Japanese guideline for diagnosis and treatment of interstitial cystitis
Abstract
Interstitial cystitis (IC) is a disease of the urinary bladder with lower urinary tract symptoms such as urinary frequency, bladder hypersensitivity and/or bladder pain and resultant serious impairment of quality of life. In Japan, assuming that IC is very rare, research activity and medical care of IC have been sparse until 2001, when the Society of Interstitial Cystitis of Japan (SICJ) and a patient support group were founded.1,2 Subsequently the International Consultation on Interstitial Cystitis Japan (ICICJ) was held in Kyoto in 2003.3 On the other hand, the etiology of IC has not been well clarified, which complicates its diagnosis and treatment at clinical settings. We have thus developed the Japanese Clinical Guideline,4 which is targeted at healthcare professionals including specialists in urology and women's health care who may engage in the diagnosis and treatment of IC. This article is the English translation of a shortened version of the Guideline for convenience of readers worldwide.
Methods
The guideline was developed by the executive members of SICJ and endorsed by the Japanese Urological Association (JUA). The principal source of references was 1350 articles that were searched by the PubMed database in 2005 with the keyword, ‘interstitial cystitis’. Other sources included the ICICJ reports of 2003,3 the International Consultation on Incontinence (ICI) report in 2004,5 and the Guideline for Chronic Pelvic Pain of the European Association of Urology (EAU).6 The grade of recommendation was determined for each treatment by examining the level of evidence, variability of effect, magnitude of effect, clinical applicability, associated mobility and cost. A clinical algorithm was prepared through discussion among the members.
Definition
There is no widely accepted definition of IC.7 The National Institute of Diabetes and Digestive Kidney Diseases (NIDDK) consensus criteria, which were developed to ensure the comparability of patients enrolled in clinical studies,8 would be too restrictive for clinical diagnosis.9 Due to its unclear definition, many terms that refer to IC or IC-like conditions have been proposed:10 interstitial cystitis (IC), painful bladder syndrome (PBS), chronic pelvic pain syndrome (CPPS), IC/PBS (PBS/IC), IC/CPPS (CPPS/IC) and bladder pain syndrome (BPS) are examples. IC has been used since the nineteenth century and is most universally recognized as a disease entity in academic, medico-legal or medico-economical societies. Meanwhile IC may intuitively indicate inflammation in the interstitum, which is not necessarily true.11 PBS is the complaint of suprapubic pain related to bladder filling, accompanied by other symptoms such as frequency, nocturia, in the absence of proven pathologies.12 CPPS refers to a symptom syndrome with chronic pelvic pain.12 BPS is also a pain syndrome proposed by the European Society for the Study of IC/PBS (ESSIC) (http://www.essic.eu/) to replace the term IC. Pain syndromes would be appropriate to refer to symptom syndromes of IC or IC-like conditions, because bladder and/or pelvic pains are characteristic to those conditions. However, they are unacceptable, because a significant proportion of IC patients are without pain and these patients would remain undiagnosed out of the scope of the pain syndromes.13 The term ‘IC/PBS’ inclusively covers diverse patient populations from those meeting NIDDK criteria to those only having PBS. However, this term is a chimera combining a disease name and a symptom syndrome name, and is thus linguistically inaccurate and misleading.
For this guideline, we defined IC as ‘a disease of the urinary bladder diagnosed by three conditions: (i) lower urinary tract symptoms such as urinary frequency, bladder hypersensitivity and/or bladder pain; (ii) bladder pathology proven endoscopically by Hunner's ulcer and/or mucosal bleeding after over-distension; and (iii) exclusion of confusable diseases such as infection, malignancy or calculi of the urinary tract.’ In addition we suggest a term ‘hypersensitive bladder syndrome (HSB)’ for the symptom syndrome associated with IC or IC-like conditions, because hypersensitivity would be more inclusive than pain in that it incorporates both patients with and without pain.
Etiology and pathogenesis
The etiology of IC is mostly unknown and many hypotheses are proposed.
Mast cell activation
Mast cells play a central role in the inflammatory process: they release potent inflammatory mediators such as histamine, leukotriene and serotonin, and also interact with IgE antibodies, other inflammatory cells and nervous systems.14,15 The mast cell count increased in the bladder tissue from IC, but the increase is not specific to IC and unclear in non-ulcer type IC.14,16,17
Glycosaminoglycan layer defects
The glycosaminoglycan (GAG) layer protects the bladder mucosa as a chemical barrier against urine. If it is defective, infiltrated urine induces submucosal inflammation, stimulating sensory nerve fibers and causing frequency and pain.18 However, the reason why the GAG layer becomes defective has been unknown.
Inhibition of cell proliferation and dysfunction of bladder epithelium
Anti-proliferative factors (APF), detected in the urine of IC patients, down-regulate gene expressions that stimulate proliferation of bladder epithelial cells and up-regulate genes that inhibit proliferation, leading to urothelial under-maturation and dysfunction.19,20 The factor is potentially a specific diagnostic biomarker for IC.
Autoimmunity
A number of reports suggest the existence of auto-antibodies in IC patients.21–24 The primary antibody detected in IC is antinuclear antibody, which is similar to auto-antibody profile in systemic autoimmune diseases such as Sjogren's syndrome.25 However, the specific auto-antibody has not been identified in IC.
Infection
Infection of a microorganism has never been identified as the direct cause of IC.26 No study has proven overproduction of urinary IgA and IgG27 or the presence of bacterial 16SrRNA genes by PCR.28 It is widely recognized that antibiotic treatment is ineffective for IC. Yet, infection can occasionally be an associated factor to initiate or worsen IC.
Neurogenic inflammation
Abnormality in the nervous system is an important potential etiology of IC. The nervous system is affected in IC patients:29–31 for example, predominance of the sympathetic nervous system, increased tyrosine hydroxylase, activation of purinergic nerves, and decreased expression of the S-100 family in Schwann cells.32–35 Ultrastructural histopathology shows characteristic features of neurogenic inflammation in IC.36
Toxic agents
Toxic agents in the urine may distort epithelial function of the urinary bladder. One hypothesis is that heat labile cationic urine components of low molecular weight exert toxic effect to bladder mucosa.37
Hypoxic condition
A decrease in the microvascular density in the submucosal layer is found in IC.38 Bladder vascular perfusion is reduced by bladder filling in IC, while it is rather increased in controls.39 Hyperbaric therapy is known to relieve IC symptoms. It is conceivable that the impaired blood circulation in the bladder is related to IC.
Interaction among pathogenic factors
No single factor of the above-mentioned etiologies could explain the entire process of IC pathogenesis. Recent findings indicate that the etiology of IC is a complex interaction among nervous, immune, and endocrine systems. For example, mast cell activation by adjacent nerve endings can be affected by estradiol and corticotrophin-releasing hormone (CRH).40 Urinary levels of tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor increased in IC.41 The co-expression of stem cell factor and interleukin-6 (IL-6) is implicated with epithelial migration of mast cells.14 A nuclear factor-kappa beta (NF-κβ) subunit is overproduced and the expression of the IL-6 gene increased five times after activation of NF-κβ in IC.42 A hypothetical pathway would be that the events such as deficient urothelium and/or injury by toxic substances or immunopotent cells may initiate inflammatory reactions including overproduction and/or under-degradation of cytokines and growth factors, infiltration of immunocompetent cells such as mast cells and up-reglation of sensory nerves, forming a vicious cycle to enhance inflammation in the bladder interstitium.43 In this regard, IC is a neuroimmunoendocrine disorder or an ‘overhealing’ disorder of the urinary bladder.
Epidemiology
Recent studies uniformly indicate a significantly high prevalence of IC, roughly 0.1% to 1.0%. In Finland, the prevalence was estimated as 10.1 cases per 100 000 population in 1975 based on positive symptoms, negative urinary tract infection and positive bladder biopsy.44 Following studies in 2002 indicated that the rate was 450 per 100 000, and more recently it was 680 per 100 000 for a probable IC and 300 per 100 000 for a definite diagnosis of IC.45,46 The prevalence of 306 per 100 000 was reported in 2007 in Austria.47 In the United States, a questionnaire survey in 1987 indicated at least 43 500 IC patients and 217 500 possibly IC patients. A report in 1994 suggested a prevalence of 0.5 percent of the total population.48 A survey with female nurses in 1999 estimated 52 to 67 per 100 000.49 A study using the managed care database showed 197 female and 41 male patients with IC per 100 000 population, respectively.50 In Japan, the prevalence was only 2 per 100 000 patients by a questionnaire survey of 300 major hospitals in 1998.51 Japanese patients tended to be older (52.9 years in average) than those in Europe and the United States.52 This may indicate that Japanese patients have spent a long time before diagnosis. However, a recent epidemiological investigation in Japan found that 1.0% of the general population experienced bladder pain every day.53
Quality of life
Interstitial cystitis is a disease that profoundly affects patients' quality of life (QOL). A study evaluated QOL of 424 patients by short form 36 (SF-36),54 reporting that about 80% of the patients felt adverse affects on daily and social lives due to IC (Table 1). Two subsequent studies confirmed that IC patients had significantly worse QOL by SF-36 scores in many aspects compared with age-matched women without IC.55,56 Significant impairment in mental health dimension was demonstrated by specific scales for depression.56 A Japanese study found severely impaired QOL in IC patients by SF-36 and the King's Health Questionnaire (KHQ) scales.57 It is even worse when compared with the patients with chronic renal failure requiring hemodialysis, Crohn's disease, systemic lupus erythematosus or rheumatoid arthritis.
| Domain of QOL | Symptom severity (No. patients) | Total patients (424) | ||
|---|---|---|---|---|
| Mild (149) | Moderate (168) | Severe (107) | ||
| Well-being | 4.0 | 6.6 | 15.0 | 7.8 |
| Daily life | 34.9 | 57.1 | 75.7 | 54.0 |
| Social life | 58.4 | 75.0 | 85.0 | 71.1 |
Diagnosis
There are no established diagnostic criteria for IC.58 Two approaches have been undertaken; one is to depend on highly specific criteria such as NIDDK research criteria59 and the other is to use symptom-based criteria to increase the sensitivity of diagnosis by advocating the terms such as painful bladder syndrome (PBS) or chronic pelvic pain syndrome (CPPS).
In this guideline, we define three requirements for IC diagnosis: symptoms such as urinary frequency, hypersensitivity and/or bladder pain, cystoscopic findings proving pathologies in the urinary bladder and exclusion of other confusable diseases explainable for the symptoms and findings. Of primary importance is to suspect IC when a patient complains of unexplainable urinary symptoms.60–62
Symptoms
Common symptoms of IC include urinary frequency, nocturia, urinary urgency, bladder hypersensitivity, bladder discomfort, and bladder pain.63 Symptoms are susceptible to dietary, environmental and mental stress with variable remissions and exacerbations.64 O'Leary-Sant Symptom and Problem Indices are most commonly used for the symptom assessment.65 The typical symptom of IC would be bladder pain, especially pain worsened during holding urine and relieved after voiding. Pain may be felt in the perineum, in the pelvis, or during sexual intercourse. However, it should be noted that a substantial portion of patients complain of no pain.66–69 A more specific symptom would be bladder discomfort at an early phase of bladder filling (hypersensitivity), which is not urgency or sudden compelling desire to void (the key symptom of overactive bladder). For this reason we suggest a term ‘hypersensitive bladder syndrome (HSB)’ to collectively describe lower urinary tract symptoms in IC.
Clinical examinations
Tenderness in the bladder may be noted by pelvic examination. Urinalysis usually shows no abnormality. Voiding recording is indispensable to confirm the decrease in voided volume or functional bladder capacity (e.g. less than 200 mL).70 In urodynamic studies detrusor overactivity may be present. Reduced bladder capacity without detrusor overactivity (bladder hypersensitivity) is suggestive of IC.71–73
Cystoscopy
Cystoscopy provides positive specific findings of IC and also can identify other pathologies in the bladder (e.g. bladder stone or cancer).63 It is important to watch the bladder mucosa from the early phase of filling. A characteristic mucosal change ‘Hunner's ulcer’ may be identified (ulcer type). It most commonly appears at the boundaries between the bladder dome and the posterior or lateral walls. An ulcer is recognized as a reddish mucosal change lacking in normal capillary structure occasionally associated with fibrin clot and scar. Even a touch by the endoscope may cause bleeding. In patients without ‘Hunner's ulcer’ the entire mucosa may appear to be fairly normal (non-ulcer type), although bladder capacity decreases in most cases.
Hydrodistension
Hydrodistension is over-distending the bladder by hydrostatic pressure under general or lumbar anaesthesia (refer to treatment section for technical details). In most cases of IC the maximum bladder capacity is decreased (e.g. less than 500 mL). The ulcerated or scarred area, if present, sometimes ruptures with distension and massive bleeding occurs on deflation. Non-ulcer type IC also shows mucosal bleeding after deflation, which varies in size, from a very limited area to the whole bladder, and in severity, from petechial hemorrhagic lesions (glomerulations) to rain fall bleeding. These findings are at present the sole objective and positive basis for IC diagnosis.63 Despite the remaining doubt as to their specificity,74 the severity of bleeding had positive correlations with IC symptoms.75 The cystoscopic findings should be recorded in a standardized way ( 1, 2).
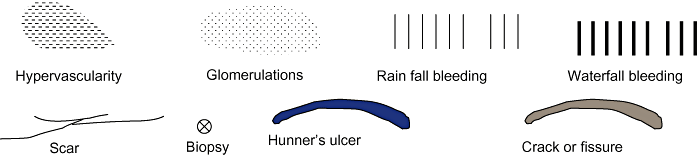
Symbols to be used for describing cystoscopic findings.
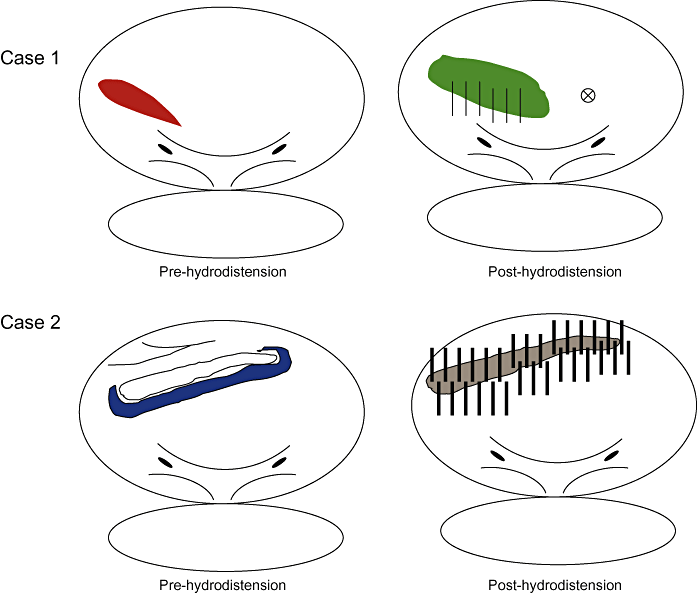
Examples of cystoscopic findings at hydrodistension. Case 1: Hypervascular area was observed on the right wall before hydrodistension. After hydrodistension, glomerulations associated with rainy bleeding appeared. The biopsy specimen was obtained from the trigone. Case 2: An ulcer extending from the right wall to the dome was found. It was accompanied by scar and hypervascular area. After hydrodistension, the ulcer turned into a crack and started waterfall bleeding.
Bladder biopsy
Bladder biopsy is not an essential part of diagnosis, as no specific histological findings for IC have been identifed.67,76–78 Mast cell infiltration is often encountered, especially in ulcer-type IC. Its clinical significance would be ruling-out intraepithelial carcinoma and clarifying the histopathology of the patient.79 Taking biopsy is recommended after hydrodistension to avoid risk of bladder rupture.79
Potassium sensitivity test
This test measures exacerbation of bladder pain after instilling potassium solution intravesically. IC patients tend to experience pain exacerbation to a greater extent, presumably due to increased permeability of the bladder mucosa to the solvent.80 The clinical value would be limited, however,81,82 due to high false-positive and false-negative rates and invasiveness of the procedure.
Urinary markers
Various kinds of urinary markers have been studied, among which an antiproliferative factor (APF) appears to be promising.83,84
Exclusion of confusable diseases
Diseases to be excluded are listed in Table 2. It should be noted that IC may coexist with these diseases.
| Bladder diseases | Overactive bladder, neurogenic bladder, bladder cancer, bladder calculus, radiation cystitis |
| Prostate and urethral diseases | Prostatic hypertrophy, prostate cancer, urethral diverticulum, urethral stricture |
| Genitourinary infections | Bacterial cystitis, urethritis, prostatitis |
| Gynecologic diseases | Endometriosis, uterine myoma, vaginitis, climacteric disturbance |
| Other conditions | Polyuria |
Recommended tests
Diagnostic tests are classified according to recommendation level in Table 3.
| Mandatory | Recommended | Optional |
| Clinical historyPhysical examinationsUrinalysis | Urine cultureUrine cytologySymptom scoresQOL scoresFrequency-volume chartResidual urine measurementProstate-specific antigenCystoscopy and/or Hydrodistension | UltrasonographyUrodynamic studyX-ray examinationPotassium testBiopsy |
- QOL, quality of life.
Treatment
The recommendation grade of each treatment is displayed in Table 4.
| Treatment | Grade of recommendation‡ | Dosage§ | |||
|---|---|---|---|---|---|
| Conservative treatment | Behavior therapy | b | |||
| Physical therapy | c | ||||
| Alleviation of tension | b | ||||
| Diet therapy | b | ||||
| Hydrodistension | b | ||||
| Oral treatment | Hydroxyzine | c | 25–75 mg/day | ||
| Amitriptyline | b | 10–75 mg/day | |||
| Suplatast tosilate | c | 300–600 mg/day | |||
| Cimetidine | c | 600 mg/day | |||
| Antibacterial agent | d | ||||
| Steroid | c | 5–15 mg/day | |||
| L–arginine | d | ||||
| Pentosan polysulfate | b | 300 mg/day | |||
| Bladder Instillation | DMSO | b | 50 mL of 50% solution | ||
| Heparin | b | 10 000 unit (10 mL) | |||
| Hyaluronic acid | d | ||||
| Chondroitin sulfate | d | ||||
| Pentosan polysulfate | d | ||||
| Capsaicin | d | ||||
| Resiniferatoxin | d | ||||
| BCG | d | ||||
| Oxybutynin | c | ||||
| Lidocaine | b | ||||
| Other Treatments | TENS | c | |||
| Sacral nerve neuromodulation | c | ||||
| Acupuncture | c | ||||
| TUR | b | For ulcer–type | |||
| Augmentation, Cystectomy | c | Last resort | |||
- † No treatments were approved for IC in Japan.
- ‡ ‡Grade of recommendation; a: strongly recommended, b: recommended, c: insufficient evidence for recommendation, d: not recommended. The grade was determined for each treatment by examining level of evidence, variability of effect, magnitude of effect, clinical applicability, mobility and cost.
- § §Only for the treatments with grade of recommendation b and c. BCG, Bacillus Calmette-Guerin; DMSO, dimethyl sulfoxide; TENS, transcutaneous electric nerve stimulation; TUR, transurethral resection.
Conservative treatment
Grade of recommendation: b or c, Level of evidence for efficacy: E
Behavioral therapy including timed voiding, controlled fluid intake, pelvic floor muscle training and bladder training improved symptoms in more than a half of the patients.85,86 (level V) Physical therapy such as biofeedback and soft tissue massage relieves urinary frequency, bladder pain or coital pain probably through the relaxation of the pelvic floor muscles.87–93 (level V) To maximize the efficacy, however, supervision by specialists may be required. Considering that mental stress aggravates the symptoms, stress reduction such as exercise, bathing, shortening working hours or joining educational programs and patient support groups would be efficacious.94–97 (level V) Support groups should be helpful for lonely and less educated patients, and are available at http://www.ichelp.com/welcome.htm or http://www.tomonoki.org/ (in Japan). Dietary manipulation by avoiding acidic beverages, coffee, spicy food, and alcohol may be beneficial.98–102 (level V) Each patient should be counseled for the food that aggravates his or her symptoms to avoid it.103–107
Hydrodistension
Grade of recommendation: b, Level of evidence for efficacy: E
Hydrodistension has been the most common treatment for IC108,109 since the first report in 1930,110 although there has been no randomized comparative study. Most reports consistently indicated about a 50% efficacy rate, but the effect often persisted only for about 6 months.111–115 (level V) Presumed mechanism is that ischemia and metabolic acidosis induced by hydropressure degenerates afferent nerves, leading to reduced bladder pain and increased bladder capacity.112,116,117 The release of growth factors from the interstitium may be also involved.
No standard method for hydrodistension or no significant difference in efficacy among the methods is known.118 (level V) Bladder rupture may occur as a morbidity during hydrodistension.110,112,119 A suggested procedure is as follows:
- 1
Lumbar anesthesia at the T6 level, preferably up to T4 level.120 (level V)
- 2
Inflate the bladder by physiologic saline by 80 cmH2O with continuous endoscopic observation for possible bladder ruptures. If the infused volume reaches 800 mL to 1000 mL before the intravesical pressure achieves 80 cmH2O, the infusion should be terminated.
- 3
After inflation of the bladder, maintain the pressure for a few minutes, drain the water, and observe the bladder mucosa for bleeding during the draining.
- 4
Procedures may be repeated, although the therapeutic significance of the repetition is unknown.
- 5
A urethral catheter should be placed in the bladder overnight.
Medical treatment
Antihistamine (Hydroxyzine)
Grade of recommendation: c, Level of evidence for efficacy: E. An experimental121 and some open-labeled studies have suggested efficacy in about half of patients121–125 (level V) with slight to moderate drowsiness as the adverse event. In a randomized comparative study, no significant difference was found between hydroxyzine and placebo, although a tendency towards a favorable effect of hydroxyzine was noted.126
Tricyclic antidepressant (Amitriptyline)
Grade of recommendation: b, Level of evidence for efficacy: C. Amitriptyline is expected to inhibit mast cell activity by blocking histamine H1 receptor and to modify pain transmission in the central nervous system by inhibiting serotonin and noradrenalin reuptake. Uncontrolled studies demonstrated improvement of frequency and bladder pain in 26% to 73% of patients.127–130 (level V) In a prospective double blind study O'Leary and Sant Symptom Scores were reduced by more than 30% in 42% of the patients in the amitriptyline group while only 13% in the placebo group.131 (II) The efficacy was demonstrated in a long-term study132 (level V). Adverse events were mild to moderate dry mouth and drowsiness.
Suplatast tosilate
Grade of recommendation: c, Level of evidence for efficacy: E. Suplatast tosilate inhibits production of IgE, IL-4 and IL-5, and is efficacious for allergic diseases. A report from Japan suggested increase in bladder volume and symptomatic improvement in a majority of patients without serious side effects.133 (level V) No significant adverse event is known.
Cimetidine
Grade of recommendation: c, Level of evidence for efficacy: C. Cimetidine is a histamine H2-receptor antagonist. Three un- controlled studies indicated about 70% efficacy rate in IC.134–136 (level V) A randomized double blind study140 on 36 patients demonstrated a significant improvement in suprapubic pain and frequency compared with a placebo group; however, the detail of the study is unclear137 (II).
Antibiotic and antibacterial agent
Grade of recommendation: d, Level of evidence for non-efficacy: C. In a randomized placebo-controlled study, antibiotics including rifampin, doxycycline, erythromycin, metronidazole, clindamycin, amoxicillin and ciprofloxacin were shown to have no significant therapeutic effect over placebo.138 Adverse effects were more often in active arms.
Steroid
Grade of Recommendation: c, Level of evidence for efficacy: E. Efficacy of predonisolone for IC symptoms is suggested in a study, but hydrodistension was carried out simultaneously with predonisolone.139 (level V) Adverse events associated with long-term administration is a major concern.128,140 (level V) It may be indicated for IC related with autoimmune diseases.
L-arginine
Grade of recommendation: d, Level of evidence for non-efficacy: C. L-arginine, a natural substrate of nitric oxide synthase (NOS), may re-activate NOS activity which is suppressed in IC, and relieve symptoms.141 (level V). Another study, however, indicated no significant change in NO levels in the bladder and no improvement in symptoms142 (level V). No significant effect was confirmed by subsequent double blind studies.143,144 (II)
Pentosan polysulfate
Grade of recommendation: b, Level of evidence for efficacy: C. The glycosaminoglycan (GAG) layer is a non-specific protective mechanism of the bladder mucosa, and its deficiency is implicated with pathogenesis of IC. Pentosan polysulfate (PPS) is believed to repair the damaged GAG layer, but only 3% to 6% of orally administered PPS is excreted in the urine.145 Two open trials showed modest symptom improvement.146,147 (level V) A placebo-controlled double blind study was conflicting.148–151 (II) PPS is efficacious, but it takes 3–6 months until the effect is noticeable, and the effect is restricted to only about a quarter of patients. Side effects are rare including hair loss, diarrhea, nausea and headache in a long-term adminstration.150
Cyclosporine
Grade of recommendation: c, Level of evidence for efficacy: C. The efficacy of cyclosporine was reported in open trials.152,153 (level V) In a randomized study comparing cyclosporine with pentosan polysulfate sodium, the response rate was 75% for cyclosporine compared with 19% for pentosan polysulfate sodium.154 (II) Further studies were warranted, as adverse events are a concern.
Intravesical instillation or bladder wall injection
Dimethyl sulfoxide
Grade of recommendation: b, Level of evidence for efficacy: C. Dimethyl sulfoxide (DMSO) is claimed to have anti-inflammatory, analgesic, muscle relaxant and collagenolytic effects. It has been empirically used to alleviate symptoms of IC. In randomized155 and non-randomized156–158 studies, improvement rates of around 80% have been reported. (II,V) Most patients recognized a garlic-like odor, and a few patients felt bladder spasm possibly due to mast cell degranulation.159 Periodic ophthalmic examinations are advisable as cataract has been reported in an animal study. Usually a solution of 50% DMSO diluted by physiological saline or distilled water is instilled into the bladder, and retained in the bladder for 10–20 minutes.160 The interval varies from a few times a week to every few months.
Heparin
Grade of recommendation: b, Level of evidence for efficacy: E. Heparin is expected to function as a glycosaminoglycan (GAG) layer on the bladder urothelium. More than half of patients experienced symptom relief without significant adverse events in open trials.161,162 (level V) A randomized comparative study is necessary to obtain conclusive evidence. Instillation with lidocaine may be more efficacious163 (level V).
Hyaluronic acid
Grade of recommendation: d, Level of evidence for efficacy: E. Hyaluronic acid, a glycoprotein, could repair the deficient GAG layer. Four reports indicated long-lasting moderate efficacy with no significant toxicity.164–168 (level V) However, a recent randomized double blind study conducted in 2004 demonstrated no better efficacy than placebo (unpublished data).
Chondroitin sulfate
Grade of recommendation: d, Level of evidence for efficacy: E. Chondroitin sulfate is also a glycoprotein. Its efficacy was reported in a single article.169 (level V)
Pentosan polysulfate
Grade of recommendation: d Level of evidence for efficacy: C. Pentosan polysulfate (PPS), instilled intravesically, may directly replenish the deficient GAG layer. A double-blind placebo-controlled study indicated its efficacy, although the sample number was small (n = 20) and the magnitude of efficacy was limited.170 (II)
Vanilloids (Capsaicin, Resiniferatoxin)
Grade of recommendation: d, Level of evidence for non-efficacy: C. Capsaicin, a C-fiber afferent neurotoxin, may alleviate the symptoms by desensitizing bladder afferents. Resiniferatoxin is more potent than capsaicin in desensitizing C-fibers with less irritation. Clinical efficacy has not been demonstrated clearly in small studies171–173 (level V) or a placebo-controlled study.174 (II) No particular toxicity was reported.
Bacillus Calmette-Guerin
Grade of recommendation: d, Level of evidence for non-efficacy: C. Bacillus Calmette-Guerin (BCG) is an immunomodulatory agent for the intravesical instillation therapy of bladder cancer. An article demonstrated beneficial effect of BCG over placebo for IC symptoms.175 (II) However, other studies including randomized double blind studies demonstrated no clinical benefit.176–178 (II) Adverse events may be serious.
Oxybutynin
Grade of recommendation: c, Level of evidence for efficacy: C. A single article indicated symptomatic resolution by intravesical instillation of oxybutynin in addition to bladder training.179 (II)
Lidocaine (with electromotive drug administration)
Grade of recommendation: b, Level of evidence for efficacy: E. Lidocaine is a local anaesthetic and relieves pain by blocking sensory nerves in the bladder, although pain relief lasts for a short time.180–183 (level V) Alkalization or electromotive drug administration (EMDA) of lidocaine is attempted to promote absorption.
Botulinum toxin
Grade of recommendation: d, Level of evidence for efficacy: E. Botulinum toxin inhibits calcitonin gene-related peptide (CGRP) release from afferent nerves and weakens pain response induced by acetic acid in rats.184 A small study indicated symptom relief in IC patients by botulinum toxin injection into the bladder wall.185 (level V) Further studies are warranted to confirm the promising efficacy.
Other treatments
Transcutaneous electric nerve stimulation
Grade of recommendation: c, Level of evidence for efficacy: E. Transcutaneous electric nerve stimulation (TENS) is expected to eliminate pain by electrically stimulating peripheral sensory nerves in the skin.186,187 Some articles reported its efficacy for the treatment of classic IC188,189 (level V); however, a randomized comparative study is lacking. TENS is applicable at the outpatient clinic.
Sacral nerve neuromodulation
Grade of recommendation: c, Level of evidence for efficacy: E. Sacral nerve neuromodulation is stimulation of the sacral root S3 or S4 by a permanent device to modulate afferent neural transmission, which results in pain relief, suppression of detrusor overactivity and stabilization of pelvic floor muscles.190,191 Several articles showed significant symptom improvement.190–194 (level V) However, it requires a surgery for implantation and replacement of the device.
Acupuncture
Grade of recommendation: c, Level of evidence for efficacy: D. Acupuncture is a traditional and relatively noninvasive therapy, although its precise mechanism of action is unclear.195 The efficacy of acupuncture for IC would be limited and transient, and repeated treatment will be required to maintain the effect.195,196 (‡V) A large placebo effect is reported.197 (‡V) It is an outpatient treatment with minimal morbidity.
(d) Transurethral resection, transurethral coagulation
Grade of recommendation: b (for ulcer type only), Level of evidence for efficacy: E. Endoscopic resection or fulguration of the bladder tissue diminishes inflammatory cells and hypersensitive sensory nerve endings, expectedly leading to symptomatic resolution. Transurethral resection (TUR) of the ulcer lesions eliminated symptoms for 1 year or longer in about a half of patients,198–200 (level V) although it is less effective for non-ulcer IC. A relapse can be managed by repeating TUR. Transurethral coagulation using neodymium-YAG laser is an alternative to TUR and comparably effective.201–204 (level V) Bladder perforation, hemorrhage, urethral stricture and VUR are known as the complications of these procedures.
Cystectomy, Augmentation, Urinary diversion
Grade of recommendation: c, Level of evidence for efficacy: E. Surgical removal of the bladder is the last resort, when other treatments have failed to relieve the disabling symptoms.205,206 Supratrigonal cystectomy combined with augmentation cystoplasty using a bowel segment (ileal, ileocoecal, right colon, or sigmoid colon) is the most common procedure, achieving a significant symptomatic improvement.205–209 (level V) Results are apparently more favorable for patients with end-stage ulcer-type IC.210 Subtrigonal cystectomy would not be appropriate because of difficulties associated with ureteral or urethral anastomosis.211 Patients undergoing cystoplasty need a long-term follow-up for possible morbidities including hydronephrosis and adenocarcinoma in the intestinal portion.212 Total cystectomy and urinary diversion (continent urinary diversion, orthotopic neobladder or ileal conduit) is another option, although necessity of self catheterization or cosmetic concern should be taken into account.213 (level V) Pelvic pain or pouch pain may persist after augmentation and cystectomy.214–216 Sporadic reports indicate that urinary diversion with the bladder unresected improved the symptoms.217,218
Assessment of therapeutic efficacy
New treatments for IC will be challenged for efficacy in the future. To enhance the research process, the inclusion criteria and efficacy assessment should be standardized.
Inclusion criteria for enrollment
The criteria (Table 5) are a slight modification of the NIDDK inclusion criteria in 1987.219 Details of inclusion criteria, especially for target symptoms and their severity, should be explicitly determined to optimize the sensitivity in detecting efficacy by the study.
| Inclusion criteria | Hunner's ulcer or glomerulations† on cystoscopy, ANDLower urinary tract symptom(s) (urinary frequency,‡ bladder hypersensitivity,‡ urinary urgency, bladder pain) |
| Exclusion criteria§ | Duration less than 3 months,¶ lower urinary tract infection in 3 months, hydrodistension in 3 months,‡ bladder instillation in 1 month,‡ neurogenic bladder,‡ lower urinary tract calculi, genital herpes, bladder cancer, prostate cancer,‡ urethral cancer, gynecologic cancer, urethral diverticulum, vaginitis, tuberculous cystitis, radiation cystitis and drug-induced cystitis |
- † Extensive and wide-spread petechial hemorrhages required in National Institute of Diabetes and Digestive Kidney Diseases (NIDDK) criteria.
- ‡ ‡Not included in NIDDK criteria.
- § NIDDK exclusion criteria deleted; age under 18, absence of urgency, capacity greater than 350 mL, involuntary bladder contractions by urodynamics, daytime frequency less than five times, nocturia less than two times, and symptoms relieved by antibiotics or anticholinergics.
- ¶ Duration less than 12 months in NIDDK criteria.
Efficacy criteria
Efficacy assessment method
There is no gold standard for the assessment. The optimal method should be defined before starting the study. The examples are given below.
- •
Aggregated symptom scores
- •
Individual symptom scores
- •
Voiding frequency
- •
Single voided volume
- •
QOL scores
- •
Overall improvement by patients' subjective impressions (Table 6)
| Have your bladder symptoms been improved or worsened by the treatment? Choose one statement which best fits to your impression. |
| 1. Markedly improved. |
| 2. Improved (moderately). |
| 3. Slightly improved. |
| 4. No change. |
| 5. Slightly worsened. |
| 6. Worsened (moderately). |
| 7. Markedly worsened. |
Potential factors that may affect the efficacy assessment
- •
Symptom severity
- •
Presence or absence of bladder pain, and its intensity
- •
Bladder capacity
- •
Presence or absence of an ulcer (ulcer type, non-ulcer type)220
- •
Gender
- •
Age
Other considerations
- •
A placebo-controlled (or standard-treatment-controlled) double-blind study is highly recommended for pharmaceutical therapy.
- •
Efficacy assessment should be carried out at 3 months after intervention. Assessment of long-term efficacy (e.g., at 6 months or 12 months) is also recommended.
Clinical algorithm
The algorithm is intended to guide the practioners to diagnose and treat patients with IC or suspicion of IC (Fig. 3).
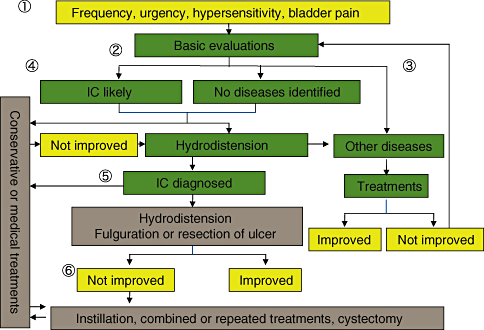
Clinical algorithm for interstitial cystitis (IC). (1) Most cases of interstitial cystitis present with symptoms such as urinary frequency, bladder hypersensitivity, and/or bladder pain. The most common symptom is urinary frequency. The basic evaluation consists of the mandatory evaluation (history taking, physical findings, and urinalysis) or recommended evaluation (urine culture, urine cytology, symptom scores, quality of life (QOL) scores, frequency-volume chart, residual urine measurement, prostate-specific antigen, and cystoscopy with or without hydrodistension). It may involve optional evaluation (urodynamic tests, imaging tests, and potassium test). (2) Diseases to be differentiated are urinary calculi, bladder cancer, prostate cancer, urethral cancer, bacterial cystitis, prostatitis, urethritis, neurogenic bladder, overactive bladder, benign prostatic hyperplasia, urethral stricture, urethral diverticulum, polyuria, and so on. Should the treatment provide no sufficient improvement, consider further evaluation for the accurate diagnosis. (3) When IC is suspected or other diseases cannot be identified, hydrodistension should be considered. Hydrodistension provides not only a diagnositic clue but a therapeutic benefit. Less invasive treatments (conservative therapy or medical treatment) may be attempted prior to hydrodistension. (4) Therapeutic hydrodistension should be carried out with sufficient anaesthesia. Ulcerative lesions, if present, should be endoscopically coagulated or resected. (5) When sufficient improvement cannot be achieved, combined treatment or repeated hydrodistension should be considered. Cystectomy should be the last resort.




