Surgical outcomes of partial nephrectomy for renal cell carcinoma: A joint study by the Japanese Society of Renal Cancer
Abstract
Objective: A joint study was undertaken by the Japanese Society of Renal Cancer to investigate the present status of partial nephrectomy in Japan and to speculate about what may be the indications for partial nephrectomy in patients with renal cell carcinoma.
Methods: Data were tabulated for 469 patients from participating medical institutions and various clinical factors were investigated with regard to disease progression (local recurrence and distant metastasis).
Results: Disease progression was observed in 21 patients (4.5%). No significant relation to disease progression was observed for sex, laterality, tumor histology, grade and tumor size. Although patients with solitary tumors displayed excellent prognosis irrespective of tumor diameter, patients with multiple tumors displayed a high likelihood of disease progression. Patients older than 77 years old and patients with imperative indication were found to have a poorer prognosis.
Conclusion: In patients with solitary tumors, partial nephrectomy can be actively performed, even if the patient displays elective indications and the tumor is >4 cm in diameter. In patients displaying multiple tumors with imperative indications, the decision whether to perform partial nephrectomy should be made by the patients and their physicians after considering the impact on curability and the quality of life.
Introduction
While the standard treatment for renal cell carcinoma (RCC) is radical nephrectomy, nephron-sparing surgery has been performed for patients with bilateral RCC, tumor in a solitary kidney or renal impairment such that radical nephrectomy is likely to significantly reduce residual renal function (imperative indications). In the past few decades, nephron-sparing surgery for patients with a healthy contralateral kidney (elective indication) is increasingly being performed, as more incidental RCC are being discovered in the early stage due to advances in diagnostic imaging and the more widespread use of screening. However, the indications as to whether to perform nephron-sparing surgery remain unclear, and the decision to perform the surgery depends on the opinions at each medical institutions.
To investigate the present status of partial nephrectomy in Japan and to speculate about what may be the proper indications for partial nephrectomy in patients with renal cell carcinoma, the Japanese Society of Renal Cancer conducted a multicenter survey to investigate various clinical factors for disease progression after partial nephrectomy.
Materials and methods
Patients who had been diagnosed with RCC and who underwent partial nephrectomy between 1 January 1990 and 31 December 1999 at medical institutions affiliated with the Japanese Society of Renal Cancer were investigated (Table 1). The indications for partial nephrectomy were determined based on criteria at each institution. Of these patients, both imperative indications such as bilateral RCC, tumor in a solitary kidney or renal impairment with RCC, and elective indications, which included having a healthy contralateral kidney, were taken into consideration. The procedure for partial nephrectomy was either open or laparoscopic with circumferential resection in normal parenchyma.
| Hokkaido University |
| Sapporo Medical University |
| Iwate Medical University |
| Morioka Red Cross Hospital |
| Chiba University |
| Tokyo University |
| Jikei Medical University |
| Jikei Medical University Aoto Hospital |
| Keio University |
| Tokyo Women's Medical University |
| Nippon Medical School |
| National Defense Medical College |
| Yokohama City University Fukuura Hospital |
| Yokosuka Kyousai Hospital |
| Chigasaki Municipal Hospital |
| Ashigarakami Prefectual Hospital |
| Mie University |
| Kyoto Prefectual University |
| Nara Prefectual Medical University |
| Tenri Yorozu Hospital |
| Okayama University |
| Hiroshima University |
| Kyushu University |
Patients were excluded as subjects if: (i) they had undergone simple enucleation along the pseudo-capsule of the tumor; or (ii) they were assessed as definitely having an hereditary tumor syndrome such as von Hippel-Lindau disease.
Age, sex, affected side, tumor diameter, number of tumors, histological classification, grade, indications for surgery, whether disease progression (both local recurrence and distant metastasis) occurred, and survival were investigated in each patient. The tumors were graded on a scale of 1–3 based on nuclear appearance according to the International Union Against Cancer (UICC) tumor node metastasis (TNM) classification with minor modifications advocated by the Japanese Urological, Pathologic and Radiological Societies.1 For histological classification, the Working Group of the UICC held in 1997 recommended avoiding use of the term granular cell carcinoma. In the present study, however, many cases were diagnosed in the 1990s following the General Rule for Clinical and Pathological Studies on Renal Cell Carcinoma, so the term granular cell carcinoma is still included.
For the statistical analysis, the characteristics of groups were compared using the χ2 test for categorical variables and using the Mann–Whitney U-test for continuous variables. Age distribution was divided to set optimal cut point according to receiver operating characteristic (ROC) curve. Kaplan–Meier survival curves were generated from the life table analysis, and survival was compared using log-rank tests. Prognostic implications of selected variables were evaluated using the Cox proportional hazards model.
Results
A total of 469 patients (361 men, 77%; 108 women, 23%) who underwent partial nephrectomy for RCC were enrolled from 23 medical institutions (Table 1). The mean follow-up period was 1445 days, with a minimum of 7 days and a maximum of 4745 days. During this period, 21 patients (4.5%) experienced disease progression (Table 2).
| No | Age | Gender | Side | Location | Multiplicity | Tumordiameter(cm) | Grade | Celltype | Prognosis | Site ofrecurrence | Time to disease progression (day) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Elective indication | |||||||||||
| 1 | 78 | M | R | Upper | S | 1.4 | 3 | Clear | Cancer death | Unknown | 296 |
| 2 | 50 | M | R | Lower | S | 3 | 2 | Granular | Alive | LN | 421 |
| 3 | 54 | M | L | Upper | S | 2 | 1 | Clear | Alive | Unknown | 1592 |
| 4 | 78 | M | L | Upper | S | 3 | 2 | Clear | Alive | Liver | 777 |
| Imperative indication | |||||||||||
| 1 | 60 | M | R | Mid | S | 2.2 | 1 | Clear | Alive | Lung | 1263 |
| 2 | 69 | F | L | Mid | S | 3 | 1 | Clear | Alive | L kidney | 1921 |
| 3 | 64 | M | L | Mid | S | 3 | 2 | Clear | Alive | Lung | 1494 |
| 4 | 56 | M | L | Mid | S | 1.7 | 1 | Clear | Cancer death | Unknown | unknown |
| 5 | 77 | M | R | L | S | 2.5 | 1 | Clear | Alive | Lung | 543 |
| 6 | 54 | M | L | Upper | S | 3 | 2 | Clear | Cancer death | Pancreas, lung, liver | 3059 |
| 7 | 63 | M | R | Unknown | S | 1.5 | 1 | Clear | Cancer death | Lung, bone, brain | 1337 |
| 8 | 82 | F | R | L | S | 3.5 | 2 | Clear | Alive | Bone | 668 |
| 9 | 63 | F | R | L | S | 1.1 | 1 | Clear | Alive | Thyroid | 674 |
| 10 | 69 | F | L | L | S | 6 | 2 | Clear | Alive | Lung, LN | 1237 |
| 11 | 79 | M | R | L | S | 3.1 | 1 | Clear | Cancer death | Lung, bone | 53 |
| 12 | 55 | M | L | Upper + L | m | 3.1 | 2 | Clear | Cancer death | Kidney, liver | 371 |
| 13 | 71 | M | B | Unknown | m | 4.5 | 2 | Clear | death by other cause | Lung | 492 |
| 14 | 59 | M | R | Mid | m | 1.8 | 2 | Clear | Cancer death | Lung | 391 |
| 15 | 33 | M | R | Upper + L | m | 2.5 | 1 | Clear | Alive | L kidney | 366 |
| 16 | 66 | M | L | L | m | 2 | 2 | Clear | Alive | Bil adrenal | 250 |
| 17 | 65 | M | L | Upper | m | 2 | 2 | Granular | Cancer death | Kidney, IVC, skin | 340 |
- M, male; F, female; L, left; R, right; S, singular; m, multiple; LN, lymph nodes; Bil, bilateral; IVC, inferior vena cava.
Associations between clinical factors and disease progression are shown in Table 3. Among the clinical factors examined, age, tumor multiplicity and operative indication were statistically significant for disease progression (P < 0.05). Patients older than 77 years old exhibited statistically poorer prognosis than the others. Among 447 patients with solitary tumors, 16 had disease progression (3.6%), whereas five patients in 22 multiple tumors experienced disease progression (22.7%). Among the known indication of 453 patients, 115 exhibited imperative indications. Disease progression occurred in 17 of these imperative patients (14.8%), and cancer deaths occurred in seven patients (6.0%). Among the 338 patients with elective indications, four (1.2%) experienced disease progression, and cancer death occurred in one patient (Table 2).
| No. of patients | No. of disease progression | Disease progression rate (%) | P-value | |
|---|---|---|---|---|
| Age distribution | ||||
| ≤53 | 162 | 2 | 1.2% | |
| 54–76 | 288 | 14 | 4.9% | 0.049* |
| 77≤ | 19 | 5 | 26.3% | 0.000** |
| Gender | ||||
| Male | 361 | 17 | 4.7% | |
| Female | 108 | 4 | 3.7% | 0.654 |
| Tumor site | ||||
| Right | 253 | 9 | 3.6% | |
| Left | 181 | 10 | 5.5% | |
| Bilateral | 23 | 2 | 8.7% | 0.500 |
| Unknown | 12 | 0 | 0 | |
| Tumor diameter (cm) | ||||
| 0–1 | 16 | 0 | 0 | |
| 1–2 | 154 | 8 | 5.2% | |
| 2–3 | 164 | 9 | 5.5% | |
| 3–4 | 77 | 2 | 2.6% | |
| 4< | 43 | 2 | 4.7% | 0.385*** |
| Unknown | 15 | 0 | 0 | |
| Tumor number | ||||
| Solitary | 447 | 16 | 3.6% | |
| Multiple | 22 | 5 | 22.7% | 0.000 |
| Cell type | ||||
| Clear cell | 377 | 19 | 5.0% | |
| Granular cell | 45 | 2 | 4.4% | |
| Spindle cell | 2 | 0 | 0 | |
| Papillary cell | 7 | 0 | 0 | |
| Other subtype | 25 | 0 | 0 | |
| Unknown | 13 | 0 | 0 | NA |
| Histological grade | ||||
| G1 | 216 | 9 | 4.2% | |
| G2 | 230 | 11 | 4.8% | 0.656**** |
| G3 | 7 | 1 | 14.3% | 0.198**** |
| Unknown | 16 | 0 | 0 | |
| Indications | ||||
| Imperative case | 115 | 17 | 14.8% | |
| Elective case | 338 | 4 | 1.2% | 0.000 |
| Unknown | 16 | 0 | 0 | |
- * ≤53;
- ** ≤76;
- *** ≤4 cm;
- **** ****G1.
The recurrence rate based on 5-year disease-free survival was 82.1% in patients with imperative indications and 98.1% in patients with elective indications (Fig. 1). The 5-year cancer-specific survival rate was 98.2% for all patients, 94.6% for patients with imperative indications, and 99.7% for patients with elective indications (Fig. 2). The age, tumor multiplicity and the types of indication were found to be independent prognostic factors for disease progression by multivariate analysis (P < 0.05, Table 4).
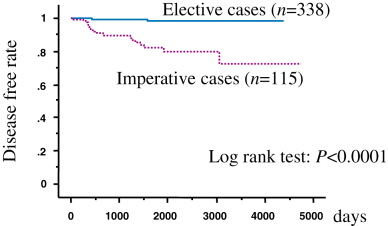
Disease-free survival.
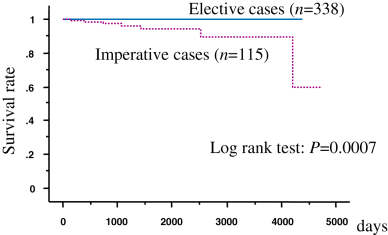
Cause-specific survival.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| P | HR | 95%CI | P | HR | 95%CI | |||
| Age (year) | ||||||||
| ≤53 | 1.00 | 1.00 | ||||||
| 54–76 | 0.049 | 4.44 | 1.00 | 19.63 | 0.163 | 2.98 | 0.64 | 13.79 |
| ≤77 | 0.000 | 29.88 | 5.69 | 156.87 | 0.000 | 26.70 | 4.64 | 153.59 |
| Sex | ||||||||
| Female | 1.00 | x | ||||||
| Male | 0.612 | 1.32 | 0.45 | 3.94 | ||||
| Multiplicity | ||||||||
| Single | 1.00 | 1.00 | ||||||
| Multiple | 0.000 | 8.18 | 3.17 | 21.10 | 0.006 | 4.48 | 1.55 | 12.94 |
| Grade | ||||||||
| G1 | 1.00 | x | ||||||
| G2 | 0.656 | 1.22 | 0.51 | 2.95 | ||||
| G3 | 0.198 | 3.89 | 0.49 | 30.84 | ||||
| Indication | ||||||||
| Elective | 1.00 | 1.00 | ||||||
| Imperative | 0.000 | 11.45 | 3.84 | 34.14 | 0.000 | 8.81 | 2.78 | 27.96 |
| Size | ||||||||
| >4 cm | 1.00 | 1.00 | ||||||
| ≤4 cm | 0.385 | 0.53 | 0.12 | 2.28 | 0.074 | 0.26 | 0.06 | 1.14 |
- HR, hazard regression; 95%CI, 95% confidence interval.
Discussion
The position of radical nephrectomy as the standard surgical procedure for RCC is indisputable. However, in the USA, nephron-sparing surgery was actively performed at some medical institutions in the 1960s,1 mostly for imperative cases. Subsequent to reports of favorable outcomes following nephron-sparing surgery in the 1990s,2,3 and an increase in the number of patients with incidental tumors, nephron-sparing surgery for elective indications began to be carried out and has become widespread. In this survey, 2740 radical nephrectomies were reported from the affiliated medical institutions, whereas 376 partial nephrectomies were performed at the same institutions. However, the indications for nephron-sparing surgery remain unclear and have not yet been standardized.
Several studies of the long-term outcomes after nephron-sparing surgery have been carried out in the USA. Novick et al. have reported a 5-year survival rate of 67% and a cancer-specific survival rate of 84% in 100 patients who underwent nephron-sparing surgery between 1956 and 1987.4 Lerner et al. compared survival rates in 185 patients who underwent nephron-sparing surgery and 209 patients who underwent radical nephrectomy between 1969 and 1992.5 The 5- and 10-year overall survival rates were 77% and 53%, respectively, for the nephron-sparing surgery group, compared with 77% and 56% for the radical nephrectomy group. The 5- and 10-year cancer-specific survival rates were 89% and 77%, respectively, for the nephron-sparing surgery group and 89% and 87% for the radical nephrectomy group. No prognostic differences were noted between the treatment groups in patients with tumor diameters ≤4 cm. A recent study comparing radical and partial nephrectomies over a 10-year period in patients with tumors of 4–7 cm in diameter also found no differences between cancer-specific survival rates in the two groups; in fact, outcomes were better for partial nephrectomy.6 However, no studies have yet investigated long-term outcomes in a large number of patients. The Japanese Society of Renal Cancer therefore attempted to investigate the present status of partial nephrectomy in Japan and to speculate the proper indications for partial nephrectomy by tabulating data from 469 patients. Data for patients in this study were provided by numerous medical institutions, and indications for surgery and actual surgical methods differed between each medical institutions. The partial nephrectomy in this study group accounted for approximately 11% of all surgeries for RCC, but varied widely from 1–31%, depending on the medical institution. Because of these backgrounds, the study focused on rates of disease progression and survival, as the results for these are clear.
Among the clinical factors examined, no significant relation to disease progression was observed for sex, affected side or, unexpectedly, tumor diameter. In the USA and Europe, a diameter of 4 cm is considered to be an important cut-off for determining prognosis in partial nephrectomy.3,5 In our study, disease progression occurred in 19 of the 412 patients (4.6%) with tumors ≤4 cm and in two of the 43 patients (4.7%) with tumors >4 cm in diameter, which shows no significant difference. The two patients with disease progression with tumors >4 cm, both had imperative indications (Table 2). These findings suggest that tumor diameter may be excluded when determining indications for partial nephrectomy, especially for elective cases. On the other hand, the recurrence rate was found to be appreciably higher in patients of older age, with multiple tumors and imperative indications (P < 0.05). The older patients may indicate less invasive treatment even with a more advanced tumor that is not suitable for nephron-sparing surgery. However, in this series, there seems to be no apparent difference in disease status between younger and older patients (Table 2). A biological difference that will cause difference in treatment outcome must be elucidated in the future. The 5-year cancer-specific survival rate differed significantly between patients with imperative indications and those with elective indications (P < 0.05). Although the imperative cases tend to have multiple and larger tumors, the indications and tumor multiplicity showed a independent prognostic significance by multivariate analysis (Table 4). Partial nephrectomy thus appears inappropriate for patients with multiple tumors in elective indications. However, in patients with imperative indications, partial nephrectomy for multiple tumors should probably be performed in some patients to ensure quality of life by obviating the need for dialysis, despite the higher possibility of disease progression. Ultimately, the decision as to whether to perform partial nephrectomy should be made by the patient and surgeon together, once the patient has been fully informed.
In this study, no differences in recurrence rate were noted based on histological classification, as 90% of patients with recurrence had clear cell carcinoma. In addition, the ratio of G3 is too small to indicate any difference in recurrence rate. Regardless, histological classification and tumor grade are unlikely to be useful in determining the indications for partial nephrectomy, as the results only become known postoperatively.
The results of this joint study have some limitations because it was retrospective and multi-institutional. The techniques and ability to perform nephron-sparing surgery may differ widely among institutions. The completion of partial nephrectomy irrespective of tumor diameter definitely depends on careful selection of suitable cases that will be clearly demarcated and peripherally located. In addition, the superior ability of the operator to perform a safe and complete resection are certainly required. However, the rapid improvements in operative procedure will enable partial nephrectomy to be carried out more effectively and thus the number of patients who will benefit from the procedure will increase in the future.
Conclusions
A multicenter study was conducted by the Japanese Society of Renal Cancer to investigate the present status of partial nephrectomy in Japan and to speculate indications for partial nephrectomy by investigating disease progression and prognosis. The results indicate that partial nephrectomy can be actively performed in patients with solitary tumors, even in patients with elective indications with tumors >4 cm in diameter. Partial nephrectomy is not suitable, however, for patients with elective indications who have multiple tumors due to the high risk of disease progression. In patients displaying imperative indications, the decision as to whether to perform partial nephrectomy should be made by the patient and their physician, taking into account the impact on curability and quality of life.
Acknowledgments
Additional board members and institutions of the Japanese Society of Renal Cancer who contributed to achieve the present study: Masamichi Hayakawa, National Defense Medical College, Tokorozawa; Go Kimura, Nippon Medical School, Tokyo; Takahisa Nakamoto, Hiroshima City Asa Hospital, Hiroshima; Yoshinobu Kubota, Yokohama City University School of Medicine, Yokohama; Takeshi Kishida, Yokohama City University Medical Center, Yokohama; Toshiro Terachi, Tokai University, Isehara; Makoto Yanagawa, Mie University, School of Medicine, Tsu; Mitsuru Nakahashi, Ashigarakami Prefectual Hospital, kanagawa; Noriaki Tokuda, Kouseikann Prefectual Hospital, Saga; Nobuo Shinohara, Hokkaido University, Sapporo.




