Dense winter bloom of the dinoflagellate Heterocapsa triquetra below the thick surface ice of brackish Lake Shihwa, Korea
Communicating editor: M. Hoppenrath.
SUMMARY
We investigated the seasonal abundance of the dinoflagellate Heterocapsa triquetra (Ehrenberg) F. Stein, as well as the relevant in situ environmental factors, in brackish Lake Shihwa, Korea. We also examined the growth rates and morphological characteristics of the species in laboratory cultures. In the field, the population densities of H. triquetra remained at low levels from late spring to early summer, and then completely disappeared from August to November 2007. Interestingly, a dense bloom of H. triquetra appeared below the ice surface on 17 January 2008; identities of the cells were confirmed by rDNA sequence comparisons. The second peak reached a density of 672 × 103 cells L−1 on 28 March 2008, at a water temperature of 9.1°C. Laboratory experiments showed that growth rates of H. triquetra increased with incremental temperature increases within the range of 10 and 20°C. The highest growth rate reached by H. triquetra was 0.62 d−1 at 20°C with a salinity of 30. Above 25°C, the dinoflagellate was unable to grow between salinities of 10 and 15, and reached only relatively low growth rates (<0.12 d−1) under other salinity conditions. However, under continuous cultures at 5°C and 8°C, H. triquetra cells retained its growth capability for more than 12 days, implying that H. triquetra can survive and grows even at very low temperatures. The equivalent spherical diameter (ESD) of H. triquetra did not change markedly between 10 and 25°C, but the equivalent spherical diameter was significantly different at 5°C. The cell volume buildup of H. triquetra at low temperatures is one of the important survival strategies to overcome the harsh environmental conditions. These characteristics make H. triquetra a consistently dominant dinoflagellate in Lake Shihwa during the cold winter season.
INTRODUCTION
The dinoflagellate Heterocapsa triquetra (Ehrenberg) F. Stein is one of the most common bloom-forming species in the coastal and estuarine waters of the world (Yamochi & Joh 1986; Kim et al. 1990; Kononen et al. 1999; Litaker et al. 2002a,b). Some Heterocapsa species are known to be harmful to oyster cultures because they produce a toxic protein complex (Matsuyama et al. 1996; Nagai et al. 1996). Conversely, H. triquetra does not produce toxins (Hansen 1995), but has caused ecological damage due to its ability to form dense blooms (Litaker et al. 2002a). Litaker et al. (2002a,b) also reported that, annually between January and March, a large bloom dominated by H. triquetra occurs in estuaries in North Carolina, USA.
In Korea, H. triquetra is a dominant species from autumn to winter in Masan Bay and Jinhae Bay (Lee et al. 1990; Lee & Yoo 1990; Lee & Han 2007). Lee et al. (2005) observed higher growth rates of H. triquetra at relatively mid-low temperatures, ranging between 10 and 25°C. They also found that the optimum salinity occurs between 15 and 40, implying that this species can adapt to a broad range of temperatures and salinities.
Lake Shihwa is an artificial lake, constructed in 1994 for the purpose of supplying water to a nearby industrial complex and agricultural area. The lake was originally designed to provide a freshwater source for the various factories along the lake, gradually replacing its original seawater with freshwater. However, after dike construction and an insufficiency of creek water flowing into the lake, the restriction of water exchange with the outside ocean resulted in serious deterioration of the lake water quality and the original plan for freshwater usage was finally abandoned (Han & Park 1999). Therefore, the resulting higher nutrient loading in the lake has caused a huge algal bloom throughout the year because the average primary productivity is about 1000 times higher than production before dike construction (KOWACO 1993; Choi et al. 1997). Algal bloom organisms are the diatoms Cyclotella, Nitzschia, and Chaetoceros in autumn and winter, and the dinoflagellate Prorocentrum minimum in spring and summer (Choi et al. 1997). Since dike construction, large blooms of Heterosigma akashiwo and H. triquetra in the upper regions of the lake frequently occur instead of the other regular bloom species (Shin et al. 2000). This is especially true for H. triquetra during the winter season. However, the reason why species population densities are maintained at high levels even under low temperature conditions is not fully understood. Some environmental changes, such as the nutrient concentrations after dike construction, are thought to be the cause of species succession.
To understand the impact of abiotic factors on the growth of H. triquetra in the brackish water of Lake Shihwa, this study focuses on: (i) the relationship between the population density and abiotic factors; (ii) a report of the sequence of the small subunit rDNA (SSU rDNA) from winter-blooming cells; and (iii) the growth rate as a function of varying temperature and salinity. We also compare our experimental results with observations of natural populations to understand the physiological ecology of this species in Lake Shihwa.
MATERIALS AND METHODS
Field study
Field sampling was conducted monthly from May 2007 to May 2008 at three sites around Lake Shihwa, designated St. 1, St. 2 and St. 3 (Fig. 1). Surface water samples were collected with a bucket after breaking through the ice layer, particularly in January and February 2008, when the surface water was frozen at St.1. Water temperature and salinity were obtained using a portable water quality sensor (U-10, HORIBA, Kyoto, Japan). Water samples were kept in a cooler for transport to the laboratory. Samples for nutrient analysis were filtered with a GF/F glass fiber filter. Filtered water samples were placed in plastic tubes and frozen. Prior to analysis, samples were thawed at room temperature and stored in dark conditions. Nutrients (NO3 + NO2, NH4, and PO4) were measured using the APHA method (1992). Sub-samples (500 mL) for phytoplankton species estimation, including the dinoflagellate H. triquetra, were immediately fixed after sampling with 2% Lugol's solution, and stored at 4°C in the dark until cells were counted using a Sedgwick-Rafter chamber.
Map of the sampling sites in Lake Shihwa. St.1 is located on the riverside. St.2 is close to the dike, and St.3 is outside the lake. The area of Lake Shihwa is 200 km2, including the uppermost tidal flats and shallow margins.
Isolation and culture of Heterocapsa triquetra
Individual cells of H. triquetra were isolated at St.1 from the surface water of Lake Shihwa on 29 April 2008, when the water temperature and salinity were approximately 20°C and 23.5°C, respectively. Four species, H. triquetra, Prorocentrum micans, Dinophysis acuminata, and Myrionecta rubra (=Mesodinium rubrum), predominated in the water sample from St.1. Each of the isolated cells was washed by serially transferring them into three droplets of f/2-Si medium. The cells were then placed into a 0.5-mL well chamber of a 96-well tissue culture plate filled with f/2-Si medium. The cells in the culture plates were incubated at 20°C under 60 µmol m−2 s−1 with a 12:12 LD (light : dark) cycle, using a cool white fluorescent light. Once dense cultures of the species were obtained, they were transferred to 100-mL glass bottles containing f/2-Si medium for one month to acclimate to laboratory conditions.
Effects of temperature and salinity on the growth of Heterocapsa triquetra
Equivalent spherical diameter measurement and morphology
In Lake Shihwa, the natural population density of H. triquetra was significantly higher under the ice in January 2008. The cell volume in January was larger than the observed cell volumes of other seasons. Certainly, these larger cells are not the normal form of H. triquetra. Thus, we used two methods to confirm the possible appearances of H. triquetra under harsh environmental conditions. First, the cell volume was examined using field samples collected in January and April with the mean equivalent spherical diameter (ESD; µm) of H. triquetra. Researchers measured the mean ESD by Flow CAM (Fluid Imaging Technologies, Inc., Yarmouth, USA), which is a continuous imaging technique designed to characterize particles in the microplankton size range (Sieracki et al. 1998; Buskey & Hyatt 2006). Under laboratory conditions, isolated H. triquetra cells collected in April were also incubated for 14 days under different water temperatures (5, 10, 15, 20 and 25°C), a salinity of 30 and an irradiance of 60 µmol m−2 s−1 under a cool white fluorescent light with a 12:12 LD cycle. The ESD of H. triquetra was measured in 300 randomly-selected cells at each temperature condition. Thecal plates of H. triquetra were observed at 1000 × magnification under a microscope (Zeiss, Axiovert 135 M, Jena, Germany) after having been stained with approximately 10 µg mL−1 of Calcofluor White (Fritz & Triemer 1985). Furthermore, we confirmed the appearance of H. triquetra using molecular methods, as described below.
DNA extraction, PCR amplification, sequencing and data analysis
We determined the molecular identity of Heterocapsa, which predominantly occurs during the winter in Lake Shihwa. We collected water samples when Heterocapsa cells were prevalent in January and February 2008. We filtered the samples with 3.0-µm-pore-size polycarbonate membranes (47 mm diameter, Millipore). Genomic DNA was extracted from the natural assemblages on the filters by using proteinase K and sodium dodecyl sulfate with a chloroform extraction and isopropanol precipitation (e.g. Harder et al. 2003). Partial SSU rDNA was amplified by polymerase chain reaction (PCR) using genomic DNA and dinoflagellate-targeting primers: a forward Pe18F632, 5′-GGA YYT CTG CYG AGG ACG AC-3′; and a reverse 18R1748, 5′-CCT TGT TAC GAC TTC TCC TTC-3′ (Ki & Han 2007). Additionally, internal transcribed spacers (ITS) to 28S rDNAs were amplified by PCR with the following primers: HTITS2F01, 5′-CAC AAC TTG CAA AGC ATC TTT G-3′; and HT28RD3, 5′-AAC TCC AGC TAT CCT GAG GGA-3′. The resultant PCR products were purified and subjected to DNA sequencing. DNA sequences determined here have been deposited into GenBank as accession numbers HQ902266 through HQ902268.
DNA sequences were briefly checked by BLAST (Basic Local Alignment Search Tool) searches of the GenBank database. Then, we further compared with our sequence data with other available data (e.g. H. triquetra and H. arctica) by measuring DNA similarities and constructing neighbor-joining (NJ) trees.
RESULTS
Field study
The water temperatures at all three sampling stations were similar and varied from 0.9 to 31°C. The highest temperature was recorded in August and the lowest in January (Fig. 2a). The salinity at St.1 greatly fluctuated because of tidal range effects, ranging from 0 to 30 (Fig. 2b). Salinity variations at St. 2 were similar to St. 3, ranging from 23.2 on 18 September to 34.5 on 17 June.
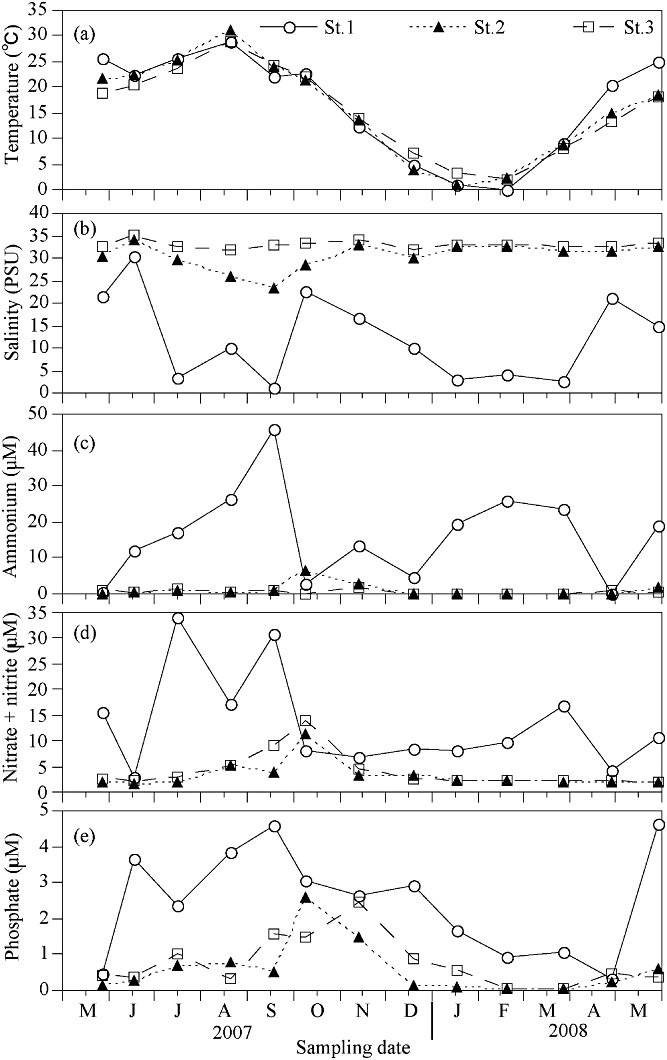
Seasonal variation of (a) temperature (b) salinity (c) ammonium (d) nitrate + nitrite, and (e) phosphate at the three Lake Shihwa sampling stations from 28 May 2007 to 30 May 2008.
There was a large temporal variation in dissolved inorganic nutrients at St. 1. The ammonium concentration at the three stations varied from 0.01 µM to 45.84 µM, averaging 16.2 µM at St. 1, 1.08 µM at St. 2, and 0.61 µM at St. 3 (Fig. 2c). The nitrate + nitrite concentration varied from 1.63 µM to 33.92 µM, averaging 13.39 µM at St. 1, 3.31 µM at St. 2, and 4.21 µM at St. 3 (Fig. 2d). The phosphate concentration was generally low, and ranged from 0.07 µM to 4.63 µM. The average phosphate concentration was 2.47 µM at St. 1, 0.59 µM at St. 2, and 0.77 µM at St. 3 (Fig. 2e). N : P ratios during the winter season were higher compared to the other three seasons (Fig. 3).
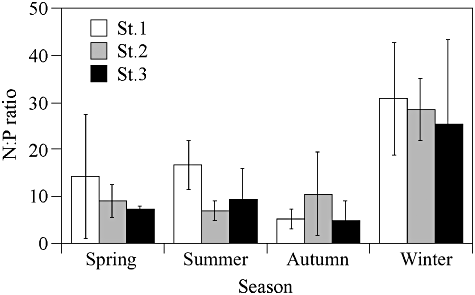
Seasonal means of N : P ratios at each station during the study period. Winter: January to March; spring: April to June; summer: July to September; and autumn: October to December. Error bars indicate ± S.D of each season.
The population of H. triquetra at Stations 2 and 3 remained at low densities from late spring to early summer (Fig. 4) and completely disappeared from August to November 2007. However, the population of H. triquetra at St.1 was not observed from the initial sampling date to November 2007. Following this, the cell abundance tended to increase from December 2007 to April 2008. H. triquetra had two abundance peaks. The first and second abundance peaks were 58 × 104 cells L−1 on 17 January and 67 × 104 cells L−1 on 28 March 2008, when the water temperature was 0.9°C and 9.1°C, respectively. In particular, among the seasonal variations, the number of cells significantly increased at temperatures below 10°C compared to other temperature conditions (Fig. 4).
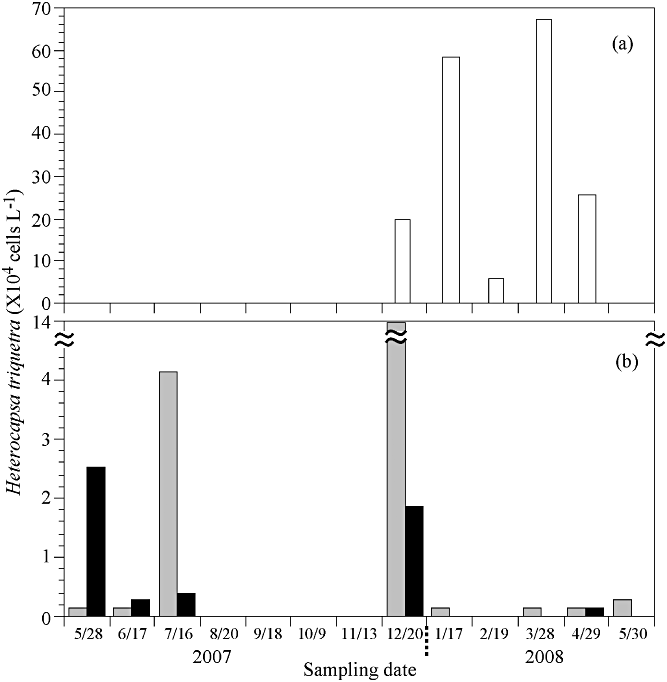
Seasonal variations in concentration of Heterocapsa triquetra at the three Lake Shihwa sampling stations (a: St. 1; b: St. 2 and St. 3) from 28 May 2007 to 30 May 2008. Gray and black boxes represent St. 2 and St. 3, respectively.
In this study, we determined partial 18S, complete ITS, and partial 28S rDNA sequences from H. triquetra cells that bloomed during the winter season, and compared these extensively with other Heterocapsa rDNA sequences. NJ trees (Fig. 5) constructed with 18S and 28S rDNA showed that our Heterocapsa and H. triquetra were grouped together with high DNA similarities (Table 2). Particularly, our 18S rDNA of Heterocapsa, which was collected in January, was completely identical to a H. triquetra sequence (GenBank no. AY421787) revealed from different Korean shorelines. Furthermore, BLAST searches showed that complete sequences of the ITSs and 5.8S rDNA, including the partial 28S rDNA, were perfectly matched to those of other H. triquetra (e.g. AB084101, AF208249, AF527816, and EF613355) from Korean, Japanese, and European seawaters. These findings suggest that the predominant Heterocapsa cells in Lake Shihwa, particularly in the cold seasons, could be discriminated from H. triquetra.
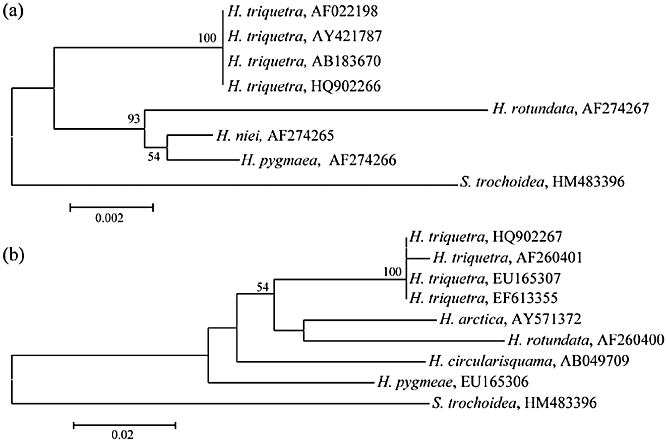
Neighbor-joining (NJ) trees constructed with (a) partial 18S rDNA and (b) 28S rDNA sequences using the Maximum Composite Likelihood model in MEGA 4.0 (Tamura et al. 2007). Our 18S and 28S datasets were separately aligned using Clustal W 1.8 (Thompson et al. 1997). The aligned sequences were trimmed at each end to the same length, and obvious base errors found only in single strands were removed manually. Finally, only unambiguous positions (1051 aligned sites for 18S; 689 for 28S) were subjected to NJ analyses. The dinoflagellate Scrippsiella-trochoidea was used as the out-group.
| No. | 18S rDNA dataset | 28S rDNA dataset | [1] | [2] | [3] | [4] | [5] | [6] | [7] | [8] |
|---|---|---|---|---|---|---|---|---|---|---|
| [1] | H. triquetra, AB183670 | H. triquetra, HQ902267 | 100 | 100 | 100 | 98.4 | 99.1 | 99.2 | – | |
| [2] | H. triquetra, AY421787 | H. triquetra, EF613355 | 100 | 100 | 100 | 98.4 | 99.1 | 99.2 | – | |
| [3] | H. triquetra, HQ902266 | H. triquetra, AF260401 | 99.4 | 99.4 | 100 | 98.4 | 99.1 | 99.2 | – | |
| [4] | H. triquetra, AF022198 | H. triquetra, EU165307 | 99.8 | 99.8 | 99.2 | 98.4 | 99.1 | 99.2 | – | |
| [5] | H. rotundata, AF274267 | H. arctica, AY571372 | 91.2 | 91.2 | 90.7 | 91.2 | 98.9 | 98.8 | – | |
| [6] | H. pygmaea, AF274266 | H. rotundata, AF260400 | 90.3 | 90.3 | 89.9 | 90.2 | 89.6 | 99.7 | – | |
| [7] | H. niei, AF274265 | H. circularisquama, AB049709 | 90.9 | 90.9 | 90.3 | 90.8 | 89 | 87.3 | – | |
| [8] | – | H. pygmeae, EU165306 | 90.3 | 90.3 | 89.7 | 90.2 | 88.8 | 90.1 | 88.9 |
Laboratory experiments
In the preliminarily experiment, 6 days of acclimation slightly increase the growth rate at each temperature condition (Fig. 6). However, there is no difference in the optimum temperature. Therefore, we had conducted the following experiments without acclimation to examine the optimum condition for the growth of H. triquetra. Thus, our results indicate the optimum condition; however, growth rates under various conditions tested in the present study are possibly underestimated. The growth rates of H. triquetra for the various temperature and salinity combinations are shown in Figure 7. The growth rates of H. triquetra increased with temperature within the range of 10 to 20°C. The highest observed growth rate of H. triquetra was 0.62 d−1 at 20°C and a salinity of 30. At 25°C, H. triquetra were unable to grow between the salinity conditions of 10 and 15. Numbers were kept relatively low, within <0.12 d−1, under other salinity conditions. At 30°C, there were continuously negative values under all salinity levels. Cells grew even at 5°C under salinity conditions between 20 and 35. Under continuous culture at 5°C and 8°C, H. triquetra cells gradually increased and retained their growth capability for more than 30 days (Fig. 8). Also, two-way anova indicated that there were significant differences in the growth rate between temperature conditions (Table 1). Salinity also affected growth rates. Therefore, the temperature-salinity interaction had an influence on the growth rates of H. triquetra.
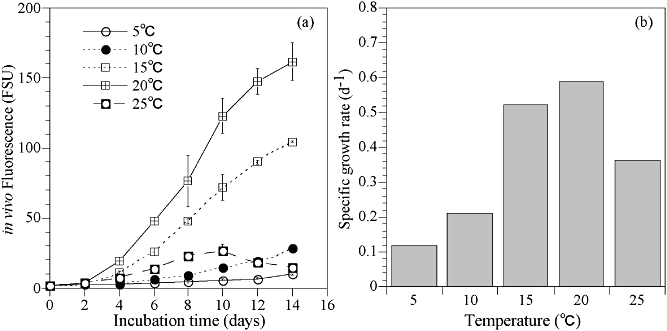
Changes in the growth curves of Heterocapsa triquetra at a salinity of 30 under a 12:12 LD (light : dark) cycle and 60 µmol m−2 s−1 for a 2-day interval under different temperature conditions (a) and the specific growth rate of H. triquetra at each temperature (b).
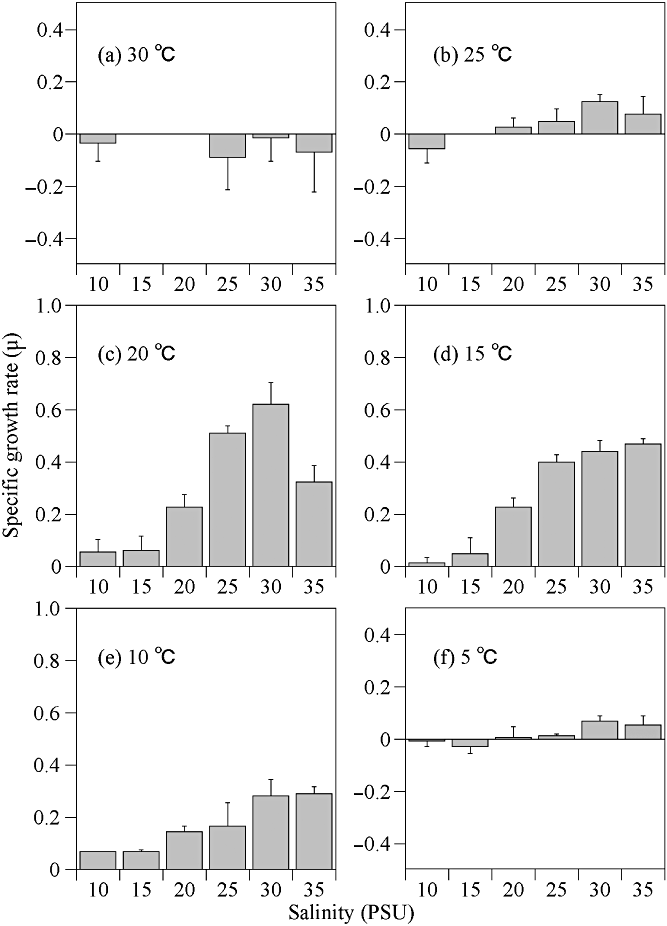
Changes in the specific growth rate of Heterocapsa triquetra under different combinations of temperature and salinity. Error bars indicate ± standard deviation (SD).
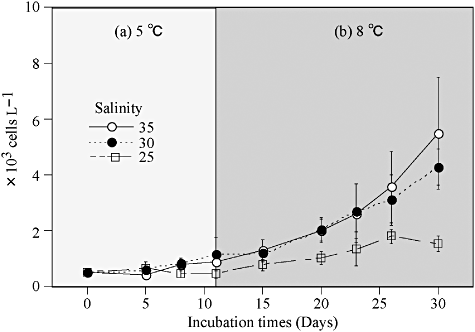
Effects on the growth of Heterocapsa triquetra under several salinity conditions at: (a) 5°C and (b) 8°C. Heterocapsa triquetra was incubated for 11 days at 5°C and then kept at 8°C.
| Factors | Heterocapsa triquetra | ||
|---|---|---|---|
| DF | F | P | |
| Temperature | 5 | 56.77 | <0.001 |
| Salinity | 5 | 118.57 | <0.001 |
| Temperature X salinity | 25 | 10.68 | <0.001 |
| Error | 72 | ||
| Total | 108 | ||
The maximum ESD of H. triquetra was 19.91 ± 2.19 µm at 5°C. On the other hand, the minimum cell ESD was 15.08 ± 1.21 µm at 15°C (Fig. 9). Although the ESD in H. triquetra did not change markedly between 10 and 25°C, the ESD differed significantly at 5°C (P < 0.001). The ESD (22.08 ± 2.30 µm) of H. triquetra cells in the field (January, 0.9°C) was larger than that of laboratory-cultured cells. On the other hand, the mean ESD (15.28 ± 1.27 µm) of H. triquetra cells in April (20.4°C) was smaller than that of the 20°C culture in the laboratory.
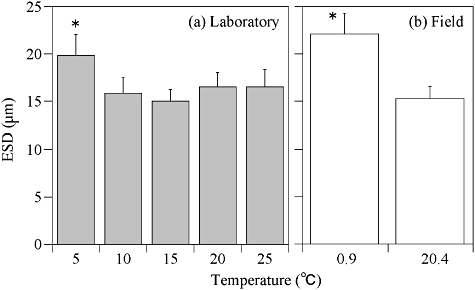
Changes in the mean equivalent spherical diameter (ESD) ± standard deviation (SD) of Heterocapsa triquetra under several temperatures of (a) laboratory and (b) field conditions. Field sample temperatures of 0.9°C and 20.4°C were obtained on 17 January and 29 April, respectively.
The cell morphology of H. triquetra is shown in Figure 10. Based on fluorescence microscopy of Calcoflour White–stained cells, the plate formula of H. triquetra is Po, cp, 5′ 3a, 7′′, 6c, 5 s, 5′′′ and 2′′′′. The position of the nucleus in H. triquetra is located at the central parts of the apical and precingular plates. The nucleus position of H. triquetra is distinguished from H. arctica, which is shown between precingular and cingular plates. Above 15°C, H. triquetra cells form normally. At 5°C, several cells shed the theca, but remain covered with the pellicle wall. Cell sizes at 5°C were clearly larger than those of high temperature cultures above 15°C.
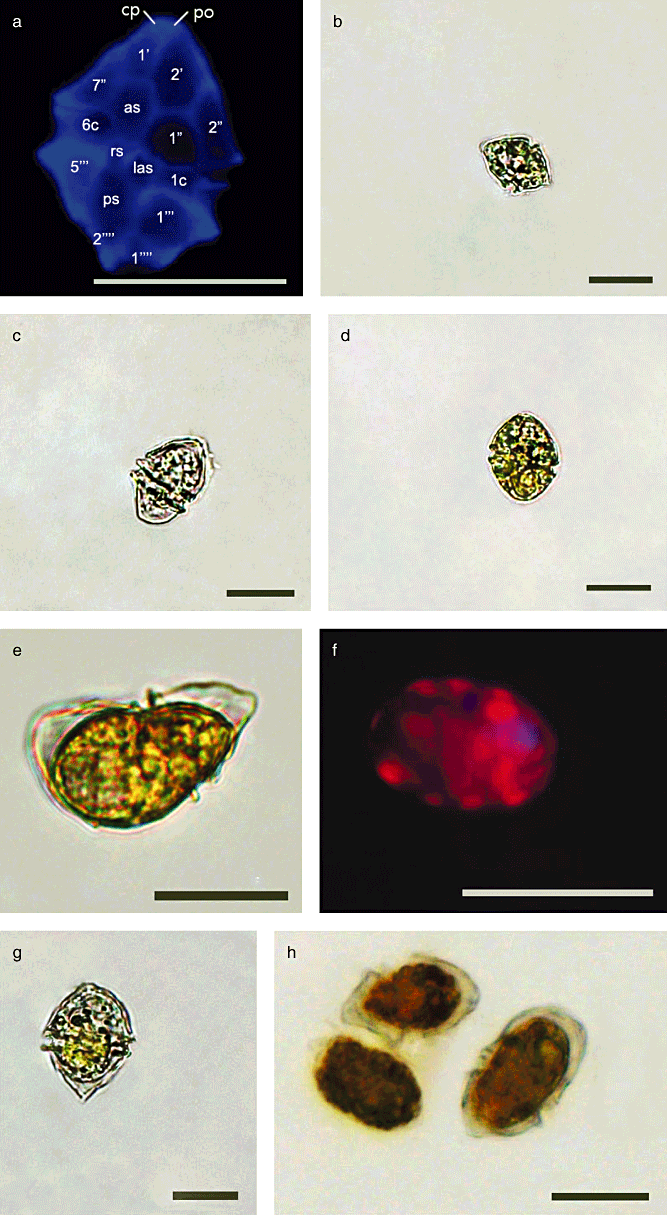
Heterocapsa triquetra cell morphology. (a) Fluorescence microcopy of Calcoflour White–stained thecae, revealing the thecal plate. Po, pore plate; cp, canal plate; 1′–5′, apical plates; 1′′–7′′, precingular plates; 1c–6c, cingular plates; as, anterior sulcal plate; las, left anterior sulcal plate; ps, posterior sulcal plate; 1′′′–5′′′, postcingular plates; 1′′′′–2′′′′, antapical plates. (b) Cell form cultured at 15°C. (c and d) Cell forms cultured at 10°C. (e, f and h) Cell forms cultured at 5°C. Cells shed the theca but remain covered with the pellicle wall. (g) Field sample in January, which was observed below the ice-water interface. All scale bars are 20 µm.
DISCUSSION
The dinoflagellate Heterocapsa triquetra has been recognized as a common bloom-forming species between the autumn and winter seasons in the coastal and estuarine waters of temperate areas (Marshall 1967; Yamochi & Joh 1986; Kim et al. 1990; Litaker et al. 2002a). However, to our knowledge, a dense bloom of the cells under surface ice has not been previously reported. In brackish Lake Shihwa, the H. triquetra population densities below the ice-water interface have significantly increased. We hypothesized about the possibility of H. triquetra bloom occurrences in harsh environmental conditions.
To obtain clear evidence in morphological discriminations of winter-blooming cells, we investigated wide-range rDNA sequences obtained from the January samples collected at St. 1 and St. 2, and found that all the genotypes were identical as judged by rDNA similarity (100% in the individual SSU, ITS, and 28S comparisons). This indicates that the winter blooms were genetically homogeneous and caused by a single species. Further molecular analyses with a BLAST search and DNA similarity studies showed that our individual sequences of the 18S, ITS, and 28S had perfectly matched to already-known sequences of H. triquetra (see Table 2, Fig. 5). Of the genus Heterocapsa, however, H. arctica also occurred in low temperatures and was reported from Finland (Rintala et al. 2010). In our current study, we compared available H. arctica sequences with our current sequences (GenBank nos. AB084095, AY571372), and found that low DNA similarities (e.g. 82.3% in complete ITS, 91.2% in partial 28S) were detected between sequence-pairs of the species (e.g. Table 2). These results indicate that the winter-blooming cells are identical to H. triquetra, rather than H. arctica.
The cells of H. triquetra collected under ice conditions had undergone random morphological changes and were observed as abnormal forms. According to Iwataki (2008), the armored dinoflagellate genus Heterocapsa is relatively small and shares congruous morphological characters such as thecal plate arrangement and the position of the nucleus and pyrenoid. This suggests that identification at the species level is very difficult. Iwataki et al. (2004) also mentioned that although the scale morphology of H. triquetra strongly resembles that of H. pseudotriquetra, the hypotheca of H. triquetra can be distinguished by an antapical horn compared to the rounded antapical horn of H. pseudotriquetra. Heterocapsa species under surface ice in January were observed with an antapical horn and were larger than normal field cells (Fig. 10B and G). In our laboratory studies, the ESD of H. triquetra at 5°C was significantly larger compared to other temperature conditions. The ESD of H. triquetra cells collected in January was also similar to those of cells grown at 5°C in the laboratory (Fig. 9). Even if these cells were morphologically changed in the harsh environment, the reproductive capability of their cells had been sufficiently maintained (Fig. 8). The cell volumes (i.e. ESD) of H. triquetra have significantly increased in low water temperature conditions, suggesting that the cell volume change is one of the important survival strategies to overcome low temperature as well as an adaptation to minimize mortality.
Although H. triquetra are characteristically found during all seasons (Olli 2004), it has a seasonal niche favorable for low temperature growth in winter (Litaker et al. 2002a). In this study, H. triquetra blooms strongly tend to occur in low temperature and salinity conditions from January to March, and completely disappear from August to November. A field study by Litaker et al. (2002b) observed the most rapid growth of H. triquetra at <12°C, and often <10°C, similar to the occurrence range in our study. However, the seasonal changes of H. triquetra in our field study differ from the pattern expected from its growth curves in our laboratory. The specific growth rate that occurred below 10°C under low salinity conditions was low (<0.1 d−1). At 5°C, the specific growth rate of H. triquetra was close to zero at salinities above 20, but was negative between salinities of 10 and 15. Also, this organism was significantly grown at temperatures ranging from 15 to 20°C, but not at 30°C, implying that the growth of H. triquetra abruptly declines at higher temperatures than the optimum. Yamaguchi et al. (1997) reported that the highest growth rate (0.95 d−1) of H. triquetra was observed with the combination of 15°C and a salinity of 15. They also pointed out that the optimum temperature for H. triquetra is considerably lower than that of other Heterocapsa species and, that the temperature optima was 15°C, or about two times higher than the result found in our laboratory experiments. In addition, the growth of H. triquetra was relatively high (0.4 d−1) even at 10°C (Yamaguchi et al. 1997). These differences between previous studies and our current research may have been induced by the physiological characteristics of the H. triquetra strain. In this study, although the dense winter bloom of H. triquetra in Lake Shihwa is not directly linked to the results of our laboratory temperature growth experiments, H. triquetra may have originated from habitats of low temperature because the cell can survive at 5°C and grow at 8°C in nutrient-rich water for more than 30 days. Therefore, the local population of H. triquetra may have the plasticity to slowly adapt to low temperatures and this ability is indirectly involved with its capability to form dense winter blooms.
In the laboratory experiment, the growth rate of H. triquetra was close to 0 when incubated at 5°C. On the other hand, we found a bloom in the lake with a water temperature of 0.9°C. As mentioned in the Results section, the growth rate at each condition may be underestimated. For an accurate estimation for the isolate, acclimation is essential. Another possible explanation for the inconsistency is a difference in the genotype. Recently, Saravanan and Godhe (2010) reported that abundance of the diatom Skeletonema marinoi peaked twice within a year. Isolates of the February population differed genetically and physiologically from isolates of the September population. Thus, it is also possible that the winter population in this study was different from the summer population, which was used for the laboratory experiment. Isolation of winter population and evaluation of their physiology and genetics should be carried out in a subsequent study.
Harmful algal blooms are a phenomenon commonly associated with eutrophication. The H. triquetra population is physiologically well-suited to take advantage of episodic nutrient input according to Litaker et al. (2002a). They also demonstrated that nitrogen-rich water is much more important in controlling the bloom development of H. triquetra (Litaker et al. 2002a) since coastal environments are increasingly influenced by dissolved inorganic nitrogen (DIN) (Ryther & Dunstan 1971; Paerl et al. 1990). Lake Shihwa is an artificial lake, in which the restriction of water exchange with the outside ocean has resulted in high nitrogen loading from river discharge. Hence, a large bloom of Heterosigma akashiwo and H. triquetra occurs frequently in the upper regions of the lake instead of a regular diatom bloom. Rudek et al. (1991) and Fujiki et al. (2004) reported similar results where increased nitrogen caused by high river–flow events creates a phosphorus deficiency. In the current field study, high population densities of H. triquetra were kept under a high nitrogen source and a high N : P ratio conditions during the winter (Fig. 3). Mallin et al. (1991) mentioned that H. triquetra is an indicator species of nitrogen enrichment in estuarine water. According to Rudek et al. (1991) and Litaker et al. (2002a), H. triquetra is capable of producing high levels of the extracellular enzyme alkaline phosphatase, thus allowing it to escape phosphorus limitation. Therefore, after dike construction, the nitrogen enrichment ecosystem is expected to be an important factor in the formation of a dense bloom of H. triquetra.
The bloom event of H. triquetra is ecologically a function of mortality rate as well as relative specific growth rates. H. triquetra population dynamics are affected by mesozooplankton grazing (Verity 1986; Kamiyama 1994; Litaker et al. 2002a). Therefore, lower grazing pressure on a given organism may create an advantage. According to Litaker et al. (2002a), since low ambient water temperature also suppresses mesozooplankton grazers, H. triquetra exploits seasonal niches to avoid grazing losses. In temperate coastal waters, mesozooplankton generally fluctuate depending on seasonal events such as the spring phytoplankton bloom. They also begin to increase by early summer and attain their peaks in late summer. The peaks are sustained until autumn, and then greatly reduced in winter (Madhupratap & Onbé 1986). H. triquetra have been recognized as a high-quality food for the Acartia spp. of copepods (Uye & Takamatsu 1990), which has been found to dominate in Korean and Japanese coastal waters (Liang & Uye 1996; Kang et al. 2007). In particular, mesozooplankton species such as genus Acartia, Sinocalanus, and Eurytemora were dominant during the winter season in Lake Shihwa (Park & Huh 1997; Yoo et al. 2010). Of these, species of the genus Acartia, such as A. omorii and A. hongi, have a broad temperature tolerance (temperature range of 5 to 24°C), with the instantaneous growth at each stage increasing exponentially with increasing temperature (Liang & Uye 1996; Yoo et al. 2010). This implies that these mesozooplankton species are not directly concerned with H. triquetra population dynamics during the winter season. Although grazing pressure was not investigated in this study, H. triquetra might have an advantage over the grazing pressure of mesozooplankton, allowing population growth in the field to occur under very low temperature conditions.
Phytoplanktonic organisms in estuaries and coastal areas are subjected to vastly fluctuating physical conditions as a result of tidal currents. Pérez and Canteras (1990) reported that while tidal conditions do not affect the specific composition of the phytoplankton community, they do affect the numbers of individuals observed, with the greatest numbers being found at low tide. In this study, a dense bloom of the H. triquetra population in January and March was also observed in low salinity conditions at low tide (tidal data not shown), which is governed by river flow after removing saltwater by tidal effects. Tidal current plays an important role in determining the nature of exchange flows, and results in dramatic salinity changes. H. triquetra is classified as a mesohaline species (Marshall & Alden 1990), even though it is functionally euryhaline (Litaker et al. 2002a). H. triquetra has wide salinity range tolerance, with salinities as low as 3 to 4 (Hobbie 1971; Mallin 1994). In most previous studies, population growth of H. triquetra occurred between salinities of 5 to 25 (Marshall & Alden 1990; Mallin 1994; Litaker et al. 2002a). In our experiments, the optimum growth of H. triquetra was observed between salinities of 25 and 35 (Fig. 5), which is dissimilar to our field data, as mentioned previously. Although the salinity gap does not provide a sufficient explanation, complicated interactions of water masses by tidal effects might have strong impacts on H. triquetra population dynamics since fast-swimming dinoflagellates such as H. triquetra have a competitive advantage in frequently-mixed waters (Lindholm & Nummelin 1999). In fact, the H. triquetra population is more robust at low salinity than previously discovered by our field results. Therefore, a dense bloom of H. triquetra might have been stimulated by low salinity conditions in addition to the cell accumulation resulting from tidal currents and high nitrogen sources after river discharge.
The dinoflagellate H. triquetra was recently considered to be a mixotrophic organism (e.g. Jeong et al. 2010), although the species has been thought to be autotrophic. Mixotrophic species gain their nutrition through a combination of photosynthesis and uptake of dissolved or organic material. Nutrition-uptake species have a competitive advantage over simple photosynthetic organisms, since this manner of feeding would enable the mixotrophic species to take nutrition from organic material when inorganic nutrients are limited. Although not investigated for their mixotrophic ability in this study, the advantage of additional nutrient sources available to H. triquetra might contribute as an important factor to overcome the unfavorable conditions found under low light conditions below the thick surface ice. Future research focusing on the mixotrophic ability of Heterocapsa species will be needed to reveal the cues for winter blooms.
In summary, a dense winter bloom of the dinoflagellate H. triquetra occurred abnormally below the ice surface of brackish Lake Shihwa. We identified H. triquetra based on the wide-range rDNA comparisons and morphological characteristics of Heterocapsa. Also, the cell volumes (i.e. ESD) of H. triquetra during winter blooming are significantly higher than those of other seasons, suggesting that cell-volume augmentations are recognized as an important survival strategy for overcoming low temperatures. Therefore, H. triquetra is stimulated by low salinity condition and might depend on environmental conditions, such as nitrogen influx from freshwater discharge.
ACKNOWLEDGMENTS
This research was supported by KORDI's projects PE98539 and PE98582 and the National Research Foundation of Korea (NRF-2008–314-C00319).




