Establishment of new severity ratings based on analysis of hospital-acquired pneumonia
SUMMARY
• The Japanese Respiratory Society issued its first guidelines for the management of hospital-acquired pneumonia in adults in 2002. Pathological and severity ratings were investigated based on the results of a national multicenter survey of hospital-acquired pneumonia, and the new severity ratings shown below were established (Fig. II-1).
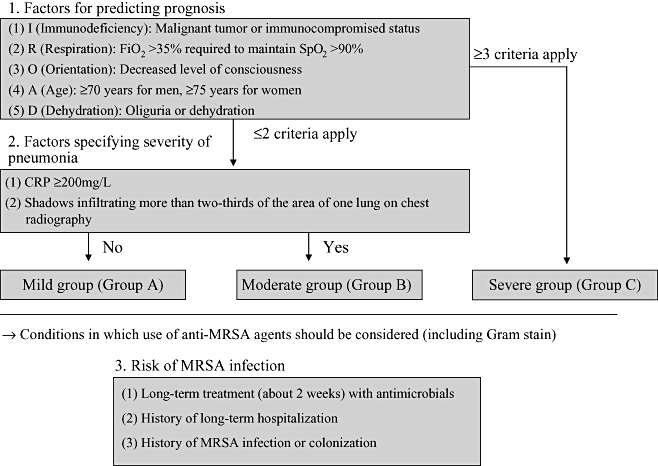
Severity ratings. MRSA, Methicillin-resistant Staphylococcus aureus.
• Severity ratings in the 2002 guidelines were based mainly on markers that predicted the effectiveness of antimicrobial treatment. In the current revision, severity is rated using markers that predict the prognosis of patients.
• Five criteria were established as factors that predict prognosis: malignant tumour or immunocompromised status; decreased level of consciousness; FiO2 >35% required to maintain SpO2 >90%; age ≥70 years in men or ≥75 years in women; and oliguria or dehydration.
• Two criteria were established as factors specifying the severity of the pneumonia itself: CRP ≥200 mg/L and shadows infiltrating more than two-thirds the area of one lung on chest radiography.
• Patients who satisfy up to two of the five criteria above to predict prognosis are classified in the mild group (Group A) if they do not satisfy either of the two criteria specifying severity of pneumonia, or in the moderate group (Group B) if they satisfy one or both of those two criteria. Patients who satisfy three or fewer of the five criteria to predict prognosis are classified in the severe group (Group C).
• When the new severity ratings were applied to the results of the national multicenter survey of hospital-acquired pneumonia, the mortality rate was found to be 12.1% (101/834) in the mild group (Group A), 24.9% (69/277) in the moderate group (Group B) and 40.8% (98/240) in the severe group (Group C). Statistically-significant differences were seen between groups, and patient classification may be useful as an indicator of prognosis (Fig. II-2).
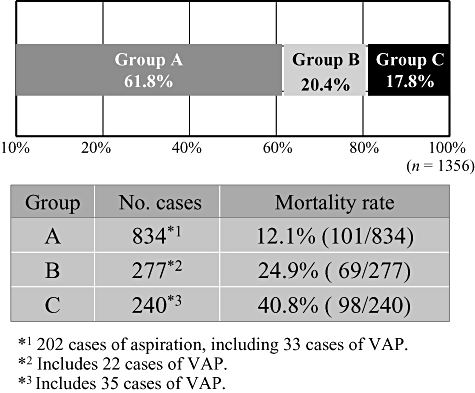
Number of cases and outcomes for each group. VAP, ventilator-assisted pneumonia.
INTRODUCTION
Hospital-acquired pneumonia is the second most common hospital-acquired infectious disease, and the one with the highest mortality rate.1 As many of these infections occur in compromised hosts and the causative microorganisms are drug-resistant strains of bacteria, treatment is difficult and the choice of antimicrobial agent is important. In the USA, the ATS has issued treatment guidelines for hospital-acquired pneumonia based on a policy of standardizing treatment.2,3 The ATS guidelines describe onset within 4 days of hospital admission as early onset, and onset after that as late onset. The medical insurance system in Japan differs from that in USA, and many patients under normal long-term hospitalization display immunocompetence relatively close to that of healthy people. Applying the treatment policies of the USA without modification would therefore be inappropriate. Standards suited to the situation in Japan were needed, and the Japanese Respiratory Society (JRS) therefore issued its first guidelines for the management of hospital-acquired pneumonia in adults in March 2002.4 In the 2002 guidelines, pathological classifications combined the presence or absence of risk factors for complicating hospital-acquired pneumonia and the severity of the pneumonia itself, and patients were divided into several groups.
After the 2002 guidelines were issued, a national multicenter survey of hospital-acquired pneumonia was conducted with the aim of investigating the validity of the guidelines.5 Patients in Groups III and IV were found to account for >90% of all patients. Only four Group I patients (0.3%) were identified in the whole study. Moreover, even among patients included in a single group, Group III, differences existed in mortality rate according to the severity of the pneumonia itself. This suggested a need to review the severity ratings and pathological classifications.5
Severity ratings in the 2002 guidelines also applied the findings of ‘Clinical evaluation methods for new antimicrobial agents to treat respiratory infections’,6 which emphasized the effectiveness of investigational agents. However, considering that prognosis is the primary end-point of pneumonia, establishing criteria that indicate prognosis would seem more appropriate, similar to the CURB-65 system of the British Thoracic Society (BTS)7 and A-DROP system of the JRS8 for community-acquired pneumonia.
Given the above reasons, new severity rating and pathological classification methods were prepared based on the national multicenter survey of hospital-acquired pneumonia.5 In investigating the new classification methods, individual risk factors corresponding to the PORT classifications in the community-acquired pneumonia guidelines of the IDSA were scored in accordance with weight, and severity ratings based on the total score were also considered. However, as our basic idea was for the content of the JRS hospital-acquired pneumonia guidelines to be adopted by practicing clinicians and widely used by non-specialists, a classification method that requires a cumbersome score calculation was considered inappropriate. The aim was therefore to develop simple classification standards that correspond to A-DROP in the JRS community-acquired pneumonia guidelines.
DEVELOPMENTAL PROCESS
1. Subjects
A survey on hospital-acquired pneumonia was conducted jointly by multiple centres in Japan from June 2002 to May 2004,5 and 1356 cases were collected from 254 hospitals as the subjects of evaluation. Among these 1356 cases, men, seniors (age ≥65 years), and patients with mechanical ventilator-associated pneumonia accounted for 69.2%, 81%, and 6.6% of all cases, respectively. Early-onset patients (onset ≤5 days before hospitalization) accounted for 9.2% of all patients, while patients with onset ≥30 days after admission accounted for 48.7%. The species presumed to be the causative microorganism in the greatest number of cases (25.5%) was Staphylococcus aureus (including MRSA), followed in order by Pseudomonas aeruginosa (18.3%) and Klebsiella (8.2%). The mortality rate at 30 days after the start of initial treatment was 19.8%.
2. Severity ratings and pathological classifications
Severity ratings were set with the focus on factors seen to affect prognosis in the hospital-acquired pneumonia survey,5 while also considering the PORT classification in the IDSA community-acquired pneumonia guidelines,9 the A-DROP system in the JRS community-acquired pneumonia guidelines,8 and the assessment items established as risk factors in the CURB-65 system of BTS community-acquired pneumonia guidelines.7
2.1 Selection of key assessment criteria (prognostic predictors)
Key assessment items, selected from among the items that were identified as most strongly affecting prognosis (morality rate), were malignant tumour or immunocompromised status, decreased level of consciousness, FiO2 >35% required to maintain SpO2 >90%, age ≥70 years in men, ≥75 years in women and ‘oliguria or dehydration’ (Tables II-1,2).
| Parameter | Odds ratio (95% CI) | P |
|---|---|---|
| Malignant tumour (yes) | 3.555 (2.497–5.061) | <0.0001 |
| Decreased level of consciousness (yes) | 2.406 (1.667–3.472) | <0.0001 |
| Cellular immunodeficiency (yes) | 2.035 (1.309–3.165) | 0.0016 |
| Sex (female/male) | 1.748 (1.214–2.519) | 0.0027 |
| Body temperature elevation (mild/moderate/severe) | 0.731 (0.589–0.908) | 0.0046 |
| CRP (mild/moderate/severe) | 1.322 (1.072–1.630) | 0.0091 |
| Oliguria (yes) | 2.863 (1.286–6.376) | 0.0100 |
| Shadow infiltration on chest radiography (mild/moderate/severe) | 1.285 (1.051–1.572) | 0.0147 |
| FiO2 >35% required (yes) | 1.567 (1.071–2.291) | 0.0206 |
| Neutropenic state (yes) | 0.553 (0.332–0.922) | 0.0231 |
| Aminoglycoside drug (administered) | 2.189 (1.057–4.533) | 0.0349 |
| Period of illness (≤4 days, 5–30 days, ≥31 days) | 1.296 (1.009–1.665) | 0.0425 |
- * Stepwise method, P < 0. 1.
- Variables used: sex, chest shadows, body temperature, dehydration, white blood cell count, CRP, severity of disease as stipulated in guidelines (cyanosis, decreased level of consciousness etc), risk factors (state in which aspiration may readily occur, chronic respiratory illness etc), special clinical conditions (neutropenic state etc), period of illness, age, type of initial medication (carbapenem antibiotics, fourth-generation cephem antibiotics etc).
| Parameter | Odds ratio (95% CI) | P |
|---|---|---|
| Malignant tumour (yes) | 0.501(0.385–0.652) | <0.0001 |
| Shadow infiltration on chest radiography (mild/moderate/severe) | 0.668 (0.570–0.783) | <0.0001 |
| Long-term administration of antimicrobials (yes) | 0.406 (0.265–0.622) | <0.0001 |
| Mechanical ventilator management required (yes) | 0.387 (0.243–0.616) | <0.0001 |
| Aspiration (yes) | 0.600 (0.436–0.826) | 0.0017 |
| Carbapenem antibiotics (administered) | 1.458 (1.141–1.863) | 0.0026 |
| Dehydration (mild/moderate/severe) | 0.792 (0.671–0.936) | 0.0062 |
| Oliguria (yes) | 0.378 (0.160–0.891) | 0.0261 |
| Chronic respiratory disease (yes) | 1.332 (1.003–1.768) | 0.0478 |
- * Stepwise method, P < 0.1.
- Variables used: sex, chest shadows, body temperature, dehydration, white blood cell count, CRP, severity of illness as stipulated in guidelines (cyanosis, decreased level of consciousness etc), risk factors (state in which aspiration may readily occur, chronic respiratory illness etc), special clinical conditions (neutropenic state etc), period of illness, age, type of initial medication (carbapenem antibiotics, fourth-generation cephem antibiotics etc).
Of these key factors, malignant tumour, decreased level of consciousness, FiO2 >35% required to maintain SpO2 >90%, and oliguria were recognized in the hospital-acquired pneumonia survey to strongly affect prognosis. The criterion of age ≥70 years in men or ≥75 years in women was established according to the A-DROP assessment items, as the men in the survey showed poor prognoses. ‘Immunocompromised status’ was taken as a single criterion based on the facts that prognosis was poor in patients with cellular immunodeficiency in the survey; distinguishing between conditions, such as cellular immunodeficiency, and humoral immunodeficiency, was difficult; recognized; cases of overlap were identified in the survey; and no conspicuous differences in the presumed causative agent were identified. As an assessment criterion, immunocompromised status was placed together with the similar condition of malignant tumour under ‘malignant tumour and immunocompromised status’. Dehydration was not a factor that indicated prognosis in the survey, but was found to affect treatment effectiveness, and so was adopted in the assessment criteria together with the similar condition of oliguria under ‘oliguria and dehydration’, in line with the assessment criteria in A-DROP.
2.2 Selection of secondary assessment criteria (factors for severity of pneumonia)
Considering the possibility that judgments made using only the key assessment criteria may miss cases with poor prognosis, CRP ≥200 mg/L, shadow infiltrating more than two-thirds of the area of one lung on chest radiography, chronic respiratory disease and aspiration, all of which are factors that indicate the severity of mainly pneumonia, were selected and considered as secondary judgment items.
CRP ≥200 mg/L and shadow infiltrating more than two-thirds of the area of one lung on chest radiography are factors that were identified as affecting prognosis in the survey. Although some studies10 have reported that CRP does not correlate with prognosis and investigations of community-acquired pneumonia, including CRP as an assessment item seems reasonable, as clear differences were seen in mortality rates calculated for groups with high and low CRP values using a reference value of 200 mg/L.
Chronic respiratory disease and aspiration were not factors indicating prognosis in the survey, but were adopted as assessment items because influences on effectiveness have been identified for these items.
2.3 Examination of the validity of severity assessment items
Cases that satisfy several or all of the key assessment items are placed in the severe group (Group C). Cases that do not satisfy several of the key assessment items are placed in the moderate group (Group B) if they satisfy either or both of the two secondary assessment items, while cases that do not satisfy either of the secondary assessment items are placed in the mild group (Group A). An investigation should be carried out to determine which of the items to select as assessment items.
Investigations were also conducted to determine what value to set as a reference value of CRP, and whether establishing aspiration and chronic respiratory disease as factors specifying severity was valid.
3. Calculation of mortality rates according to severity
This was evaluated by prognosis at 30 days after the start of initial treatment. ‘Death’ was defined as death regardless of the cause, and the mortality rate was calculated according to level of severity. A general target was set so that mortality rate in the severe group would be about 30%, with reference to mortality rate (33–50%) in the ATS/IDSA hospital-acquired pneumonia guidelines,3 in which the majority of cases involved VAP, and the mortality rate (29.2%)9 of Group V in the PORT classifications of the IDSA community-acquired pneumonia guidelines. Analysis was performed using Fisher's exact test, with a two-sided significance level of α= 0.05.
CONCLUSION
1. Investigation of severity ratings
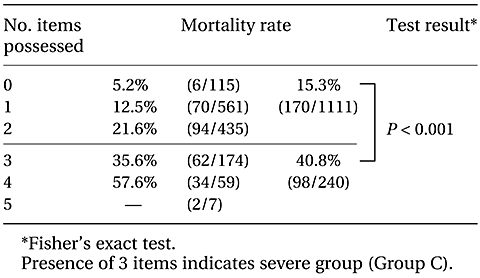
1.1 Stratification by key assessment item
A breakdown of the mortality rate by number of key assessment criteria possessed by patients is shown in Table II-3. Patients were classified using possession of three criteria as a reference, as this was the number closest to the target mortality rate (30%). This classification gives a mortality rate of 40.8% in the ‘≥3 more criteria’ group, significantly higher than the mortality rate of 15% in the ‘≤2’ group. In the following totals, patients with ≥3 key assessment criteria were placed in the severe group (Group C), and those with ≤2 were placed in the mild group (Group A) or moderate group (Group B).
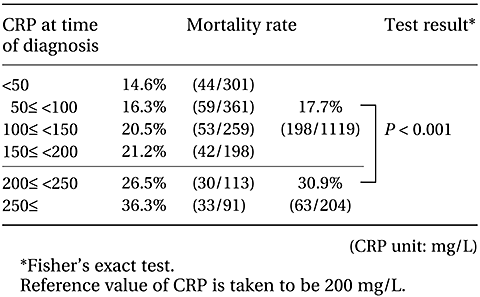
1.2 CRP reference value
A breakdown of mortality rates according to CRP level at the time of diagnosis is shown in Table II-4. CRP of 200 mg/L is closest to the target mortality rate (30%), and patients were classified using this as a reference value. The mortality rate was then 30.9% in the ‘≥200 mg/L’ group, significantly higher than the mortality rate (17.7%) in the ‘<200 mg/L’ group. In the tally below, sorting according to CRP was performed using 200 mg/L as the reference value.
1.3 Mortality rate by number of factors specifying the severity of pneumonia in mild-moderate groups
In addition to the secondary assessment parameters of CRP ≥200 mg/L and shadow infiltrating more than two-thirds of the area of one lung on chest radiography in the severity ratings, respective mortality rates were calculated for Criterion a (which includes aspiration and chronic respiratory disease), Criterion b (which includes only chronic respiratory disease), Criterion c (which includes only aspiration), and Criterion d (which includes neither aspiration nor chronic respiratory disease). The results are shown in Table II-5.
| Criterion | Category | No. cases | Mortality rate | Test result* | ||
|---|---|---|---|---|---|---|
| a | Mild group | 372 | 12.4% | (46/371) | P= 0.063 | |
| Moderate group | 743 | 16.8% | (124/740) | |||
| Severe group | 241 | 40.8% | (98/240) | — | ||
| b | Mild group | 518 | 12.2% | (63/515) | P= 0.009 | |
| Moderate group | 597 | 18.0% | (107/596) | |||
| Severe group | 241 | 40.8% | (98/240) | — | ||
| c | Mild group | 636 | 12.1% | (77/635) | P= 0.001 | |
| Moderate group | 479 | 19.5% | (93/476) | |||
| Severe group | 241 | 40.8% | (98/240) | — | ||
| d | Mild group | 838 | 12.1% | (101/834) | P < 0.001 | |
| Moderate group | 277 | 24.9% | (69/277) | |||
| Severe group | 241 | 40.8% | (98/240) | — | ||
- * Fisher's exact test.
The mortality rate of the moderate group was 16.8% (124/740) when Criterion a was applied, 18.0% (107/596) with Criterion b, 19.5% (93/476) with Criterion c and 24.9% (69/277) with Criterion d. Significant differences were seen between mortality rates of the moderate and mild groups, except when Criterion a was used.
When classifying based on these criteria, the mortality rate was 12.1–12.4% in the mild group, 16.8–24.9% in the moderate group and 40.8% in the severe group. The mortality rate of the moderate group was closest to the median value between the mortality rates of the mild and severe groups. Simple criterion d was thought to be the most appropriate, and was thus adopted in the new severity classification. The resulting severity rating resembles that seen in Figure II-1.
INVOLVEMENT OF MRSA
In the ATS/IDSA hospital-acquired pneumonia guidelines, the use of anti-MRSA agents is recommended in the empirical therapy when a recognized risk of MRSA or a high incidence in the community is present.3 Although there was a high rate of Staphylococcus aureus, mainly MRSA, as the presumed causative microorganism in the multicenter survey, the percentage of cases in which anti-MRSA agents were used in the empiric therapy was low, at 3.8%.
Considering that both clinical response and prognosis were poor in patients from whom MRSA was isolated,5 it is thought that treatment for patients with a risk of MRSA should include anti-MRSA agents appropriate to the early stage.
In the multicenter survey, a significant risk of drug-resistant strains of bacteria was seen in patients with suspected MRSA infection from Gram stain or other means and one of the following:
- •
Long-term administration of antibacterial agents (about 2 weeks)
- •
History of long-term hospitalization
- •
History of MRSA infection or colonization
- •
Aggressive use of combined anti-MRSA agents from an early stage may lead to better outcomes (Table II-6).
| • Period of illness | ||||
| <15 days(n= 172) | ≤15 days(n= 395) | Test result* | ||
| Staphylococcus aureus + | 64 (37.2%) | 141 (35.7%) | NS | |
| MSSA | 13 (7.6%) | 22 (5.6%) | NS | |
| MRSA | 40 (23.3%) | 99 (25.1%) | NS | |
| • Mechanical respirator management | ||||
| No(n= 503) | Yes(n= 64) | Test result* | ||
| Staphylococcus aureus + | 185 (36.8%) | 20 (31.3%) | NS | |
| MSSA | 30 (6.0%) | 5 (7.8%) | NS | |
| MRSA | 125 (24.9%) | 14 (21.9%) | NS | |
| • Long-term administration of antimicrobials | ||||
| No(n= 501) | Yes(n= 66) | Test result* | ||
| Staphylococcus aureus + | 180 (35.9%) | 25 (37.9%) | NS | |
| MSSA | 34 (6.8%) | 1 (1.5%) | NS | |
| MRSA | 116 (23.2%) | 23 (34.8%) | P= 0.047 | |
| • Period of hospitalization | ||||
| <5 days(n= 49) | ≤5 days(n= 518) | Test result* | ||
| Staphylococcus aureus + | 16 (32.7%) | 189 (36.5%) | NS | |
| MSSA | 5 (10.2%) | 30 (5.8%) | NS | |
| MRSA | 6 (12.2%) | 133 (25.7%) | P= 0.037 | |
| Significant difference in MRSA isolation frequency after 5 days of hospitalization. | ||||
| • Period of mechanical respirator management | ||||
| <5 days(n= 13) | ≤5 days(n= 51) | Test result* | ||
| Staphylococcus aureus + | 4 (30.8%) | 16 (31.4%) | NS | |
| MSSA | 3 (23.1%) | 2 (3.9%) | NS | |
| MRSA | 1 (7.7%) | 13 (25.5%) | NS | |
| No significant difference in MRSA isolation frequency between early ventilator-assisted pneumonia (VAP) and late VAP. | ||||
- * Fisher's test.
- + Includes MSSA, MRSA.
- MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, Methicillin-susceptible Staphylococcus aureus.
RELATION BETWEEN NEW SEVERITY RATINGS AND SEVERITY RATINGS FROM THE 2002 GUIDELINES
In the new severity ratings, patients are divided into three distinct groups with indicators of prognosis. However, the mild group includes a certain percentage of patients who were considered severe in the 2002 guidelines, and the severity of disease in these patients may be underestimated.11 Verification of the validity of the newly established guidelines under conditions of clinical use will thus be important, and efforts will be needed to conduct regular reviews with the aim of further improving the guidelines.
CONFLICT OF INTEREST
No conflict of interest has been declared by The Committee for the Japanese Respiratory Society guidelines for the management of respiratory infections.