity for α1A-AR,59,60 indicating that storage symptoms in BPH are mediated by urethral α1A- and α1 L-AR.
BPE, LUTS, and BPO
The pathology of BPH consists of three elements: BPE, LUTS, and BPO.4 Because LUTS can result from a variety of different diseases and conditions (Table 2), there is no clear correlation between LUTS and BPE or BPO.61,62 Whether there is a correlation between BPO and LUTS sometimes depends on the site of origin of prostatic nodules. For example, in the case of middle lobe hyperplasia, intravesical prostatic protrusion, rather than BPE, is correlated with LUTS and BPO.63 Urodynamic studies show a weak correlation between BPO and LUTS, whereas DO is correlated with the degree of BPO.64 Storage symptoms, in particular urge incontinence, strongly suggest DO.65–67
| 1. Prostate and lower urinary tract |
| Prostate: BPH, prostatitis, prostate cancer |
| Bladder: bacterial cystitis, interstitial cystitis, bladder cancer, bladder stones, bladder diverticulum, OAB, other (age-related detrusor underactivity) |
| Urethra: urethritis, urethral stricture |
| 2. Nervous system |
| Cerebral: cerebrovascular disorder, dementia, Parkinson's disease, multiple system atrophy, brain tumor |
| Spinal cord: spinal cord injury, multiple sclerosis, spinal cord tumor, spinal infarction, spinal degenerative disease, spina bifida |
| Peripheral nerves: diabetic neuropathy, post-pelvic surgery |
| Other: aging, autonomic hyperactivity |
| 3. Miscellaneous |
| Drug related, polyuria, sleep disorders, psychogenic |
- BPH, benign prostatic hyperplasia; OAB, overactive bladder.
Voiding dysfunction associated with BPO:
Voiding symptoms in patients with BPH associated with BPO occur as a result of resistance to urine flow. Specifically, in response to BPO, the bladder smooth muscle hypertrophies to maintain urine flow. When it can no longer compensate, a reduced expression of connexin 43, the structural protein in the gap junctions between smooth muscle cells, impairs the synchronization of contractions, resulting in contractile dysfunction.68 Even after any obstruction has been removed, such as by surgery, voiding symptoms persist in one-third of patients.69 In particular, voiding symptoms are often caused by bladder contractile dysfunction in patients >70 years of age (48%) and are even more common in those with a history of urinary retention.70 BPO cannot be demonstrated in 60% of patients >80 years of age with voiding dysfunction.71,72 This is likely to be due to an age-associated decrease in bladder contractility and increase in collagen.
Storage dysfunction associated with BPO:
With BPH, the cycle of urine storage and voiding causes repeated bladder distension, raised intravesical pressure, ischemia, and reperfusion, eventually resulting in various changes in the urothelium, nerves, and smooth muscle. Impaired vesical blood flow results in oxidative stress, with free radicals causing damage to the urothelium, nerves, and smooth muscle.73 Patients with persistent DO following transurethral resection of the prostate (TURP) have ongoing impairment of perfusion of the lower urinary tract,74 suggesting a correlation between impaired perfusion and storage dysfunction.
- 1
Changes in nerves: the nerves in the bladder wall (pelvic nerve post-ganglionic fibers) are particularly vulnerable to ischemia and undergo partial denervation. With this denervation, the bladder smooth muscle becomes supersensitive to acetylcholine (ACh).75 Furthermore, in sensory nerves subjected to ischemia, the number of neurokinin receptors increases,76 resulting in increased sensory sensitivity.
- 2
Changes in bladder smooth muscle: in the normal bladder, adjacent smooth muscle cells are coupled and undergo autonomous contractions. However, in patients with BPO, the increased expression of the gap junction protein connexin 43 induces synchronization of smooth muscle cells, increasing the strength of contractions.77 The increase in the strength of autonomous contractions is also thought to be mediated by changes in the interstitial cell networks in the suburothelial and muscle layers,78 the release of various urothelial mediators, supersensitivity to ACh,79 and/or the activation of the RhoA/Rho-kinase pathway.80
- 3
Urothelial-derived mediators: urothelial cells not only release neurotransmitters, but also have various receptors and ion channels on their surface, thereby influencing bladder function.81 Important mediators released by the urothelium include adenosine triphosphate, nitric oxide, prostaglandin, and ACh. These mediators are released in response to distension, ischemia, inflammation, and/or oxidative stress, mediated by transient receptor potential vanilloid 1,82 epithelial sodium channel,83 acid-sensing ion channels,84 muscarinic, and other purinergic receptors,85 or AR on the bladder urothelium. These bladder urothelial cells, as well as C fibers in the bladder suburothelium, respond to these stimuli by increasing their afferent input, thereby influencing storage function.86
- 4
Afferent stimuli from the urethra: enlargement of the prostate leads to distension and irritability of the urethra, with storage symptoms mediated by the stimulation of afferent nerves to the urethra. The induction of the micturition reflex by intraurethral prostaglandin E2 is inhibited by α-AR antagonists,59 suggesting that urethral sensory nerves play an important role in the onset of storage symptoms.
Conditions other than BPO:
It is not uncommon for middle-aged to elderly men with LUTS to have BPE but not BPO.62 In particular, voiding symptoms in elderly men are caused by bladder contractile dysfunction rather than obstruction.70–72 Anesthesia of the prostatic urethra has been reported to increase bladder volume,87 suggesting that urethral sensory nerves are responsible for storage symptoms.
Complications of BPH
Recurrent urinary retention
The incidence of acute urinary retention is significantly higher in patients with BPH who have a prostatic volume ≥31 mL or serum PSA levels ≥1.6 ng/mL. These patients are more likely to progress to invasive treatment.45 The risk of acute urinary retention increases with age and it is often associated with DU.70
Macroscopic hematuria
Macroscopic hematuria is present in 12% of patients in whom surgery is indicated for BPH.88 A possible cause of the macroscopic hematuria is increased microvessel density due to the increased expression of vascular endothelial growth factor associated with the enlargement of the prostate.89,90
Bladder calculi
Bladder calculi associated with BPH are thought to be caused by urinary stasis,91 although the underlying mechanism is unclear.
Recurrent UTI
Increased PVR volumes and endoscopic manipulation in the lower urinary tract are thought to contribute to UTI.
Postrenal renal failure
Although renal failure is a rare complication of BPH (<1%), the Japanese Clinical Guidelines for male LUTS and the EAU BPH Guidelines both recommend the measurement of serum creatinine levels as part of the initial assessment.4,8 Diabetes mellitus and hypertension are common causes of kidney failure in patients with BPH.92
Diagnosis
Summary
Basic evaluation and tests for men suspected of BPH include a clinical history, assessment of symptoms and QOL using validated questionnaires (i.e. the core lower urinary tract symptoms score [CLSS], IPSS, and OABSS), a physical examination, a urinalysis, a uroflowmetry, PVR measurement, serum PSA determination, and a prostate ultrasonography. Case-sensitive or elective tests include keeping a bladder diary, pressure-flow studies, serum creatinine measurements, and ultrasonography of the upper urinary tract.
Basic tests
The initial evaluation of men suspected of having BPH should be in accord with the guidelines for male LUTS.4
A clinical history should be obtained for any current illness, as well as for medications and a past history that may influence voiding functions, such as neurological diseases, diabetes mellitus, and intrapelvic or urological surgery. Symptoms and QOL should be assessed using validated questionnaires, including the IPSS and the BHP impact index.93–95 OAB symptoms should be evaluated by the overactive bladder questionnaire96 or the OABSS.97 The CLSS,98 a simple non-disease-specific tool addressing 10 important symptoms, may be useful for the initial assessment of LUTS.
Physical examinations of the lower abdomen may detect urinary retention, as evidenced by the distension of the abdominal wall. A digital rectal examination of the prostate enables the size and indurations of the prostate to be evaluated. Anal tonus and perineal sensation may be attenuated by coexisting neurogenic deficits.
Urinalysis should be negative in BPH. The presence of hematuria or pyuria, or both, should be evaluated further. Uroflowmetry is a non-invasive quantitative evaluation of voiding conditions, with a reduced flow rate indicating the obstruction of the bladder outlet or DU. A PVR measurement can be made using transabdominal ultrasonography rather than by inserting a catheter.
The determination of serum PSA levels is useful to predict PV or the clinical progression of BPH, or both.99 However, it should be kept in mind that serum PSA values are also increased in cases of prostate cancer, acute urinary retention, and prostatitis. Anti-androgens and 5α-reductase inhibitors can reduce serum PSA values by approximately half.100
Prostate ultrasonography is recommended for the accurate evaluation of the volume and shape of the prostate.101 Trans-abdominal ultrasonography using a general ultrasound instrument is less invasive and can simultaneously detect PVR and bladder pathology. Transrectal ultrasonography, which requires specialized equipment, evaluates the detailed internal structure of the prostate. PV is usually calculated by an approximation of the ellipsoid formula and is predictive of BPH progression.102
Elective tests
The bladder diary records micturition times and the volume voided over a period of 24 h. It is particularly useful for men with daytime and nocturnal frequency to identify a reduction in the volume voided and (nocturnal) polyuria. Pressure-flow studies are standard urodynamic tests that accurately evaluate both BOO and detrusor contractility. Because this test requires the insertion of a catheter, it is recommended only when the test results may affect therapeutic outcomes. Serum creatinine concentrations may be elevated in men with urinary retention or incidental renal dysfunction. An upper urinary tract ultrasonography is recommended for men with abnormal urinalysis, large PVR, renal insufficiency, or a history of other urological diseases.
Other tests
Endoscopy of the urethra and bladder is helpful for the evaluation of morphological changes, such as adenoma protrusion into the bladder and bladder trabeculations. It is recommended for men with planned surgery and suspicion of other diseases, such as urethral stricture, bladder stones, and cancer. Retrograde urethrography is able to detect a urethral stricture. Further evaluations are indicated when other diseases and conditions are suspected.
Treatment
The treatment for BPH is basically an amelioration of impaired QOL by improving LUTS.
Grades of recommendation
The grades of recommendation for treatments (Table 3) were determined via committee discussion and consensus, based on the level of evidence (Table 4), as well as variability of conclusions, the magnitude of effect, clinical applicability, adverse events, and cost. Grades of recommendation for individual treatments are shown in Table 5.
| Grade | Nature of recommendation |
|---|---|
| A | Highly recommended to do |
| B | Recommended to do |
| C | No firm evidence for recommendation |
| C1 | Can be considered |
| C2 | Not recommended |
| D | Recommended not to do |
| Reserved | Unable to decide grade of recommendation |
| Level of evidence | Type of evidence |
|---|---|
| 1 | Evidence obtained from multiple large-scale randomized controlled trials (RCT) |
| 2 | Evidence obtained from a single RCT or low quality RCT |
| 3 | Evidence obtained from non-randomized controlled studies |
| 4 | Evidence obtained from observational studies |
| 5 | Evidence obtained from case studies or expert opinions |
| Treatment | Grade |
|---|---|
| Pharmacotherapy | |
| α1-adrenoceptor antagonists (α1-blockers) | |
| Tamsulosin, naftopidil, silodosin, terazosin,† urapidil† | A |
| Prazosin† | C1 |
| 5α-reductase inhibitors | |
| Dutasteride | A |
| Finasteride | Reserved (not approved) |
| Anti-androgens | |
| Chlormadinone, allylestrenol | C1 |
| Others | |
| Eviprostat®, Cernilton®, Paraprost®, Chinese herbal medicines (Hachimi-jio-gan, Gosha-jinki-gan) | C1 |
| Flavoxate, antidepressants, cholinergics, phosphodiesterase 5 inhibitors | Reserved (not approved) |
| Surgical interventions | |
| Open prostatectomy (sub-capsular enucleation) | B |
| Transurethral resection of the prostate | A |
| Transurethral incision of the prostate | B |
| bipolar-TURP | A |
| Holmium laser enucleation of the prostate | A |
| Photoselective vaporization of the prostate by KTP laser | B |
| Holmium laser ablation of the prostate | B |
| Transurethral detachment of the prostate, transurethral enucleation with bipolar system | C1 |
| Interstitial laser coagulation of the prostate | C1 |
| High-intensity focused ultrasound | C1 |
| Transurethral needle ablation | C1 |
| Transurethral microwave thermotherapy | B |
| Urethral stent | C1 |
| Transurethral ethanol ablation of the prostate | Reserved (not approved) |
| Botulinum toxin injection | Reserved (not approved) |
| Other treatments | |
| Lifestyle modification | B |
| Watchful waiting | B |
| Supplements | C2 |
| Indwelling catheter | Reserved |
| Intermittent catheterization | B |
- † See “Guidelines for Management of Male Lower Urinary Tract Symptoms”.8
Pharmacotherapy
1 α1-adrenoceptor antagonists
α1-adrenoceptor antagonists relieve outlet obstruction by inhibiting contractions mediated by prostatic α1-AR, thereby ameliorating LUTS of BPH.4,103α1-adrenoceptor antagonists, also referred to as α1-blockers, can result in the rapid relief of LUTS. The use of α1-adrenoceptor antagonists can result in a 16–25% (2.0–2.5 mL/s) increase in maximum flow rate and a 30–40% (4–6 point) reduction in the average IPSS.4,7,8,103,104 Adverse reactions to alfuzosin and tamsulosin, such as postural hypotension and asthenia, have been reported to be as low as that reported for placebo (4–10%). Other adverse events include ejaculatory dysfunction and intraoperative floppy iris syndrome. All α1-adrenoceptor antagonists have a similar efficacy in appropriate doses, with the effects being dose dependent.7,8,104 In Japan, tamsulosin, naftopidil, silodosin, terazosin, urapidil, and prazosin have been approved for use.103 Note that the recommended doses of some drugs are different in Japan to those in Europe and the USA.
Tamsulosin
Recommendation grade: A
There is adequate evidence to support its efficacy for BPH (Level 1).
Tamsulosin is an α1A/α1D-AR subtype-selective antagonist, with an affinity for α1A- and α1D-AR 15.3-, and 4.6-fold higher, respectively, than for α1B-AR.105 A Japanese randomized clinical trial (RCT) of tamsulosin in the treatment of BPH demonstrated its superiority to placebo, and reported that the optimal dosage was 0.2 mg/day.106 Studies in Western countries have reported optimal doses of 0.4 mg/day.104,107 Adverse reactions to tamsulosin, including postural hypotension (0.19%), were uncommon in the Japanese study (2.87%).
Naftopidil
Recommendation grade: A
There is adequate evidence to support its efficacy for BPH (Level 1).
Naftopidil is an α1D/α1A-AR subtype-selective antagonist, with an affinity for α1D-, and α1A-AR 16.7- and 5.4-fold higher, respectively, than for α1B-AR.105 The efficacy of naftopidil in the treatment of BPH has been demonstrated in RCT comparing it with both placebo and other agents.108,109
Silodosin
Recommendation grade: A
There is adequate evidence to support its efficacy for BPH (Level 1).
Silodosin is a selective α1A-adrenoceptor antagonist, with an affinity for α1A-, and α1D-AR 583- and 10.5-fold higher, respectively, than for α1B-AR.110 An RCT in Japan indicated significantly larger decreases in IPSS and QOL scores following treatment with silodosin compared with placebo.111 Pooled results from two Phase III studies of silodosin for the treatment of BPH have been reported in the USA.112
2 5α-reductase inhibitors
Dutasteride
Recommendation grade: A
There is adequate evidence to support its efficacy for definite BPH (≥30 mL) (Level 1). Caution is required in evaluating the PSA value, since dutasteride affects PSA levels.
Dutasteride inhibits both isoforms (type 1 and type 2) of 5α-reductase and suppresses the production of dihydrotestosterone (DHT). In Japan, a Phase II RCT was conducted with 284 BPH patients aged ≥50 years, a prostatic volume ≥30 mL, an IPSS ≥8, and a maximal urine flow ≤15 mL/s.113 They were administered dutasteride 0 mg (placebo), 0.05 mg, 0.5 mg, or 2.5 mg daily for 24 weeks. An approximately 90% decrease in serum DHT levels was observed after 2 weeks' treatment in the 0.5 and 2.5 mg groups, as was seen in overseas trials.114 A Japanese Phase III RCT allocated participants to receive dutasteride 0.5 mg daily (n= 193) or placebo (n= 185) for 52 weeks.100 In the dutasteride group, a significant decrease in prostatic volume was seen after 24 weeks' treatment, and significant decreases were seen in IPSS and maximal urine flow after 36 weeks' treatment. After 52 weeks' treatment, IPSS had improved by 5.3 points, maximal urine flow by 2.2 mL/s, and prostatic volume by 22% over baseline. Although uncommon, adverse events related to sexual function were rather more common in the dutasteride group and PSA levels decreased to an average 46.1%. These results are very similar to those from an overseas study.115
Finasteride
Recommendation grade: reserved (not approved)
There is adequate evidence to support its efficacy (Level 1). 116 However, in Japan finasteride is indicated only for the treatment of androgenic alopecia and is not approved for BPH.
3 Anti-androgens
These drugs inhibit the pituitary function and the testicular production of testosterone, as well as testosterone uptake and DHT binding to androgen receptors in the prostate. Although covered by insurance, the evidence to support their efficacy for BPH is far from adequate and the incidence of sexual dysfunction is high. Careful patient selection and follow-up are necessary when prescribing these agents.
Chlormadinone
Recommendation grade: C1
There is insufficient evidence to support its efficacy in BPH (Level 3). It is, however, thought to have a similar clinical effect to finasteride (not approved in Japan for BPH), whose efficacy has been confirmed in overseas studies. Various adverse reactions can occur, including sexual dysfunction.
In a comparative trial of chlormadinone 50 mg/day versus Eviprostat, symptomatic improvement was reported by 90% of patients and the prostatic volume decreased by 50% in the chlormadinone group (n= 20), while symptomatic improvement was reported for 70% of patients and the prostatic volume decreased by 30% in the Eviprostat group (n= 20).117 Double-blind comparative trials of chlormadinone and finasteride (not approved in Japan for BPH) found similar clinical efficacy for both agents, including prostate shrinking effects.118,119
Important adverse reactions include congestive heart failure, thrombosis, hepatic dysfunction or diabetes mellitus occur, so treatment should not be continued indefinitely.
Allylestrenol
Recommendation grade: C1
There is insufficient evidence to support its efficacy (Level 3). Sexual dysfunction can occur.
Allylestrenol is considered to have a similar efficacy to chlormadinone. In randomized comparative studies of chlormadinone versus allylestrenol, there were no significant differences between the two in terms of efficacy, but allylestrenol had less effect on sexual function.120–122 Important adverse reactions include the loss of libido and sexual dysfunction, so treatment should not be continued indefinitely.
4 Other oral medications
Eviprostat®
Recommendation grade: C1
There is some evidence to support its efficacy, although the studies are old and its efficacy is inferior to that of α1-adrenoceptor antagonists (Level 2). Recent studies have reported its usefulness in combination therapy with α1-adrenoceptor antagonist. Adverse reactions are rare and minor.
Although the studies were conducted in 1975, there is evidence to support its efficacy from double-blind trials123,124 and a retrospective study suggesting its efficacy in the treatment of BPH.125 RCT conducted with Eviprostat and α1-adrenoceptor antagonists such as naftopidil109 and tamsulosin126 have demonstrated the inferior efficacy of Eviprostat. However, symptomatic improvement has been reported with the addition of Eviprostat to patients on tamsulosin with persistent pelvic discomfort,127 and patients refractory to naftopidil.128
Cernilton® (cernitine pollen extract)
Recommendation grade: C1
Efficacy is suggested for symptoms such as nocturia, but there is no evidence of improvement in objective findings (Level 1). There are few adverse reactions.
Comparison with placebo and Paraprost it is suggested that cernilton is efficacious in relieving nocturia, but no improvement was seen in the urinary flow rate or PVR volume (I, II).129–131 One study found cernilton useful in the treatment of chronic abacterial prostatitis and chronic pelvic pain syndrome.132
Paraprost®
Recommendation grade: C1
There is insufficient evidence to support its efficacy (Level 2). There are few adverse reactions.
An RCT comparing Paraprost and prazosin found no significant difference in the degree of improvement in symptoms or PVR volume, although Paraprost was inferior in improving urinary flow rate.133
Chinese herbal medicines (Hachimi-jio-gan, Gosha-jinki-gan)
Recommendation grade: C1
There is insufficient evidence to support their efficacy, although some studies have reported the usefulness of gosha-jinki-gan in combination with other agents (Level 2).
Hachimi-jio-gan has been indicated for the treatment of BPH, despite any clear supporting evidence. Gosha-jinki-gan is also a Chinese herbal drug that consists of hachimi-jio-gan with additional herbal ingredients. A crossover, non-blinded RCT in which gosha-jinki-gan was added when OAB symptoms persisted despite treatment with tamsulosin, found a significant improvement in QOL in the combination therapy group.134 A study in which gosha-jinki-gan was administered to patients with prostate disease such as BPH, with an inadequate response to α1-adrenoceptor antagonist therapy, found significant improvements in urinary flow rate, IPSS, and QOL scores.135
Flavoxate
Recommendation grade: reserved (not approved)
There is insufficient evidence to support its efficacy (Level 2). Flavoxate is not approved in Japan for BPH.
Although flavoxate has only weak anticholinergic activity, it also acts as a calcium channel blocker and to inhibit the central micturition reflex. A placebo-controlled RCT (n= 70) found no significant difference between flavoxate and the placebo in efficacy.136 Another study found a significant decrease in nocturnal frequency after flavoxate was administered to patients with BPH whose nocturia had not improved with α1-adrenoceptor antagonist therapy.137 There were almost no adverse events.
Antidepressants
Recommendation grade: reserved (not approved)
Tricyclic antidepressants have not yet been approved for the treatment of BPH. There is scant evidence supporting their efficacy (Level 5). Adverse events include arrhythmia and drowsiness.
Theoretically, imipramine should be effective for the treatment of various forms of urinary incontinence, but no studies to date have demonstrated its efficacy in the treatment of BPH.
Anticholinergics
Recommendation grade: reserved (not approved)
There is evidence to support the efficacy and safety of anticholinergic monotherapy for the treatment of BPH with OAB symptoms (Level 1). However, anticholinergic agents can induce voiding difficulty and acute urinary retention, so caution is required in patients with BPH complicated by BOO or voiding difficulties. Anticholinergic agents are not covered by medical insurance for BPH in Japan. They may be used in combination with α1-adrenoceptor antagonists (see clinical question CQ 6 below).
Of two placebo-controlled RCT of anticholinergics in male patients without obvious symptoms of BOO, one reported their superiority to placebo, and the other failed to show any such superiority. The frequency of acute urinary retention was less than 1% in the treatment group, however; similar to that in the placebo group (I, II).138,139 A trial of anticholinergic monotherapy in the treatment of male patients with LUTS and DO confirmed their superiority to placebo, with no cases of acute urinary retention.140
However, anticholinergic agents can induce voiding difficulty and acute urinary retention, so caution is required when considering anticholinergic monotherapy in male patients, particularly those with obvious BOO.4 Anticholinergic agents are not covered by medical insurance for the treatment of BPH, and should be administered with caution to patients with BPH complicated by BOO or voiding difficulties.
The Japan Neurogenic Bladder Society “Practice guidelines for overactive bladder” recommend the use of anticholinergic agents.3 The “Guideline for management of male lower urinary tract symptoms” recommends that anticholinergic therapy should be given under the supervision of a urologist.4 Accordingly, anticholinergic monotherapy should only be considered in male patients with OAB symptoms with no (or only mild) BPH, under strict urological supervision. In patients with BPH accompanied by OAB symptoms, however, α1-adrenoceptor antagonists should be administered first, and an anticholinergic agent can be added if the symptoms are refractory to adrenoceptor antagonist monotherapy.
Cholinergic agents
Recommendation grade: reserved (not approved)
The efficacy of these agents is suggested for patients whose symptoms fail to respond to TURP, or with neurogenic bladder (Level 5), despite other contradictory studies (Level 2). There is no evidence to support their efficacy for BPH or no approval of their use by medial insurance in Japan. Serious adverse events such as cholinergic crises, angina pectoris, and arrhythmias, have been reported.
Cholinergic agents are thought to enhance detrusor contractility. They are not covered by medical insurance for the treatment of BPH and their use is restricted to urinary retention and underactive bladder. A meta-analysis of the use of cholinergic agents in the treatment of underactive bladder, including male and female patients, found they were superior to placebo in three of 10 RCT, and not superior in seven.141 In two RCT conducted with healthy male adults and post-prostatectomy patients, these agents' superiority to placebo was not demonstrated.142,143 In Japanese studies conducted with patients without BOO, a study with small numbers of participants found distigmine effective in patients with difficulty voiding following TURP144 and another study reported that combination cholinergic and α1-adrenoceptor antagonist therapy was more effective than monotherapy in the treatment of underactive bladder.145 Adverse events include abdominal pain and diarrhoea, and cholinergic crises, with sweating, miosis and respiratory failure, have also been reported.141
Phosphodiesterase-type 5 inhibitors
Recommendation grade: reserved (not approved)
There is adequate evidence to support the efficacy of sildenafil and tadalafil (Level 1). However, they are not approved for BPH in Japan.
Phosphodiesterase-type 5 (PDE5) inhibitors block degradation of cGMP and increase nitric oxide activity. Relaxation of the smooth muscle of the urethra and prostate is also mediated by nitric oxide,146 so we can expect PDE5 inhibitors to ameliorate LUTS.
In a placebo-controlled RCT in men with ED and LUTS, sildenafil (n= 189) significantly improved IPSS, QOL scores and the BPH impact index in comparison to placebo (n= 180).147 No significant change was seen in urinary flow rates, however. Another small-scale RCT did see a significant improvement in urinary flow rates.148 An RCT with three treatment arms, alfuzosin 10 mg daily, sildenafil 25 mg daily, and combination therapy, found significant improvements in IPSS and ED in the combination therapy group in comparison with the monotherapy groups.149 A large-scale RCT was undertaken in which patients with BPH and LUTS were allocated to placebo (n= 211), tadalafil 2.5 mg daily (n= 208), tadalafil 5 mg daily (n= 212), tadalafil 10 mg daily (n= 216), or tadalafil 20 mg daily (n= 209). Significant improvement in the IPSS was seen at all dosages in comparison to placebo, and the optimum dosage of tadalafil was determined to be 5 mg daily.150 Urinary flow rates improved, although the difference was not significant. Another RCT confirmed the efficacy of tadalafil, with significant improvement in symptoms in patients with ED and moderate to severe symptoms of BPH.151,152 In a crossover trial with 27 patients comparing tamsulosin (0.4 mg/day) monotherapy with tamsulosin plus tadalafil (20 mg/day) combination therapy (both for 45 days), significant improvement in the IPSS and QOL score was seen in the combination therapy group. However, no difference was seen between the groups in maximal urine flow or PVR volume.153 Safety was excellent in both trials. These agents are not approved for BPH in Japan.
5 Conservative therapies
Conservative therapies include lifestyle modification, watchful waiting, and supplements.
Lifestyle modification
Recommendation grade: B
There is adequate evidence supporting the efficacy of this treatment (Level 2). Invasiveness is almost completely absent and the financial burden is low.
Recommended lifestyle modifications include: (i) the provision of education and reassurance (e.g. an explanation of bladder and prostate physiology, reinforcing the fact that it is not malignant, inviting patients to attend educational services); (ii) the restriction of excessive fluid intake (avoiding over-consumption of water, limiting intake of coffee and alcohol);154 (iii) bladder training, prompted voiding; and (iv) other (avoiding spicy foods, keeping bowels regular, regular exercise, avoiding sitting for extended periods and chills of the lower body, provision of information about medications that affect micturition).4,155
Watchful waiting
Recommendation grade: B
The evidence to support the usefulness of this approach is inadequate (Level 3). However, treatment may be unnecessary for patients with BPH with no symptoms or complications, and there are few disadvantages to not intervening early with appropriate follow-up.
Although there have been no studies providing clear evidence for this approach, even if BPH is present but the symptoms are mild, there are no complications, and prostatic cancer has been excluded, then aggressive treatment may not be indicated. Some form of lifestyle modification is provided when watchful waiting is the approach taken with patients with mild symptoms.156,157 Annual assessment using the IPSS is recommended during the period of watchful waiting.8 With long durations of watchful waiting, 85% of patients remain stable after 1 year, dropping to 65% after 5 years.7,8 The degree of bothersomeness of symptoms and PVR volume have been identified as predictive factors for the cessation of watchful waiting.43,158
Supplements
Recommendation grade: C2
Evidence to support this approach is lacking (Level 5), and inconsistent (Level 1). Safety is uncertain, and the cost to patients is high.
Most health foods and alternative remedies are handled as over-the-counter medications. Most are sold as combinations of multiple active ingredients, and the influence of the combinations on efficacy and safety is unknown. Costs are borne by patients, as these medications are not covered by medical insurance.
6 Other treatments
Indwelling catheterization
Recommendation grade: reserved
Although an indwelling catheter allows urine to pass out of the bladder, complications and impairment of QOL are common. Indwelling catheterization is indicated only if other treatments are impractical (Level 5).
Indwelling catheterization is performed in cases of urinary retention or severe difficulty with urination,70 or when other treatments for BPH are impractical. Indwelling catheterization is associated with an increased risk of urethral trauma, decreased QOL, UTI and bladder stones.159 An alternative is insertion of a suprapubic catheter (cystostomy).4
Intermittent catheterization
Recommendation grade: B
There is evidence for the superiority for intermittent catheterization compared with indwelling catheterization in terms of preventing UTI and the early recovery of bladder function following surgery for urinary retention (Level 2).
With intermittent catheterization QOL is considered to be superior to indwelling catheterization.4 In an RCT controlled against an indwelling catheter group (n= 40), the incidence of symptomatic UTI was significantly lower in the intermittent catheterization group (n= 40).160 In a study conducted with patients with an indwelling catheter due to chronic urinary retention, compared with a group who underwent TURP as soon as their renal function recovered (n= 17) and a group who underwent the same procedure after changing over to intermittent catheterization (n= 24), the bladder function recovered significantly earlier in the latter group.161
Surgical treatment (surgical therapy)
Summary
Surgical treatment for BPH is indicated in cases of: (i) insufficient response to medical therapy; (ii) the presence of moderate to severe symptoms; and (iii) the presence of (or concern about) comorbidities, such as urinary retention, UTI, hematuria, and bladder stones. Surgical modalities are divided into three groups: (i) resection/ablation or vaporization; (ii) thermal coagulation; and (iii) other techniques. Resection/ablation or vaporization is generally more effective, but may be more often associated with perioperative complications. Although its precise definition is ambiguous, minimally invasive surgical treatment appears to refer to techniques that are less invasive than an open prostatectomy and TURP. The selection of surgical techniques depends on the features of BPH as well as patients' characteristics, the availability of equipment, and the surgeon's experience. The recommendation grade for each of the techniques described was determined in comparison with TURP, the standard surgical procedure for BPH.
Open surgery (enucleation of the adenoma)
Recommendation grade: B
This classic technique may be associated with a high incidence of perioperative complications, but provides sustained efficacy, especially for large prostates.
The enlarged adenoma is detached manually from the surrounding prostatic tissue (surgical capsule) via an incision made on the bladder or directly on the prostate capsule. This procedure is used as a reliable and effective treatment, especially for large BPH.8,162 The technique is associated with a relatively high incidence of complications, including hemorrhage requiring blood transfusion (8.2–25.6%), reoperation (1.1%),163 surgical wound infection (2–6.9%), UTI (2.6–8.6%), and sepsis (8.6%).164 The postoperative re-treatment rate is generally low.165
TURP
Recommendation grade: A
TURP is the standard, most extensively performed surgical technique for the treatment of BPH. It is usually applicable to BPH of up to moderate size (<50–80 mL) and provides a sustained effect. Complications include hemorrhage and hyponatremia from irrigation fluids (TUR syndrome).
With a transurethrally inserted endoscope, the adenoma is resected by a loop electrode with a high-frequency current. Electrolyte-free irrigation fluids are used to secure a clear view. This procedure is technically established and is regarded as the standard for BPH of moderate size (<50–80 mL) with a high efficacy and superiority to any of the transurethral surgical techniques developed up until the 1990s.8,162,166 Complications of TURP include hemorrhage requiring blood transfusion (2.0–4.8%) and hyponatremia resulting from the absorption of irrigation fluid (0–1.1%).167 The efficacy is sustained over the long term: in an 8-year follow-up survey on more than 20 000 men, the re-resection rate was only 7.4%.168
Transurethral incision of the prostate (TUIP)
Recommendation grade: B
This technique involves cutting the prostate at the 5 and 7 o'clock positions of the bladder neck to open the prostatic urethra. It is applicable to relatively small-sized prostates (<20–30 mL).
The bladder wall and the prostate are cut from the ureteral orifice to the verumontanum with a depth reaching the prostatic capsule.169,170 An RCT has demonstrated that TUIP is as effective as TURP for relatively small BPH (<20–30 mL) without middle lobe hyperplasia.171–173 TUIP has been associated with a shorter operation time, comparable short-term (up to 12 months) efficacy, and a lower incidence of complications, such as hemorrhage requiring blood transfusion and retrograde ejaculation.172 However, sustainability is inferior to that following TURP, with a reported reoperation rate for TUIP of 7.6–9.6% over 4–5 years.166,172
Bipolar transurethral resection of the prostate in saline (bipolar TURP)
Recommendation grade: A
This technique is as effective as conventional TURP, with a lower incidence of hyponatremia.
Bipolar TURP is similar technically to conventional TURP, except that it uses saline as the irrigation fluid and the endoscope sheath as the return current collector (i.e. a bipolar electrode).174–179 Numerous RCT of BPH of 40–55 mL have shown there is a comparable operation time and efficacy between bipolar TURP and conventional TURP,180–189 with a significantly lower incidence of hyponatremia for the former.180,183,185,188 Long-term follow-up results, including the duration of effect, are still awaited.190,191
Holmium laser enucleation of the prostate (HoLEP)
Recommendation grade: A
HoLEP is applicable regardless of PV and there is sufficient evidence for its effectiveness and the sustainability of efficacy. It is comparable to open surgery or TURP in any aspect, including complications.
A holmium laser is irradiated through an endoscopic channel to either resect a prostatic adenoma or to detach an adenoma from the surgical capsule for enucleation (HoLEP). The holmium laser is readily absorbed by water and is capable of cutting, coagulating, and producing shock waves depending on the distance to the target tissue. Normal saline can be used as the irrigation fluid.192–201
Numerous RCT have compared HoLEP and TURP202–209 and have demonstrated that HoLEP is associated with a longer operation time (62.1–94.6 vs 33.1–73.8 min for HoLEP vs TURP, respectively),204,206,208 less hemorrhage, and significantly a shorter duration of catheterization and hospitalization.203,204 In four RCT of HoLEP compared with open surgery for large prostates (113–124 mL),210–213 HoLEP exhibited comparable efficacy and significant superiority to open surgery in terms of blood transfusion rate and the duration of catheterization and hospitalization. The long-term re-treatment rate following HoLEP did not differ significantly compared with TURP or open surgery.192,203 HoLEP can be performed safely in men with a large prostate (>100 mL) and those that are on anticoagulants.193–195 Some reports suggest a high incidence of postoperative urinary incontinence, ejaculation disorders, and urethral stenosis following HoLEP.193,214
Photoselective vaporization of the prostate by KTP laser (PVP)
Recommendation grade: B
This technique is associated with a low risk of hemorrhage and can be performed safely even on large prostates. There is sufficient evidence of the effectiveness and sustainability of laser vaporization of the prostate, although tissue sampling is impossible, unlike TURP.
A KTP or holmium laser is delivered endoscopically to vaporize the adenoma.215–223 Three RCT investigating the effects of PVP compared with TURP have demonstrated that PVP is as effective as TURP and is associated with less hemorrhaging and a shorter duration of catheterization and hospitalization.224–226 PVP may require a longer operation time than TURP when applied to large prostates.225 An RCT comparing PVP with open surgery in men with prostates >80 mL showed that although PVP required a longer operation time (80 vs 50 min, respectively), it was superior to open surgery with respect to the blood transfusion rate (0 vs 13.3%, respectively) and duration of catheterization and hospitalization, with no significant differences between the two techniques with regard to any other complications.227 With regard to long-term outcome, PVP may be associated with a relatively high reoperation rate: 10.4% for prostates <80 mL and 23% for prostates >80 mL.219 A histological examination is not feasible because the prostate tissue is vaporized.
Transurethral enucleation with bipolar system
Recommendation grade: C1
This technique consists of the transurethral detachment and enucleation of the adenoma without a laser. It may be effective regardless of PV, but has not been evaluated sufficiently in comparison with other treatment options or in terms of long-term outcomes.
Adenoma detachment is achieved with a resectoscope or a special loop through the bipolar system,228 or with a special detachment device through conventional TURP.229,230 The detached adenoma is either resected without enucleation, as in TURP, or enucleated and then minced and collected with a morcellator, as in HoLEP. A 3-year RCT comparing this technique with TURP reported comparable efficacy in both.231
Interstitial laser coagulation of the prostate (ILCP)
Recommendation grade: C1
This treatment is as effective as TURP and is feasible, with rare serious adverse reactions. Benefits are not sustained, however, with further treatment or re-intervention required in almost half of all patients in long-term follow-up.
ILCP delivers laser energy (from a neodymium : yttrium aluminum garnet or diode laser), via an applicator, into the prostate to produce coagulation necrosis within the adenoma, sparing its urethral surface. Although TURP results in greater improvements in Qmax, RCT have found that ILCP and TURP are comparable in terms of improvements in LUTS and QOL.232,233 Good, long-term clinical outcomes following ILCP have also been reported.234,235 The most common post-treatment adverse events are transient urinary retention and irritative symptoms. ILCP had a minimal impact on sexual function. Subsequent surgical interventions occurred at a rate of 3–16% within 1 year after the primary treatment.232,236 Over longer follow-up periods, reported rates of re-interventions, including drugs and surgery, are 30–50%.234,235
Transrectal high-intensity focused ultrasound (HIFU)
Recommendation grade: C1
There have been few reports of the efficacy and safety of HIFU. Although the safety profile is relatively favorable, further treatment or re-intervention is required in roughly half of patients in long-term follow-up.
HIFU irradiates highly focused ultrasound transrectally into the prostate to create coagulation necrosis. Several clinical studies have reported that HIFU significantly improves symptoms, QOL, and Qmax.237–239 HIFU was comparable to transurethral microwave thermotherapy (TUMT) and transurethral needle ablation (TUNA) in improving LUTS over a 2-year follow-up period.240 Common post-treatment adverse events following HIFU include transient urinary retention, hematuria, and hematospermia.8 Thermoinjury of the rectum requiring surgical repair is an uncommon severe adverse event.8 The re-intervention rate ranged between 44 and 58% 2–4 years after HIFU.241,242
Transurethral needle ablation (TUNA®)
Recommendation grade: C1
Symptomatic improvement is achievable with TUNA as much as with TURP in the short to medium term with an increased risk of postoperative irritability and urinary retention. Re-treatment, including surgery, is required in nearly half of cases.
TUNA therapy uses low-level radiofrequency energy delivered via needles into the prostate to induce necrotic lesions in the adenoma, thereby reducing BOO. Several clinical studies have reported significant improvements in the symptom scores (an average reduction rate of 40–70%) and Qmax (26–126%) over both short-term243–245 and long-term246 follow-up periods. In RCT with 12–18 months follow-up comparing TUNA and TURP, no significant differences were found between the two in terms of improvements in symptom and bother scores.246,247 Another RCT comparing TUNA and TURP demonstrated stable treatment outcomes over a 5-year follow-up period.248 Common post-treatment adverse events associated with TUNA include transient urinary retention and worsening storage symptoms in the early post-treatment period.249,250 Reported treatment failure rates requiring subsequent further treatment, including pharmacotherapy and surgical intervention, are of the order of 21–39% over 2–5 years.241,246
Transurethral microwave therapy (TUMT)
Recommendation grade: B
This is the most extensively verified minimally invasive surgical treatment. Medium-term efficacy has been demonstrated with a high-energy deliver system, even for patients with large prostatic volumes or urinary retention. Perioperative and postoperative safety is superior to TURP, although less than a half of patients require re-intervention.
TUMT uses a special transurethral catheter with a microwave antenna to deliver microwaves into the prostate to produce coagulation necrosis and subsequent improvement in BOO. Numerous studies have been published on TUMT using various devices or technical specifications under different treatment protocols. In one clinical trial, TUMT therapy using the first-generation device Prostasoft® 2.0 was less effective than TURP in reducing LUTS.251 However, TUMT, performed with high-energy delivery systems, including Prostatron® (Prostasoft 2.5 and 3.5),252–254 TherMatrx®, Targis system®, and CoreTherm®,255–257 was comparable to TURP in terms of improving LUTS and QOL over short-term and long-term follow-up periods, although TURP resulted in a greater improvement in Qmax than TUMT.252 Arguments against TUMT as an alternative to TURP include the morbidity associated with TUMT,256 including prolonged catheterization periods,258 a higher incidence of dysuria or urgency,259 and urinary retention.260 Conversely, the incidences of hematuria or clot retention,261 blood transfusions, hyponatremia, ED, retrograde ejaculation,261–263 and urethral stricture are less for TUMT than TURP. The re-treatment rate (surgery or pharmacotherapy) after high-energy TUMT is reported to be 3–6% within 1 year.264,265 Over longer follow-up periods (up to 5 years), 10–40% of patients required re-intervention.257,266–268
Urethral stent
Recommendation grade: C1
Although the procedure is quickly efficacious with minimal invasiveness, urethral stenting may be associated with complications that require stent removal. It is indicated in high-risk patients, in whom surgical interventions or other invasive therapies are contraindicated.
Prostatic stents are placed in the prostatic urethra under endoscopic control to dilate the obstructed urethra. Temporary or permanent stents can be used. Long-term data are available regarding the safety and efficacy of permanent urethral stents.269 As their name suggests, temporary stents are used for the treatment of urinary retention after TUMT for short periods.270,271 The clinical use of permanent stents is limited by significant complications, including encrustation (calcification), discomfort or urethral pain, bleeding, and the migration of the stents.272–275
Transurethral ethanol ablation of the prostate (TEAP)
Recommendation grade: reserved (not approved)
The efficacy of TEAP has been demonstrated for symptoms and urinary flow rates, although few long-term studies with large numbers of participants and no RCT comparing with standard therapies are available. It is not covered by medical insurance in Japan.
As part of the TEAP procedure, anhydrous ethanol is injected under urethroscopic control into the prostate. The injected ethanol induces coagulation necrosis and a reduction in tissue volume. Several clinical studies have demonstrated significant improvements in symptom scores, QOL, and Qmax over both short-term and long-term follow-up periods.276–278 Common post-treatment adverse events associated with TEAP include urinary retention, hematuria, and irritative symptoms.276,278–280 Bladder necrosis requiring urinary diversion has been reported in two cases.276 The re-intervention rate ranges from 7% after 1 year276 to 40% after 3 years.277 RCT comparing TEAP with standard surgery are required before this procedure can be considered a reasonable alternative treatment for BPH.
Botulinum toxin injection
Recommendation grade: reserved (not approved)
There is adequate evidence to support its efficacy, although its long-term efficacy is uncertain. Botulinum toxin is not approved in Japan for BPH.
Botulinum toxin inhibits ACh release at the neuromuscular junction, causing the paralysis and atrophy of striated and smooth muscles. It also inhibits ganglionic and postganglionic fibers of the autonomic nervous system, inducing tissue atrophy and apoptosis.281 Through the latter mechanism, direct intra-prostatic injections of botulinum toxin can be expected to be beneficial in patients with BPH.
The usefulness of intra-prostatic botulinum toxin injections is suggested in studies with patients with BPH in a poor general condition,282,283 and in an open labelled study with 77 patients.284 Their results showed 2 months post-injection a 63.9% decrease in the IPSS, a 51.6% decrease in PSA, a 42.8% decrease in prostatic volume, a 55.9% decrease in PVR volume, and a significant improvement in urinary flow rates. An RCT that allocated patients refractory to pharmacotherapy to a continued pharmacotherapy (n= 30) or botulinum toxin therapy (n= 30) reported decreased prostatic volumes and improvements.285
BPH treatment and sexual function
Summary
Treatments for BPH may impact adversely on sexual function. Surgical interventions often result in ejaculatory dysfunction; α1-adrenoceptor antagonists sometimes cause ejaculatory dysfunction; and the use of 5α-reductase inhibitors and anti-androgens is associated with multiple sexual dysfunctions, with the frequency being higher for the latter.
Sexual dysfunction related to surgery
Ejaculatory dysfunction is the most common sexually related adverse event following surgery. The frequency of ejaculatory dysfunction has been reported to be 80% following open surgery, 65–70% following TURP,7,286 70–80% following HoLEP or holmium laser ablation of the prostate,8,287 and 40% following TUIP.8 The frequency of ejaculatory dysfunction following thermotherapy has been reported to range between 6.1 and 51.4%.288,289 In contrast, ED following surgery is relatively rare, with rates of 12.5, 2–10, and 0% reported for open surgery, TURP, and HoLEP, respectively.7,286,287,290
Sexual dysfunction related to medical treatment
Erectile dysfunction (ED)
RCT have shown that ED is not caused by α1-adrenoceptor antagonists7 and that these antagonists may even reduce the risk of ED.291,292 The rates of ED have been reported to be 8.1–30.9% for patients on finasteride8,293 and the rate of ED was significantly greater for men on dutasteride than on placebo (4.7 vs 1.7%, respectively) during the first 6 months of treatment.294 In a Japanese RCT evaluating dutasteride, the frequency of ED was 2% compared with <1% in the placebo group.286 Other studies have reported rates of ED of 6.96–53.7% in Japanese men on anti-androgens.121,295
Ejaculatory dysfunction
The rate of ejaculatory dysfunction in men on α1-adrenoceptor antagonists has been reported to be 0.4–30%,7,111,296,297 with the frequency higher in men using α1A-adrenoceptor antagonists. In men on 5α-reductase inhibitors, the rate of ejaculatory dysfunction has been reported to range between 2.1 and 4.4%39,116 and anti-androgens have been reported to cause ejaculatory dysfunction in as many as 50% of Japanese men using these drugs.118
Decreased libido
Decreased libido is associated with 5α-reductase inhibitors, with reported rates of 1.0–7.7%,7,293,298 but not with α1-adrenoceptor antagonists.7 Anti-androgens have been reported to decrease libido in 1.5% of Japanese patients with BPH.118
Clinical study
This section details the standard criteria necessary for any clinical study for BPH.
Main inclusion and exclusion criteria
Inclusion criteria
- •
Men aged ≥50 years (can be ≥40 years)
- •
Clinical signs and symptoms of BPH
- •
Moderate to severe LUTS (i.e. IPSS ≥8)
- •
QOL disturbance due to LUTS (i.e. QOL score ≥2)
- •
Prostate enlargement (i.e. PV ≥20 mL)
- •
Suspected lower urinary tract obstruction (i.e. Qmax <15 mL/s)
Exclusion criteria
- •
Suspicion or presence of diseases other than BPH
- •
Persisting effects of pre-treatments for BPH†
- •
A history of any diseases affecting lower urinary tract function
- •
Conflicts with the characteristic of the treatment
Note: †Patients on BPH medications should stop treatment before enrollment into a study (12 months for 5α-reductase inhibitors and anti-androgens, 4 weeks for α1-adrenoceptor antagonists and anticholinergics). Those using gonadotropin-releasing hormone agonists and antagonist should be excluded.
Severity of BPH
Despite a high prevalence of BPH, standard international diagnostic criteria have not been established for the disease or its severity. To this end, we recommend the Japanese criteria.299 Briefly, the severity of BPH is evaluated in four domains: (i) symptoms; (ii) QOL; (iii) function; and (iv) anatomy. The items in these four domains are assessed using the IPSS, QOL index, uroflowmetry (Qmax, PVR), and transrectal ultrasonography (PV), respectively. As indicated in Tables 6 and 7, the severity of BPH is divided into three categories: mild, moderate, and severe. These categories are useful as a common scale of the severity of BPH in clinical studies.
| Measure | Domains used to assess severity of BPH | ||||
|---|---|---|---|---|---|
| Symptoms | QOL | Function | Anatomy | ||
| IPSS | IPSS QOL index | Qmax (mL/s) | PVR volume (mL) | Prostate volume (mL) | |
| Severity of BPH | |||||
| Mild | 0–7 | 0, 1 | ≥15 | <50 | <20 |
| Moderate | 8–19 | 2–4 | ≥5 | <100 | <50 |
| Severe | 20–35 | 5, 6 | <5 | ≥100 | ≥50 |
- IPSS, international prostate symptom score; PVR, postvoid residual urine; Qmax peak urinary flow rate; QOL, quality of life index.
| Overall severity | No. of domains in Table 6 evaluated as: | ||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Mild | 4, 3 | 0, 1 | 0 |
| Moderate | Other combinations | ||
| Severe | Any | Any | 2, 3, 4 |
In addition, the severity of symptoms in BPH can be measured using the OABSS300 and CLSS.98
Efficacy of treatments in BPH
Standard international criteria to determine the efficacy of treatments for BPH have not been established. Again, we strongly recommend the use of the Japanese criteria.301 Briefly, the efficacy of treatment for BPH is also evaluated in four domains: (i) symptoms; (ii) QOL; (iii) function; and (iv) anatomy. The efficacy of treatment is determined as excellent, good, fair, or poor and is assessed using clinical measures for: (i) symptoms (ratio of post-treatment : pre-treatment IPSS); (ii) QOL (ratio of post-treatment : pre-treatment QOL); (iii) function (ratio of post-treatment : pre-treatment Qmax); and (iv) anatomy (ratio of post-treatment : pre-treatment PV [see Table 8]). The overall efficacy is determined as the median of efficacy for three domains, symptoms, QOL, and function.
| Measure | Domains used to assess efficacy with respect to | |||
|---|---|---|---|---|
| Symptoms | QOL | Function | Anatomy | |
| IPSS post/pre | QOL pre-post | Qmax(mL/s) post-pre | PV post/pre | |
| Efficacy | ||||
| Excellent | ≤0.25 | ≥4 | ≥10 | ≤0.5 |
| Good | ≤0.50 | 3 | ≥5 | ≤0.75 |
| Fair | ≤0.75 | 2, 1 | ≥2.5 | ≤0.9 |
| Poor | >0.75 | ≤0 | <2.5 | >0.9 |
- Standard criteria for therapeutic efficacy. The overall efficacy is determined as the median of efficacy for three domains: symptoms, quality of life (QOL) and function. PV, prostate volume; Qmax peak urinary flow rate.
In addition, to evaluate the efficacy of treatment on symptoms of BPH, changes in the OABSS and CLSS after treatment can be used, with particular emphasis on one of the symptoms of BPH (e.g. nocturia).302 The efficacy of treatment on QOL can be assessed using changes in the BPH impact index, King's health questionnaire, and SF-36, although the criteria for efficacy using these scales have not yet been standardized.
Other recommendations
The points listed below should be attended to in Phase 3 studies of the efficacy of medical treatments for BPH:
- •
Double-blind RCT using placebos or standard treatments as the control group are recommended.
- •
Although the primary endpoints should be evaluated at 3 months, patients should be monitored for efficacy and safety for up to 12 months to assess the duration of the clinical effect of the drugs (the objective of which is to decrease PV).
CQ 1: When is a bladder diary recommended as part of the assessment of BPH?
A bladder diary is recommended for men with daytime or nocturnal frequency (Grade B). The diary records individual voiding prospectively, enabling the accurate evaluation of voiding time, individual volumes voided, and total urinary volume. This information is useful for the differential diagnosis of urinary frequency, which can be classified as a decrease in the volume voided, polyuria, or both.4,300 Ideally, the diary should be kept over a period of 3–7 days, although keeping the diary over 1 or 2 days may be sufficient.8
CQ 2: What examination is recommended for the anatomical evaluation of the prostate?
Ultrasonography is recommended for the anatomical evaluation of the prostate (Grade A). Compared with a digital rectal examination and other imaging tests, ultrasonography is more accurate and minimally invasive.4,101,303 Trans-abdominal ultrasonography is easily performed and is readily able to detect bladder pathology, whereas trans-rectal ultrasonography permits the detailed imaging of the inner structures. The type of ultrasonography performed depends on the equipment available, as well as on the objective of the examination. PV is predictive of both clinical progression and the therapeutic outcomes of surgical and medical treatment.303,304
CQ 3: When and how is evaluation of the upper urinary tract recommended?
Evaluation of the upper urinary tract is not to be performed routinely. It is recommended for men with abnormal urinalysis, a large amount of PVR, renal insufficiency, or a history of other urological diseases (Grade B). In these cases, ultrasonography is recommended as the initial method of assessment.4,303 Renal ultrasonography in 556 men with BPH detected hydronephrosis, renal cysts and renal cancer in 2.5, 11.7% and 0.18% of men, respectively.305
CQ 4: What considerations are recommended when assessing serum PSA values?
Serum PSA concentrations should be determined because higher PSA concentrations are indicative of prostatic cancer or enlarged PV.4,303,306 Serum PSA concentrations are increased in men with enlarged adenoma, prostate cancer, urinary retention, and prostatitis,4,303,306 but can be reduced to approximately 50% by long-term treatment with anti-androgens or 5α-reductase inhibitors100,294,303 (Grade A).
CQ 5: Is long-term therapy with α1-adrenoceptor antagonists recommended?
The efficacy and safety of α1-adrenoceptor antagonists up to 1 year has been reported in many studies. However, there is a relative paucity of long-term data over 3 years regarding the maintained efficacy of these drugs (Grade B). Most long-term studies into the efficacy of α1-adrenoceptor antagonists are open-label extensions of previous short-term trials or retrospective studies in real-life clinical practice. The study designs are not consistent.
There are eight long-term studies (over 3 years; range 4–10 years) for the treatment of α1-adrenoceptor antagonists in the literature.307–314 During follow-up, approximately 18, 64, and 36–80% of patients withdrew from the studies after 2, 3, and >4 years, respectively. The reasons for their withdrawal included an insufficient therapeutic response (14–54%), loss to follow-up for unknown reasons (16–48%), satisfaction with their condition (1–8%), the detection of prostate cancer (4–54%), adverse effects (1–17%) and urinary retention (3%).307,308,310–313 Eight percent of patients with treatment failure stopped medication or changed to other drugs, and 8–25% underwent surgery. The risk factors for treatment failure were severe LUTS, low urinary flow rate, large prostate (>30–40 mL), large PVR or a history of urinary retention, concomitant OAB symptoms, urodynamically proven BOO, and insufficient effects after using short-term therapy.311–314 Recently, the results of two long-term (4-year) RCT comparing the effects of 5α-reductase, an α1-adrenoceptor antagonist, and combination therapy on clinical progression in BPH/LUTS and prostatic enlargement have been reported (see CQ 7).46,315
CQ 6: Is combination therapy with α1-adrenoceptor antagonists and anticholinergics recommended for men with OAB?
There is adequate evidence supporting the efficacy and safety of combination therapy with α1-adrenoceptor antagonists and anticholinergics for BPH associated with OAB (BPH/OAB; Grade A).
For male OAB symptoms, monotherapy with α1-adrenoceptor antagonists is effective and may be a first-line treatment,4 although the efficacy of α1-adrenoceptor antagonists is limited for patients with DO.316 The efficacy and safety of anticholinergic monotherapy have also been confirmed in the treatment of BPH/OAB.140 In a placebo-controlled RCT comparing tamsulosin, tolterodine ER, and their combination in men with BPH or OAB, the benefits were significantly greater in the combination therapy group, with only a mild increase in PVR volumes and a 1% incidence of urinary retention.139 Combined therapies with anticholinergics and α1-adrenoceptor antagonists can be effective in cases in which α1-adrenoceptor antagonists were ineffective in improving storage symptoms, with urinary retention being rare.317–320 However, it should be noted that most of these studies were conducted in Caucasian men, with strict exclusion criteria, specialist supervision, and relatively short-term observational periods. There remains a concern about exacerbation of voiding difficulties and possible urinary retention with a more widespread and longer use of anticholinergics with or without α1-adrenoceptor antagonists in the practical setting.4
Note: Recently two Japanese studies reported that combination therapies with tamsulosin plus anticholinergics are more effective for BPH and OAB than tamsulosin monotherapy, with lower doses of anticholinergics associated with better outcomes.321,322
CQ 7: Is combination therapy with an α1-adrenoceptor antagonist and a 5α-reductase inhibitor recommended?
Combination therapy is recommended for relatively severe disease, e.g. prostatic volume ≥30 mL (Grade B). The CombAT study randomly assigned 4844 patients with BPH (prostatic volume ≥30 mL, 1.5 ≤ PSA ≤ 10 ng/mL, 5 ≤ maximal urine flow ≤15 mL/s) to three groups and administered them with either dutasteride, tamsulosin or combination therapy for 4 years.315 The average change in the IPSS score was −6.3 points in the combination therapy group, which was significantly greater than that of −3.8 points in the tamsulosin group and −5.3 points in the dutasteride group (both P < 0.001). The increase in maximal urine flow of 2.4 mL/s seen in the combination therapy group was also significantly greater than that in the tamsulosin group (0.7 mL/s, P < 0.001) and the dutasteride group (2.0 mL/s, P < 0.05). The cumulative incidence of clinical progression was 12.6% in the combination therapy group, 21.5% in the tamsulosin group and 17.8% in the dutasteride group (both P < 0.01 for time until progression). The cumulative incidence of acute urinary retention or surgical intervention for BPH was 4.2% in the combination therapy group, 11.9% in the tamsulosin group and 5.2% in the dutasteride group, with a significant difference between the combination therapy group and the tamsulosin group for time until intervention (P < 0.001), but with no significant difference between the combination therapy and dutasteride groups (P= 0.18).
A study with 327 patients with BPH (using criteria similar to the above study) administered tamsulosin plus dutasteride combination therapy to all patients for 24 weeks, then randomly assigned patients either to continue combination therapy or to cease tamsulosin (replacing it with placebo) and take dutasteride monotherapy for 36 weeks.323 As a result, for the 82 patients with a pre-treatment IPSS ≥20, the aggravation of symptoms was reported by 14% of the continued combination therapy group (n= 42), and 42.5% of the dutasteride monotherapy group (n= 40) (P-value unknown).
A study of the 5α-reductase inhibitor, finasteride (not indicated for BPH in Japan), the Medical Therapy of Prostatic Symptoms study randomly assigned 3047 patients with BPH (IPSS ≥ 8 points, 4 ≤ maximal urine flow ≤ 15 mL/s) to one of four groups: placebo, doxazosin (α1-adrenoceptor antagonist), finasteride or combination therapy. They monitored clinical progression (IPSS, urinary retention, impaired renal function, recurrent UTI) over a mean follow-up period of 4.5 years.46 In comparison with the placebo group, the risk of clinical progression in the doxazosin, finasteride and combination therapy groups was reduced by 39% (P < 0.001), 34% (P= 0.002), and 66% (P < 0.001), respectively. The risk reduction seen in the combination therapy group was significantly greater than that in either monotherapy group (both P < 0.001). The cumulative rates of progression were 17% in the placebo group, 10% in the doxazosin and finasteride groups, and 5% in the combination therapy group. We can also expect an additive effect from combination therapy in Japanese patients, from similar results in the Asian sub-population as in all patients in the CombAT study,324 and from a similar (additive) effect for dutasteride, regardless of whether or not the patients had previously been administered tamsulosin, in a sub-analysis of a Japanese Phase III trial.100 We await the results of future trials conducted with Japanese patients, including examinations of cost effectiveness.
CQ 8: What urodynamic test is recommended for men undergoing surgical treatment for BPH?
It has been reported that 25–30% of men undergoing prostatectomy have an unfavorable outcome.325,326 BOO, DU, and DO are all important prognostic variables for the surgical outcomes of BPH.327 Symptom improvement is less likely for men with no or equivocal BOO compared with men with evident BOO.328,329 Both DU without BOO and DO without BOO strongly predict treatment failure for TURP.330,331 A higher degree of BOO without DO and/or DU is associated with improvements in both symptoms and QOL.332 Thus, urodynamic examinations, including pressure-flow studies and cystometry, are recommended to delineate BOO, DU, and DO (Grade B).329,333 In contrast, there is a view that the information obtained from pressure-flow studies does not improve the surgical outcome sufficiently to justify the cost and invasiveness of the procedure.334 Predicting BOO using simpler parameters, such as uroflowmetry and PV, may be a viable alternative.335
CQ 9: What measures are recommended for persistent symptoms after surgical treatment (predominantly TURP)?
Appropriate treatments should be selected after evaluating possible causes other than BOO using urodynamic studies, including pressure-flow studies and recording a frequency–volume chart (Grade B). DO induced by BOO generally improves postoperatively, but DO without accompanying BOO often persists after surgery331 or DO may arise independently as a result of the surgery.336 DU is present in 20–30% of men with LUTS,337 and the surgical outcome for these patients is poor.327 In a long-term postoperative study,336 BOO recurred in only 12.4% of patients treated with TURP, whereas DU was present in 36.5% of the men complaining of LUTS after surgery. Nocturia is a symptom with low specificity for BPH338 that is often caused by polyuria. Thus, postoperative recurrence of LUTS is not necessarily attributable to BOO, but rather to overlooked or developing DO, DU, or polyuria.336
CQ 10: What foods and dietary habits are recommended for patients with BPH?
There is a known relationship between dietary habits and the future risk of surgical intervention for BPH (increased by cereals and meat, decreased by vegetables) (Grade C1). In a study that compared 6092 patients who had either undergone surgery for BPH or had an IPSS ≥15 with 7800 patients with an IPSS of 8–14, a negative correlation was seen between vegetable consumption and the severity of BPH (no significant correlation was seen with fruit). Looking at individual nutrients, a negative correlation was seen between the consumption of fruit and vegetables rich in β-carotene, lutein and vitamin C and BPH.339 A study comparing 1369 patients who underwent surgery for clinically diagnosed BPH with 1451 controls found a negative correlation between the consumption of onion and garlic and BPH.340 A similar controlled trial found that the consumption of starch increased and that of polyunsaturated fatty acids decreased the risk of BPH.341 Another study found that a diet high in cereals and meat and low in vegetables and legumes increased the risk of BPH.342 A comparison of dietary habits over the previous 10 years in 406 patients with surgically treated BPH and 462 controls found a significant negative correlation between the consumption of vegetables, tofu (bean curd) and red meat and the risk of surgery.343 A survey of dietary habits in 1369 patients with surgically treated BPH and 1451 controls found that carotene reduced the risk of surgery, vitamin C and iron tended to reduce the risk, whereas salt and zinc increased the risk. No correlation was seen between folic acid, lycopene, lutein or zeaxanthin, vitamin D or E, or retinol and the risk of surgery.344
CQ 11: Is a reduced alcohol intake recommended in patients with BPH?
It is preferable that patients with BPH symptoms avoid consuming large amounts of alcohol (Grade B). A survey comparing lifestyle habits in 398 patients with surgically treated BPH and 471 controls found a negative, but not a significant, correlation between alcohol consumption (>30 g/day) and BPH symptoms.345 A study comparing 1369 patients with BPH resistant to pharmacotherapy, with a maximal urine flow ≤15 mL/s, and 1451 controls also found a negative correlation between alcohol consumption and the onset of BPH symptoms. As a possible mechanism, the authors suggested that the hormonal status of consumers of alcohol (e.g. decreased androgen levels) may play a part.346 A study comparing 1813 patients with surgically treated BPH, 1786 patients with IPSS ≥15 points, and 20 840 controls (IPSS ≤7 points) found a negative correlation between moderate alcohol consumption (30.1–50 g/day) and the severity of BPH symptoms, and a weaker negative correlation with high alcohol consumption (>50 g/day).347 A Japanese study with 432 patients found no correlation between prostatic volume and alcohol intake.348 Urinary retention can be triggered by alcohol consumption in patients with BPH.
CQ 12: What treatments are recommended for urinary retention by BPH?
Either the insertion of an indwelling catheter or intermittent catheterization should be indicated. Subsequently catheter removal may be attempted after the administration of an α1-adrenoceptor antagonist. Surgical intervention is likely to be necessary for a large prostate (Grade B). In the short term, catheterization or the insertion of an indwelling catheter will be required in cases of urinary retention. Using intermittent catheterization is recommended only for urinary retention due to transient causes (e.g. the use of anaesthetic or α-sympathomimetic agents), and an α1-adrenoceptor antagonist at the attempt to remove the indwelling catheter.7
A retrospective survey of long-term results in 248 patients in whom indwelling catheters were successfully removed after treatment with an α1-adrenoceptor antagonist following acute urinary retention, with a mean follow-up period of 33 months, reported a failure rate of 11.6, 14.3, 28.4, and 50.5% at 6, 12, 24 and 60 months, respectively.349 Multivariate analysis revealed a prostatic volume ≥50 mL and a PSA level ≥10 ng/mL at the time of acute urinary retention, as predictive factors for surgical intervention.
In a study comparing surgery and pharmacotherapy as treatments to allow catheter removal in 72 patients with an indwelling catheter, multivariate analysis showed that patients with PSA >2.9 ng/mL, a large prostate size on digital rectal examination, and a volume drained at the time of catheterization >1000 mL, were best managed by surgical intervention.350
CQ 13: What measures are recommended for men with symptomatic BPH in whom usual treatments are not to be indicated due to their deteriorating activities of daily life?
Urethral stents, intermittent catheterizaion, and indwelling urethral or suprapubic catheters should be considered as management options for such men (Grade B). The management for BPH is challenging in men with severe symptoms that are resistant to medical therapy or with conditions such as recurrent urinary retention, bladder stones, intractable UTI, and hematuria. Metallic stents can be placed in the prostatic urethra under endoscopic control. Although this is an effective, less invasive procedure for improving symptoms and objective parameters,269–271 the clinical use of permanent stents is limited owing to the associated complications, including encrustation, discomfort or urethral pain, infection, bleeding, and stent migration.8,272,273,351–355 Intermittent self-catheterization is safe and useful with minimal complications.160 It is therefore a reasonable management option for men at high risk of surgical intervention when the voiding difficulties are resistant to medical treatment. However, it requires manual dexterity and may not be a good option for men with severe mental disabilities or disabilities in their upper limbs. Urethral indwelling catheters are useful for prompt management, yet they are associated with inevitable infection, urethral erosion, strictures, and fistula formation. Suprapubic cystostomy is an alternative measure that avoids the complications caused by indwelling urethral catheters.
CQ 14: What therapeutic strategies are recommended to avoid sexual dysfunction as an adverse event?
Surgical treatment or α1-adrenoceptor antagonists are recommended to avoid ED. To prevent ejaculatory dysfunction, surgical treatment, α1A-adrenoceptor antagonists, 5α-reductase inhibitors or anti-androgens should be avoided. To retain libido, 5α-reductase inhibitors or anti-androgens especially should be avoided (Grade B). ED as an adverse event is rare for surgery (0–12.5%),7,287,290 and comparable with placebo for α1-adrenoceptor antagonists.7 Ejaculatory dysfunction has been reported to be 50–80% post-surgery,7,8,286,287 and 1.6% to 22.3% in Japanese men using α1-adrenoceptor antagonists, particularly α1A-adrenoceptor antagonists.111,356 Decreased libido and ejaculatory dysfunction are observed in men taking 5α-reductase inhibitors or anti-androgens,122,294 and are more pronounced in the latter.122
Conflict of interest
Yukio Homma received research grants from Astellas, Asuka, DaiichiSankyo, Ono, Pfizer, and Takeda, and received lecture fees from Astellas and GlaxoSmithKline; Momokazu Gotoh received research grants from Astellas, DaiichiSankyo, GlaxoSmithKline and Takeda, and received lecture fees from Astellas; Osamu Yokoyama received research grants from AsahiKasei, Astellas, GlaxoSmithKline, Ono and Pfizer, and received lecture fees from Astellas, Kissei and Ono; Naoya Masumori received lecture fees from GlaxoSmithKline; Akihiro Kawauchi has no conflict of interest; Tomonori Yamanishi has no conflict of interest; Osamu Ishizuka received research grants from Asahi Kasei; Narihito Seki received research grants from Astellas and Kyorin, and received lecture fees from Astellas; Toshiyki Kamoto has no conflict of interest; Atsushi Nagai received research grants from Kissei and Pfizer; Seiichiro Ozono received lecture fees from Astellas. Funding of the committee was provided by The Japanese Urological Association.
Appendix
Appendix: Abbreviations
| ACh | acetylcholine |
| AR | adrenergic receptor |
| AUA | American Urological Association |
| BOO | bladder outlet obstruction |
| BPE | benign prostatic enlargement |
| BPH | benign prostatic hyperplasia |
| BPO | benign prostatic obstruction |
| CLSS | Core lower urinary tract symptoms score |
| CQ | clinical question |
| DHT | dihydrotestosterone |
| DO | detrusor overactivity |
| DU | detrusor underactivity |
| EAU | European Association of Urology |
| ED | erectile dysfunction |
| HIFU | high-intensity focused ultrasound |
| HoLEP | holmium laser enucleation of the prostate |
| ILCP | interstitial laser coagulation of the prostate |
| IPSS | International Prostate Symptom Score |
| JUA | Japanese Urological Association |
| LUTS | lower urinary tract symptoms |
| OAB | overactive bladder |
| OABSS | Overactive Bladder Symptom Score |
| PSA | prostate-specific antigen |
| PV | prostate volume |
| PVP | photoselective vaporization of the prostate by KTP laser |
| PVR | postvoid residual urine |
| Qmax | peak urinary flow rate |
| QOL | quality of life |
| RCT | randomized controlled trial |
| TEAP | transurethral ethanol ablation of the prostate |
| TUIP | transurethral incision of the prostate |
| TUMT | transurethral microwave thermotherapy |
| TUNA | transurethral needle ablation |
| TURP | transurethral resection of the prostate |
| UTI | urinary tract infection |




