Staging and diagnosis of non-small cell lung cancer: Invasive modalities
Abstract
Abstract: During the staging process of lung cancer, accurate mediastinal lymph node staging is one of the more important factors that affects patient outcome. Several different invasive and non-invasive modalities exist for mediastinal staging. Invasive tests include mediastinoscopy, thoracoscopy, transbronchial needle aspiration, transthoracic needle aspiration, endoscopic ultrasound-guided fine-needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration. Each of the invasive tests has limitations for particular locations, has particular risks and requires specific skills. Invasive tests are often used to confirm the staging of lung cancer, but are also used to obtain a diagnosis. The best approach depends upon the clinician’s assessment of the patient. This review discusses the invasive staging tests that are available, with particular emphasis on newer modalities, especially endobronchial ultrasound-guided transbronchial needle aspiration. In addition, the current advances in diagnostic bronchoscopy for lung cancer will be reviewed.
INTRODUCTION
Despite advances in surgical and multimodality treatment, lung cancer is still the leading cause of death from malignant diseases worldwide.1 The diagnosis and staging of non-small cell lung cancer (NSCLC) are usually undertaken together, because the approach to achieving a diagnosis usually depends on the presumed stage of the disease.2 However, after an anatomical pathological diagnosis of NSCLC has been made, accurate staging of the disease is important not only to determine the prognosis but also to decide the most suitable treatment plan. The most significant treatment decision is between those patients who can benefit from surgical resection or those who should receive chemotherapy or radiation or both.
The TNM staging system is used for staging of NSCLC. T stands for local tumour extension, N for lymph node metastasis and M for distant metastases. A new international staging system was proposed by Mountain in 1997.3 For the description of the N factor, the lymph node map by Naruke et al. and its revisions are often used and are useful in understanding the mediastinum (Table 1).4,5 Although a complete workup for metastases is important for staging, the presence of lymph node metastases remains one of the most adverse factors for prognosis in NSCLC. The presence of mediastinal lymph node involvement indicates the presence of stage IIIA or IIIB, which suggests inoperability and/or the need for treatment by chemotherapy and/or radiotherapy.
| Superior mediastinal nodes | Highest mediastinal | 1 |
| Upper paratracheal | 2 | |
| Pre-vascular and retrotracheal | 3 | |
| Lower paratracheal | 4 | |
| Aortic nodes | Sub-aortic (A–P window) | 5 |
| Para-aortic (ascending aorta or phrenic) | 6 | |
| Inferior mediastinal nodes | Subcarinal | 7 |
| Paraesophageal | 8 | |
| Pulmonary ligament | 9 | |
| N1 nodes | Hilar | 10 |
| Interlobar | 11 | |
| Lobar | 12 | |
| Segmental | 13 | |
| Subsegmental | 14 |
- Adapted from Mountain and Dresler.4
Mediastinal lymph node staging can be divided into non-invasive (imaging) and invasive (sampling) procedures. CT, magnetic resonance imaging, PET and PET–CT are used for non-invasive imaging.6–11 Other imaging modalities in specialized centres have reported the use of transesophageal ultrasonography (EUS) and endobronchial ultrasound (EBUS) using a radial probe for detecting even small mediastinal lymph nodes.12,13 Despite the advancement in the latest imaging techniques, while non-invasive tests can identify nodes suspicious for malignancy, they do not provide definitive tissue diagnosis. Cytological or histological confirmation of suspected metastases (invasive staging) is required.
Invasive techniques utilize surgical open biopsy or needle biopsy to obtain tissue samples to confirm the diagnosis of metastatic disease (Table 2). Tissues obtained by surgical open biopsies are usually bigger than needle biopsied specimens. However, surgical biopsies require general anaesthesia and are more ‘invasive’ than needle biopsies. Surgical open biopsy can be performed by standard cervical mediastinoscopy, extended cervical mediastinoscopy, anterior mediastinotomy or video-assisted thoracoscopic surgery (VATS). Needle biopsy techniques include transbronchial needle aspiration (TBNA), transthoracic needle aspiration (TTNA), endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and, most recently, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA).
| Method | Accessible nodal stations |
|---|---|
| Standard mediastinoscopy | Superior mediastinal, subcarinal |
| Extended mediastinoscopy | Aortic nodes |
| Anterior mediastinotomy† | Aortic nodes |
| VATS | Superior mediastinal (right), subcarinal, aortic nodes |
| TBNA | Superior mediastinal, subcarinal, N1 nodes |
| TTNA | Superior mediastinal (anterior) |
| EUS-FNA | Subaortic, inferior mediastinal |
| EBUS-TBNA | Superior mediastinal, subcarinal, N1 nodes |
- † Chamberlain procedure.
- EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration; TBNA, transbronchial needle aspiration; TTNA, transthoracic needle aspiration; VATS, video-assisted thoracoscopic surgery.
In patients suspected of having NSCLC, the method of achieving a diagnosis depends on the size and location of the primary tumour, the presence of metastases and the overall clinical presentation of the patient. Patients with metastatic disease may be most efficiently diagnosed by a biopsy of the site of metastasis. NSCLC with extensive infiltration of the mediastinum may be diagnosed by one of the techniques introduced above that has the most favourable results. For the diagnosis of the primary tumour, various techniques are available, including sputum cytology, bronchoscopic biopsy and TTNA. In patients with a central lesion, sputum cytology is recommended as a reasonable first step in the diagnostic workup,2 although to confirm diagnosis of lung cancer, bronchoscopy is the most sensitive way. In the case of a peripheral lesion, TTNA has a much higher sensitivity than bronchoscopy and is the recommended choice of procedures.2 However, recent advances in bronchoscopy have improved the diagnostic yield of peripheral lesions suspicious of lung cancer.
This paper reviews the current concepts in invasive staging of NSCLC and also presents an overview of the recent developments in EBUS for mediastinal lymph node staging. In addition, the current advances in diagnostic bronchoscopy for lung cancer will be reviewed.
TECHNIQUES OF INVASIVE MEDIASTINAL STAGING
Cervical mediastinoscopy
Mediastinoscopy has remained the ‘gold standard’ in invasive staging tests of the mediastinum.14–19 It is usually performed under general anaesthesia in the operating room. In most centres, patients are usually discharged the same day if they are in a stable condition. The patient’s neck is hyperextended to facilitate insertion of the scope. A standard cervical mediastinoscopy involves a small skin incision above the suprasternal notch, and dissection is carried down to the pretracheal fascia. A blunt digital dissection is performed with the index finger down to the subcarinal level with direct palpation of the vessels and abnormal lymph nodes if present. The scope is then passed down the pretracheal plane into the mediastinum. The operator looks directly into the mediastinoscope or by a video-assisted view. In teaching hospitals, the latter technique allows for both the teacher and the surgeon the opportunity to explore the mediastinum with a low risk of morbidity.20–23 With a blunt suction instrument, both paratracheal nodal stations (stations 2R, 2L, 4R, 4L) and anterior subcarinal nodes (station 7) are sampled. Pretracheal nodes (stations 1, 3) are also accessible, but posterior subcarinal (station 7), aortopulmonary window (station 5), para-aortic (station 6), and inferior mediastinal (stations 8, 9) nodes cannot be sampled. After haemostasis the wound is closed in layers.
The complications of mediastinoscopy are extremely low (morbidity 1.5% and mortality 0.4%) if performed by experienced surgeons.20,21 However, there is the potential for catastrophic complications. Minor complications include left recurrent nerve injury (0.7–0.9%), pneumothorax (0.5–0.7%) and wound infection. Major complications include bleeding due to injury of major blood vessels (0.1–0.2%), tracheobronchial injury and oesophageal trauma.20–22
The specificity of mediastinoscopy is reported to be 100%. However, these values cannot be fully assessed because patients with a malignant lymph node on mediastinoscopy have not been subjected to any further procedures to confirm the results. However, it is clear that the false positive rate of mediastinoscopy is very low. The sensitivity, on the other hand, has been reported to be in the range of 80–85%, with an average false negative rate of 10%. In a meta-analysis of 14 studies with 5687 evaluable patients of cervical mediastinoscopy, the overall sensitivity was 81% (95% CI: 76–97%) and the negative predictive value was 91% (range 58–97%).24 The false negative cases of mediastinoscopy can be explained in part by the presence of nodes that are not accessible by the mediastinoscope.14,15 However, it is also affected by the technical error of the surgeon during the procedure. All of the paratracheal and subcarinal lymph node stations (2R, 2L, 4R, 4L, 7) should always be examined and at least one node from each station should be biopsied unless none are present.
There is still debate as to whether mediastinoscopy should be performed on all operable NSCLC with or without evidence of enlarged lymph nodes on CT. Those in favour of routine mediastinoscopy cite the low complication rate of mediastinoscopy and the high false negative rate of CT in detecting lymph node metastases as the reason.20 On the other hand, a large multicentre study by the Canadian Lung Oncology Group found that performing routine mediastinoscopy for all patients failed to preclude significantly larger numbers of patients from unnecessary thoracotomy.25 Recently, with the introduction of PET for imaging of the mediastinum, routine mediastinoscopy may not be suggested for all NSCLC, especially for clinical stage I NSCLC staged by PET and CT.26
Although there is still debate surrounding the role of mediastinoscopy in lung cancer staging, the indications for performing the procedure should include all NSCLC patients otherwise eligible for surgery who have a suspicious mediastinal lymph node on CT or PET and patients with normal CT or PET who nonetheless require assessment of the mediastinal lymph node metastases. These patients include those with: (i) the presence of a centrally located tumour or large peripheral tumour (T2 or T2), (ii) histologically proven adenocarcinoma, and (iii) marginal function tests for major lung resection.27,28 Contraindications for mediastinoscopy include patients with severe cervical arthritis who are limited to overextension of the neck and patients with cutaneous tracheostomy.
Repeat mediastinoscopy is a diagnostic procedure for preoperative nodal staging in patients with insufficient first mediastinoscopy, with recurrent or second primary lung cancer, and restaging after induction chemotherapy or chemoradiotherapy. Because of the presence of inseparable adhesions and fibrosed tissue, repeat mediastinoscopy may not be performed in some patients. However, it can be accomplished in most cases with care and patience. Recent studies have shown that in patients, after induction chemotherapy, repeat mediastinoscopy is less sensitive than the primary mediastinoscopy because of the adhesion and fibrotic tissues.29–31 Patients with persistent N2 or N3 disease in repeat mediastinoscopy have a poor survival.30,31
Extended mediastinoscopy
The major limitation of standard cervical mediastinoscopy is its inability to access the aortopulmonary window and para-aortic lymph nodes (stations 5, 6). A complementary procedure to standard mediastinoscopy is extended mediastinoscopy, originally described by Kirschner and popularized by Ginsberg et al.32 It is a single-staging procedure for the assessment of the mediastinum in patients with left upper lobe tumours. After a standard cervical mediastinoscopy, the mediastinoscope is inserted through the suprasternal notch and directed lateral to the aortic arch. Lymph node biopsies can be taken from stations 5 and 6. The only complications reported are one aortic injury and one stroke.32–35 The sensitivity is reported to be 69–81%, and a false negative rate of 9–11% has been obtained.32,33 The drawback is that it has only been used in a few centres and is not a routine procedure for all thoracic surgeons.
Anterior mediastinotomy
Left anterior mediastinotomy was originally introduced by McNeill and Chamberlain in 1966 (also known as the Chamberlain procedure).21,36 It was used for the diagnosis and resectability of left upper lobe tumours or anterosuperior mediastinum. It is performed under general anaesthesia in the operating room. The patient is placed in supine position and a skin incision is made in the second or third intercostal space just to the left of the sternum. The internal mammary artery is retracted and preserved and the scope is inserted. The costal cartilage may be removed for optimal view, but usually visualization is carried out between the ribs. After exploration, biopsies are taken from stations 5 and 6. The reliability of this procedure has not been fully investigated, probably because of the difficulties in the visualization of the anterior mediastinum and its lack of use in clinical practice. There are only few reports recently on the value of the procedure combined with cervical mediastinoscopy.37,38 The overall sensitivity for detecting mediastinal lymph node involvement of the anterior mediastinum is reported to be 63–86%, but when it is coupled with a standard cervical mediastinoscopy, the combined sensitivity raises significantly to 87%.39–41 The reported complications are very low, which include superficial wound infections, bleeding and pneumothoraces.
Video-assisted thoracoscopic surgery
Video-assisted thoracoscopic surgery is an effective tool for diagnosing NSCLC in patients with peripheral lung nodules suspicious for malignancy. By a no-touch technique, VATS can assist in locating and resecting the nodule. For biopsy purposes, VATS is usually performed through 5–20 mm skin incisions placed at three sites in the intercostal space. The thoracoscope is inserted through one of the lower ports and different types of forceps are inserted through the other ports. VATS requires general anaesthesia with double-lumen endotracheal intubation to obtain atelectasis of the ipsilateral lung. The presence of a strong pleural adhesion is a contraindication of this procedure.
The role of VATS for staging mediastinal lymph nodes is still controversial.27,42 The lymph nodes accessible by VATS include mediastinal lymph nodes on the ipsilateral side. The highest superior nodes are not accessible. The left paratracheal lymph nodes (stations 2L, 4L) are usually technically difficult to biopsy. As an advantage over standard cervical mediastinoscopy, VATS allows biopsy of aortopulmonary (station 5) and para-aortic (station 6) lymph nodes with a high diagnostic accuracy.43,44 However, these nodes can be biopsied using extended mediastinoscopy or anterior mediastinotomy. Only paraesophageal (station 8) and inferior pulmonary ligament (station 9) nodes are more readily accessible using VATS. The disadvantage compared with mediastinoscopy is that VATS allows only exploration of the ipsilateral side.
In addition to mediastinal staging, VATS may provide additional information on tumour status, pleural carcinomatosis or malignant pleural effusions. Although there are no guidelines for using VATS in lung cancer staging,28 routine use of VATS before thoracotomy for lung cancer can eliminate unnecessary surgery and guide surgeons to determine the optimal level for thoracotomy.45
Transbronchial needle aspiration
Transbronchial needle aspiration for mediastinal staging is performed through the bronchoscope under local anaesthesia. It can be performed as an outpatient procedure with no significant morbidity.46–48 The needle catheter, which comes in various sizes, is passed through the working channel of the bronchoscope and guided to the area of interest. The needle is then advanced through the tracheobronchial wall into the lymph node. TBNA can be readily performed on the hilar and mediastinal lymph nodes adjacent to the tracheobronchial wall. Aspiration biopsies are then obtained. Rapid on-site cytological evaluation of the aspirates improves the yield, is cost-effective and eliminates unnecessary passes during the procedure.49 However, conventional TBNA is a blind procedure preventing target visualization and therefore the yield for TBNA varies widely (14–91%).24 A meta-analysis of 12 studies in 910 evaluable patients showed a sensitivity of 76%.24 The specificity was very high (96%). Although the sensitivity was moderate, the patients included in the TBNA studies had enlarged mediastinal lymph nodes. The high false negative rate makes TBNA less useful for staging of the mediastinum. Therefore, TBNA would probably be the preferred minimally invasive method for patients with radiographic evidence of enlarged mediastinal lymph nodes adjacent to the airways, as bronchoscopy is usually performed in lung cancer patients and assessment for endobronchial lesions can be performed during the same procedure.
Imaging of mediastinal lymph nodes during the procedure increases diagnostic yield. In particular, the use of CT fluoroscopy during TBNA has been reported to be of benefit in the evaluation of mediastinal lymph nodes.50–52 However, the use of CT guidance can be costly, requires the use of a CT suite, and patients as well as the examiner are exposed to radiation. These disadvantages may preclude the widespread use of the procedure. EBUS using a radial probe has been shown to improve the yield of TBNA.53,54
Transthoracic needle aspiration
Transthoracic needle aspiration is a procedure usually carried out by the interventional radiologist. Under local anaesthesia, a needle is inserted percutaneously under CT or fluoroscopic guidance. The procedure is relatively safe and well tolerated by most patients. Depending on the size of the needle used, core histological biopsies can be obtained in addition to cytological specimen. TTNA can be used for the diagnosis of suspected lung cancer of peripheral parenchymal masses as well as for the diagnosis and staging of the mediastinum. The sensitivity of TTNA for the staging and diagnosis of the mediastinum has been reported to be 91% (meta-analysis of five studies in 215 patients).24 However, the false negative rate was 20–50%, and patients enrolled in the studies generally had enlarged mediastinal lymph nodes. Therefore, TTNA may not be the technique of choice for the staging of the mediastinum but still remains useful for confirming the diagnosis of enlarged mediastinal masses.
Because of the anatomy of the mediastinum with major thoracic vessels or the heart proximal to the mediastinal lymph nodes, there are limitations on which lymph nodes are accessible by TTNA. Contraindications for TTNA are COPD, poor lung function, clotting disorders and having a single lung.55–57 Iatrogenic pneumothorax is the most frequent complication (5–60%), and because lung cancer patients often suffer from chronic airway diseases such as COPD, TTNA may not be an option for these patients. Implantation of tumour cells is rare but has been reported.58,59
Endoscopic ultrasound guided fine-needle aspiration
Endoscopic ultrasound guided fine-needle aspiration became an established method for examining the mediastinum in patients with lung cancer in the early 1990s, and the role of EUS-FNA in lung cancer staging continues to evolve.60–65 The EUS scope has an ultrasound transducer on the tip that allows ultrasound imaging of structures adjacent to the gastrointestinal tract, especially the posterior mediastinum and upper retroperitoneum. The mediastinal levels that are accessible include the aortopulmonary (station 5), subcarinal (station 7), paraoesophageal (station 8) and inferior pulmonary ligament (station 9). EUS-FNA is an outpatient procedure that is performed under conscious sedation. A 19- or 22-gauge fine aspiration needle is passed through a working channel and directly into the target under real-time ultrasonography. There is direct visualization of the needle during the aspiration. The overall risk of EUS-FNA is approximately 0.5% and may include perforation of the bowel wall or posterior pharynx, infection, haemorrhage and cardiac or respiratory complications related to the sedation medications.
In a meta-analysis of 14 studies, the sensitivity of EUS-FNA was 81–97% and the specificity was 83–100% for the diagnosis of posterior mediastinal lymphadenopathy in NSCLC.6 It also spares more invasive staging procedures such as mediastinoscopies and thoracotomies.64 However, the major drawback of EUS-FNA is the high false negative rate. Therefore, EUS-FNA should be performed primarily on patients with radiological evidence of mediastinal lymphadenopathy.
Recent comparisons of EUS-FNA and PET for the evaluation of the posterior mediastinum have shown that EUS-FNA is either equal or more accurate than PET.66,67 These studies suggest that because of the high false positive rate of PET, EUS-FNA should be performed for histological confirmation of the mediastinal lymph node status.
Another field of interest is the role of molecular staging in lung cancer. EUS-FNA has been shown to detect the presence of lung cancer-associated genes in mediastinal lymph node aspirates from patients with NSCLC.68 Overexpression of the KS1/4 gene in biopsied lymph nodes using RT-PCR may play a potential role in identifying a disease.68 In addition, EUS-FNA may play a role in the identification of gene promoter hypermethylation, which may enable the detection of micrometastases in mediastinal lymph nodes.69
Endobronchial ultrasound-guided transbronchial needle aspiration
The new convex probe endobronchial ultrasound (CP-EBUS) is used to perform EBUS-TBNA (XBF-UC260F-OL8, Olympus, Tokyo, Japan) (Fig. 1). The CP-EBUS is integrated with a convex transducer (7.5 MHz) which scans parallel to the insertion direction of the bronchoscope. Images can be obtained by directly contacting the probe or by attaching a balloon on the tip and inflating with saline. The ultrasound image is processed in a dedicated ultrasound scanner (EU-C2000, Olympus, Tokyo, Japan) and is visualized along with the conventional bronchoscopy image on the same monitor. The ultrasound images can be frozen and the size of lesions can be measured in two dimensions by the placement of cursors. This system also has the Doppler mode. The outer diameter of the insertion tube of the flexible bronchoscope is 6.7 mm and that of the tip is 6.9 mm. The angle of view is 90° and the direction of view is 30° forward oblique.
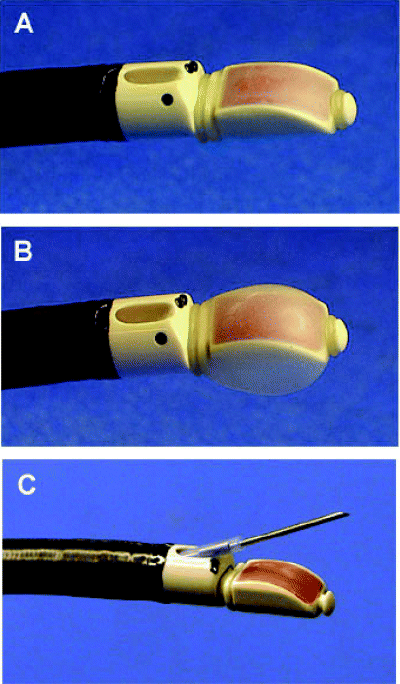
Convex probe endobronchial ultrasound. The tip of the convex probe endobronchial ultrasound (Olympus XBF-UC260F-OL8, Olympus, Tokyo, Japan) has a linear curved array ultrasonic transducer of 7.5 MHz (a). The balloon attached to the tip of the bronchoscope is inflated with normal saline (b), and a dedicated transbronchial needle aspiration needle is inserted through the working channel (c).
Endobronchial ultrasound-guided transbronchial needle aspiration is performed under local anaesthesia and conscious sedation in an outpatient setting. CP-EBUS is inserted into the airway to identify the lesion of interest. The surrounding structures are also visualized with the use of the Doppler mode, which is useful confirming blood vessels. EBUS-TBNA is performed by inserting a dedicated 22-gauge TBNA needle, which is equipped with an internal sheath, through the working channel of the bronchoscope. The lesion of interest is punctured under direct EBUS guidance. The inner diameter of this needle is nearly equal to that of a conventional 21-gauge needle, which allows the sampling of histological cores in some cases. The needle is also equipped with an internal sheath, which is withdrawn after passing the bronchial wall, avoiding contamination during EBUS-TBNA. The needle can be visualized through the optics and on the ultrasound image. After the initial puncture, the internal sheath is used to clean out the internal lumen clogged with bronchial membrane. The internal sheath is removed and negative pressure is applied by a syringe. The needle is moved back and forth inside the lesion. Finally, the needle is retrieved and the internal sheath is used once again to push out the histological core. The aspirated material is smeared onto glass slides. Smears are air-dried and fixed in 95% alcohol. Dried smears are evaluated by an on-site cytopathologist to confirm adequate cell material. Furthermore, Papanicolaou staining and light microscopy are carried out by a cytopathologist. Histological specimens obtained are fixed in formalin before being sent to the pathology department.
Endobronchial ultrasound-guided transbronchial needle aspiration has access to all of the mediastinal lymph node stations accessible by mediastinoscopy as well as N1 nodes. Lymph node stations accessible are the highest mediastinal (station 1), the upper paratracheal (station 2R, 2L), the lower paratracheal (station 4R, 4L), the subcarinal (station 7), as well the hilar (station 10), the interlobar (station 11) and the lobar (station 12) lymph nodes (Fig. 2).
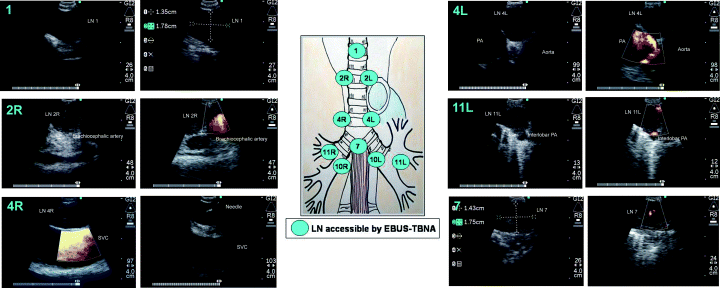
Lymph node (LN) stations accessible by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). EBUS-TBNA has access to all of the mediastinal LN stations accessible by mediastinoscopy as well as N1 nodes. The Doppler mode enables differentiation of LN from large vessels. LN stations accessible are the highest mediastinal (station 1), the upper paratracheal (station 2R, 2L), the lower paratracheal (station 4R, 4L), the subcarinal (station 7), as well the hilar (station 10), the interlobar (station 11) and the lobar (station 12) LN.
The convex probe EBUS was first reported to be useful in the visualization and TBNA of hilar lymph nodes in surgically resected lung cancer specimens before its clinical use.70 After 4 years of clinical use, a growing number of researches have shown its usefulness and accuracy for mediastinal lymph node sampling.71–78
A preliminary report on the clinical application in 70 patients showed that EBUS-TBNA was successfully performed to obtain samples from mediastinal (n = 58) and hilar (n = 12) lymph nodes. The sensitivity, specificity and accuracy of EBUS-TBNA in distinguishing benign from malignant lymph nodes were 95.7%, 100% and 97.1%, respectively.72 This was the first report to show that EBUS-TBNA can be safely performed under local anaesthesia with a high yield. The first article to report the diagnostic yield of EBUS-TBNA in a prospective study for lymph node staging of lung cancer not only showed the high yield, but also the impact of this procedure in patient management.73 In 105 patients, EBUS-TBNA was successfully performed to obtain samples from 163 lymph nodes. With respect to the correct prediction of lymph node stage, EBUS-TBNA had a diagnostic accuracy rate of 96.3%. In the 20 suspected lung cancer cases, mediastinal lymph node was used for the tissue diagnosis of malignancy as well as staging. In addition, as a result of EBUS-TBNA, 29 mediastinoscopies, eight thoracotomies, four thoracoscopies and nine CT-guided TTNA were avoided. EBUS-TBNA spares invasive staging procedures, which has a major impact on patient management in lung cancer.
More recently, a multicentre study of a larger number of patients showed the effectiveness and accuracy of EBUS-TBNA for the evaluation of mediastinal lymph nodes.76 In 502 patients, 572 lymph nodes were punctured using the CP-EBUS, resulting in successful diagnoses in 535 lymph nodes (94%). The sensitivity was 94% and the specificity was 100%.76 It has also been shown to be useful not only for the assessment of enlarged lymph nodes, but for also for the assessment of lymph nodes in patients with a radiologically normal mediastinum.77
Although recent advances in imaging, such as PET with 18F-fluorodeoxyglucose, has been shown to be more accurate for the evaluation of the mediastinum compared with CT, tissue confrimation of PET-positive lesions are recommended to prove that the lesions are truly malignant.79 A more recent study comparing EBUS-TBNA, CT and PET for lymph node staging of lung cancer showed a higher yield in favour of EBUS-TBNA.78 A total of 102 potentially operable patients with proven (n = 96) or radiologically suspected (n = 6) lung cancer were included in the study. The sensitivities of CT, PET and EBUS-TBNA for the correct diagnosis of mediastinal and hilar lymph node staging were 76.9%, 80.0% and 92.3%, respectively. The The specificities were 55.3%, 70.1% and 100%. The diagnostic accuracies were 60.8%, 72.5% and 98.0%. EBUS-TBNA was proven to have a higher sensitivity as well as specificity compared with CT or PET, for mediastinal staging in patients with potentially resectable lung cancer.
Combining EBUS-TBNA and EUS-FNA
Endobronchial ultrasound-guided transbronchial needle aspiration and endoscopic ultrasound-guided fine-needle aspiration are complementary with EBUS, having better access to anterior and superior mediastinal lymph nodes and EUS having better access to posterior and inferior mediastinal lymph nodes. Theoretically, by combining EBUS and EUS, the whole mediastinum is accessible. Two important studies report the combined use of the two minimally invasive modalities to evaluate the mediastinum.74,75 For mediastinal staging, EUS provided additional information to EBUS-TBNA in 20 lung cancer patients with enlarged mediastinal lymph nodes or mediastinal lesions.74 In a larger study of 33 patients for the staging of lung cancer, a total of 119 lesions were sampled by EUS-FNA (n = 50) and EBUS-TBNA (n = 60).75 When EBUS-TBNA samples were compared with EUS-FNA samples, 11 additional cancer diagnoses and three samples with suspicious cells were obtained by EBUS-TBNA that had not been obtained by EUS-FNA. Conversely, EUS-FNA diagnosed 12 additional cancer diagnoses, one suspicious and one specific benign diagnosis in addition to EBUS-TBNA. With a combined approach, the accuracy for the diagnosis of mediastinal cancer was 100%.
ADVANCES IN DIAGNOSTIC BRONCHOSCOPY FOR LUNG CANCER
Current techniques in diagnostic bronchoscopy
Diagnostic bronchoscopic procedures are performed through the working channel of the bronchoscope. Depending on the size of the channel, various accessories can be introduced into the channel for biopsies. Biopsy forceps for tissue diagnosis, needles for transbronchial needle aspirations and brushes for cytology are most commonly used. The sensitivity of bronchoscopic biopsy for central, endobronchial lesions have been reported to be very high (88%) in a review of 30 studies.80 However, the yield for peripheral lesions is not so promising. A meta-analysis from 30 studies showed that brushing provided the highest sensitivity (52%), followed by transbronchial biopsies (TBB) (46%) and BAL (43%). TBNA showed a higher sensitivity (67%); however, because of the limited number of studies, the data require cautious interpretations. The overall sensitivity for all modalities was 69%.80
From our own experience in bronchoscopic diagnosis of 1003 primary lung cancers, TBNA had the highest sensitivity (86.4%), followed by brushing (64.8%) and TBB (72.0%). With the combined procedures, the overall sensitivity was 92.7%. TBNA had a high sensitivity of 75.9% for even peripheral lesions 2 cm or less in diameter.81 The differences in the yield of transbronchial biopsies are probably associated with the fact that when accessing peripheral lesions without direct visualisation, the yield in less experienced hands can be suboptimal.
Endobronchial ultrasound
The application of EBUS in the field of bronchoscopic practice was first introduced in 1992.82 Currently, EBUS has broadened the diagnostic possibilities for bronchial and mediastinal pathology. The miniature 20-MHz radial probe (UM-3R, Olympus) (Fig. 3) is used as guidance for the diagnosis of peripheral intrapulmonary lesions.83 As the air content of the lung parenchyma completely reflects the ultrasound signal, pulmonary masses can be precisely located by EBUS. Pulmonary lesions have a hypoechoic texture and a sharply defined border because of strong reflective interface between the aerated lung and the lesion.
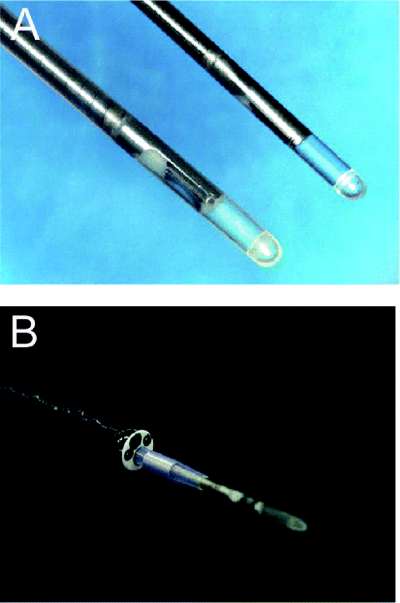
Ultra-miniature radial probe used for peripheral intrapulmonary lesions. Two different sizes of 20-MHz mechanical radial-type probe for peripheral lesions are available (a). The probe is placed into a guide-sheath (GS) and the GS-covered probe can be inserted into a 2.0-mm working channel of a fiberoptic bronchoscope (b).
The first prospective study on EBUS guidance for TBB in 50 patients showed that approaching peripheral lesions through EBUS guidance yielded a similar success rate as fluoroscopy guidance (80% vs. 76%).84 EBUS guidance was at least equivalent to fluoroscopy without the accompanying radiation exposure. This study was confirmed by a similar study (n = 50) where the diagnostic yield of EBUS-guided TBB was 76%.85 More recently, a large-scale, prospective, randomized study in 221 patients compared EBUS-guided TBB with TBB in patients with lesions <3 cm which showed a sensitivity of 79% in the EBUS group and 55% in the TBB group (P = 0.004).86
With the introduction of the ultra miniature 20-MHz radial probe (UM-S20-20R) (Fig. 3), the probe can now be placed into a guide-sheath (GS), and the GS-covered probe can be inserted into a 2.0-mm working channel of a fiberoptic bronchoscope. The GS-covered probe is advanced to the peripheral lesion to obtain an EBUS image. After localizing the lesion, the probe is removed, leaving the GS in place. A biopsy forceps and a bronchial brush are introduced into the GS to perform pathological and cytological examination. By using the EBUS-GS method, authors have shown a high yield for even small peripheral lesions and fluoroscopically invisible peripheral lesions.87,88 The use of EBUS-GS and virtual bronchoscopic navigation for TBB of small peripheral pulmonary is another growing field of interest.89
Electromagnetic navigation
Electromagnetic navigation is a new technology that involves creating an electromagnetic field around the chest and localizing endoscopic accessories using a microsensor overlaid upon previously acquired 3D CT images (superDimension/Bronchus System; superDimension Ltd, Hertzliya, Israel). It navigates the placement of endoscopic accessories within the lung. In a preliminary study in a swine model, this device was shown to be accurate in the range of 4.5 mm in locating artificial peripheral lung nodules, with no adverse effects.90 These results were tested in humans in pilot studies, which showed that electromagnetic navigation was safe and effective in detecting peripheral lung lesions.91,92 Most recently, a prospective, single-centre study has been reported to determine the ability of electromagnetic navigation to sample peripheral lung lesions and mediastinal lymph nodes. The mean peripheral lesions and lymph node size were 22.8 ± 12.6 mm and 28.1 ± 12.8 mm. The yields were 74% and 100% for peripheral and lymph nodes, respectively. Pneumothorax occurred in two subjects.93 Although these are still pilot studies, the numbers and results are promising. Further larger studies are needed to prove the efficacy of the system.
CONCLUSION
Accurate staging remains essential for the management of patients with NSCLC. Various invasive methods available for staging have been addressed in this paper. Perhaps the most important issue is how to determine patients who are eligible for curative surgical resection. Recent developments in both invasive and non-invasive staging modalities are changing the accuracy of the overall outcome. One of the problems is how we should incorporate the new modalities such as PET and EBUS-TBNA into the algorithms for lung cancer staging. Hence, we have constructed a new algorithm for the evaluation of the mediastinum (Fig. 4).
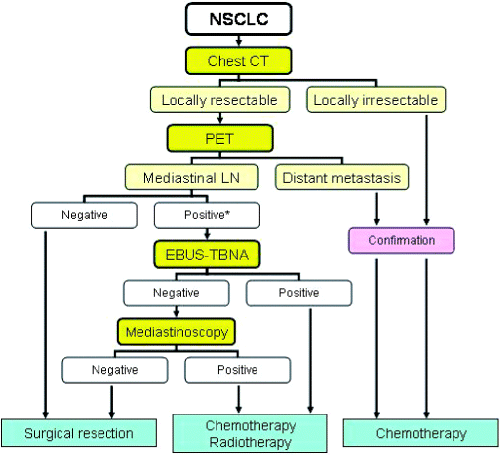
Algorithm for the staging and treatment of lung cancer. EBUS-TNA, endobronchial ultrasound-guided transbronchial needle aspiration; LN, lymph node. *Consider endoscopic ultrasound-guided fine-needle aspiration or video-assisted thorascopic surgery.
Patients with suspected or known NSCLC should be initially evaluated by CT. The resectability should be one of the points of focus when evaluating the CT. If the tumour is resectable on CT, PET is performed to detect unsuspected distant metastasis. When both CT and PET show negative findings in the mediastinum, patients will go directly to surgical resection. An EBUS-TBNA of enlarged lymph nodes on CT- or PET-positive lymph nodes is performed if the lymph nodes of interest are within the reach of EBUS-TBNA, that is, in the highest mediastinal, paratracheal, subcarinal or hilar lesions. If a result from cytology is negative for malignancy, mediastinoscopy should be considered for confirmation of the absence of mediastinal involvement. If there are enlarged and/or PET-positive lymph nodes in stations 5, 6, 8 or 9, other invasive procedures such as EUS-FNA or VATS are performed to exclude mediastinal involvement.




