Enhanced activation in the extrastriate body area by goal-directed actions
Abstract
Aim: Neuroimaging studies on biological motion have established the view that the posterior superior temporal sulcus (pSTS) is involved in detecting intention of others. Those studies have consistently reported other regions such as body-selective extrastriate body area (EBA) and motion-sensitive middle temporal, in close proximity to pSTS. Whether EBA responds only to static body parts or has a more extended role as part of a system for inferring intention of others has remained an elusive issue. The aim of the present study was to investigate the role of EBA in processing goal-directed actions.
Methods: Twelve healthy volunteers participated in the present study. Using sports-related motions as visual stimuli, brain activations were examined during observation of goal-directed actions and non-goal-directed actions on functional magnetic resonance imaging.
Results: Compared to non-goal-directed actions, goal-directed actions produced greater activations in EBA along with the mirror neuron system.
Conclusions: EBA might contribute to understanding others' actions by representing the dynamic aspects of human motions.
NEUROIMAGING STUDIES HAVE established the view that the posterior superior temporal sulcus (pSTS) plays a crucial role in processing biological motion,1–4 and it has been suggested that the pSTS constitutes a part of the human mirror neuron systems (MNS) through which observed actions of others are internally represented,5,6 and has a more general function in social cognition such as detecting intention of others7–9 and behavior of agents.3 But passive viewing of biological motion has consistently activated other regions of the posterior temporal–occipital cortex including body-selective extrastriate body area (EBA)10 and motion-sensitive middle temporal (MT),11 in close proximity to pSTS.12–14
Studies about biological motion have used point-light animation of simple action, and scrambled or occluded motion has been used in control condition. Therefore, the use of low-level stimuli as controls would make it difficult to clarify whether EBA and MT are, respectively, involved only in body and motion-sensitive low-level visual processing or lie in a part of a system for inferring the action and intention of others, such as STS. In the present study we compared brain activation in response to more complex meaningful biological motion with that to complex non-meaningful biological motion. We used sports-related motion and sports-unrelated motion for meaningful and non-meaningful biological motion, respectively, because sports-related motion is meaningful and goal-directed, whereas sports-unrelated motion itself could be meaningful biological motion but become non-meaningful and non-goal-directed in the context of sports game rules. For example, carrying the ball with a certain aim in daily life or in a certain sport (e.g. rugby) is a natural and goal-directed action, but becomes non-goal directed when accompanied by the aim to win a soccer game, because handling the ball is against the rules of soccer.
Although the issues regarding the precise role of EBA are still controversial,15 recent studies have suggested an extended role for the EBA, involving not only static visual perception of body parts but also the planning, execution and imagination of actions,16,17 and that the EBA is located at the entry of the human MNS.17,18 We hypothesized that sports-related goal-directed motion would produce greater activation than sports-unrelated non-goal-directed motion in EBA along with STS and MNS.
METHODS
Participants
Twelve healthy volunteers (mean age 29.4 ± 4.5 years) participated in the present study. All subjects were Japanese and right-handed. All participants had played basketball in elementary or junior high school, but did not play basketball regularly thereafter. The participants were free of any criteria for neuropsychiatric disorders based on unstructured psychiatric screening interviews. None of the participants was taking alcohol at the time, nor did they have a history of psychiatric disorder, significant physical illness, head injury, neurological disorder, or alcohol or drug dependence. All participants underwent magnetic resonance imaging to rule out cerebral anatomic abnormalities. After complete explanation of the study, written informed consent was obtained from all participants, and the study was approved by the Institutional Ethics Committee.
Materials
Two types of video clips were provided (basketball-related [BR] and basketball-unrelated [BU] motion). Examples of the video clips are shown in Fig. 1. Because a series of basketball plays consists of several actions and several players, it is difficult to provide a natural stream of control video clips (BU motion) consisting of identical numbers and directions of actions to BR motion. Therefore, we used some actions that are the components of a series of actions of a basketball game, aiming to make it easier to provide control actions (BU motion). BR motion consisted of three types of scenes (player shooting a free throw, player dribbling, two players performing man-to-man defense/offence). BU motion also consisted of three types of scenes (player rolling a basketball, player carrying a basketball, one player crossing in front of another without interaction). In order to make BR and BU motion as similar as possible, all players in the video clips performed in front of a basket goal on a basketball court, and the number of persons, objects, motion direction and speed were matched, that is, rolling a basketball, carrying a basketball, and crossing in front of another without interaction corresponded to shooting a free throw, dribbling, and man-to-man defense, respectively. The video clips were projected via computer and telephoto lens onto a screen mounted on a head-coil. The subjects were instructed to pay attention to the video clips and to press a selection button with the right index finger when they watched the free-throw scene and the basketball-rolling scene, indicating that they had paid attention to them. The experimental design consisted of five blocks for each of the two conditions (BR and BU motion) interleaved with 20-s rest periods. During the rest condition, participants viewed a crosshair pattern projected to the center of the screen. In the BR and BU motion 24-s blocks, three scenes were presented twice for 4 s each. The order of BR and BU motion conditions was fixed across the subjects.
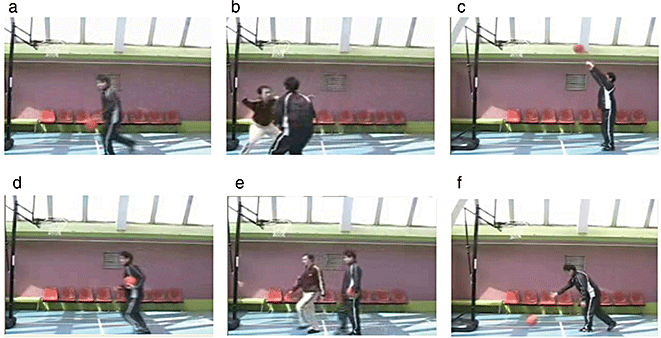
Sample of still frames from (a–c) basketball-related motions and (d–f) basketball-unrelated motions. (a) Dribbling; (b) man-to-man; (c) shooting; (d) carrying; (e) crossing; (f) rolling.
Image acquisition
Images were acquired with a 1.5-Tesla Signa system (General Electric, Milwaukee, WI, USA). Functional images of 115 volumes were acquired with T2*-weighted gradient echo planar imaging sequences sensitive to blood oxygenation level-dependent (BOLD) contrast. Each volume consisted of 40 transaxial contiguous slices with a slice thickness of 3 mm to cover almost the whole brain (flip angle, 90°; TE, 50 ms; TR, 4 s; matrix, 64 × 64; field of view, 24 × 24 cm). High-resolution, T1-weighted anatomic images were acquired for anatomic comparison (124 contiguous axial slices, 3-D spoiled gradient-recalled acquisition in a steady state sequence, slice thickness 1.5 mm, TE, 9 ms; TR, 22 ms; flip angle, 30°; matrix, 256 × 192; field of view, 25 × 25 cm).
Analysis of functional imaging data
Data analysis was performed using a statistical parametric mapping software package (SPM02; Wellcome Department of Cognitive Neurology, London, UK) running with MATLAB (Mathworks, Natick, MA, USA). All volumes were realigned to the first volume of each session to correct for subject motion and were spatially normalized to the standard space defined by the Montreal Neurological Institute template. After normalization, all scans had a resolution of 2 × 2 × 2 mm3. Functional images were spatially smoothed with a 3-D isotropic Gaussian kernel (full width at half maximum, 8 mm). Low-frequency noise was removed by applying a high-pass filter (cut-off period, 192 s) to the functional MRI (fMRI) time series at each voxel. A temporal smoothing function was applied to the fMRI time series to enhance the temporal signal-to-noise ratio. Significant hemodynamic changes for each condition were examined using the general linear model with boxcar functions convoluted with a hemodynamic response function. Statistical parametric maps for each contrast of the t-statistic were calculated on a voxel-by-voxel basis.
To assess the specific condition effect, we used the contrasts of BR motion minus BU motion. A random effects model, which estimates the error variance for each condition across the subjects, was implemented for group analysis. This procedure provides a better generalization for the population from which data are obtained. Contrast images were obtained from single-subject analysis and entered into group analysis. A one-sample t-test was applied to determine group activation for each effect. A statistical threshold of P < 0.05 corrected for multiple comparisons across the whole-brain was used, except for a priori hypothesized regions thresholded at P < 0.001 uncorrected (only clusters involving ≥10 contiguous voxels are reported). These a priori regions of interest included the biological motion-related regions (STS, MT and EBA), human MNS (inferior parietal lobule [IPL] and inferior frontal cortex). We also assessed the contrasts of BU motion minus BR motion to investigate possible brain activations in response to the BU motion condition relative to BR motion condition.
RESULTS
Behavioral results
All subjects paid attention to the video clips and pressed the button appropriately (100% accuracy).
FMRI results
BR motion minus BU motion condition produced activations in the bilateral posterior temporal–occipital cortex including bilateral EBA (x = 58, y = −60, z = 2, t = 4.86) and MT (x = 54, y = −66, z = −12, t = 8.38), right STS (x = 56, y = −22, z = −2, t = 6.58), bilateral premotor cortex (x = −48, y = −4, z = 40, t = 4.94), and bilateral IPL (x = −34, y = −50, z = 54, t = 7.25; coordinates and t-score refer to the peak of each brain region; Fig. 2). A one-sample t-test of BU motion minus BR motion contrasts indicated no significant activation at a height threshold of P < 0.001, uncorrected, and an extent threshold of 10 contiguous voxels.
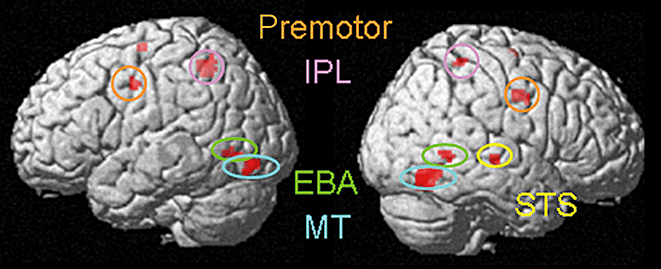
Brain activations in response to sports-related motion minus sports-unrelated motion. Significant activations in extrastriate body area (EBA), middle temporal (MT), superior temporal sulcus (STS), inferior parietal lobule (IPL) and premotor areas are shown. Within the images, L indicates left and R indicates right.
DISCUSSION
This study demonstrated that BR motion produced greater activation in the posterior temporal–occipital cortex (MT and EBA), STS and IPL than BU motion. BR motion was complex goal-directed biological motion with understandable intention, whereas BU motion was complex non-goal-directed biological motion. Therefore, the greater activation of STS was fairly predicted because it is widely accepted that STS is involved in detection of goal-directed actions and intention of others,3,8,9 and even a walking robot could activate STS.19 The greater activation of IPL, as a part of human MNS, was also predicted. Human neuroimaging and monkey studies have supported the view that when we observe others' actions, the action is internally represented through our own motor system including MNS.5,18,20 It has been suggested that MNS may participate in understanding and imitation of action through a mechanism by which observed actions are automatically matched with internal motor representation (action repertoire),5,6,21–23 and IPL neurons respond differently to similar actions with various intentions.24
The novel finding in the present study is that EBA and MT responded more strongly to BR motion than BU motion, although both BR motion and BU motion were complex biological motions containing an identical number of bodies or body parts. Neuroimaging studies about biological motion have demonstrated that STS plays a crucial role in processing biological motion and is important for detecting intention of others. But the studies have consistently reported the involvement of other brain regions such as EBA and MT,25,26 and the exact role of these regions in processing biological motion has been unclear.
Originally, EBA was identified as an area that responds selectively to human bodies and body parts. In that study, at the same time, EBA responded more strongly to natural motion than to artificial motion.10 Thereafter, the role of EBA in processing human actions has been the focus of many discussions. The static representation hypothesis is that EBA responds simply to static snapshots of the individual posture that comprise whole-body actions.27 In contrast, the dynamic representation hypothesis is that EBA is directly involved in representing the dynamic aspects of human motions as part of a system for inferring the action and intention of others.17,18 Astafiev et al. demonstrated that EBA also responded to self-produced body movements, even if the body part is not visible.16 Jackson et al. reported that, compared to observation of actions, EBA activation was enhanced during imitation.17 Furthermore, the motivation to act has been shown to modulate EBA activity.28 These studies proposed an extended role for EBA, involving the planning, execution and imagination of actions. In favor of the latter hypothesis, the present result suggests that EBA might contribute to the understanding of goal-directed actions, being located at the entry of human MNS.
MT has been known to respond selectively to moving stimuli,11 and an fMRI study reported that MT responded equally to meaningful and non-meaningful actions,19 suggesting that MT processes low-level physical properties or information of moving stimuli. But it was reported that MT responded to static images of implied motion29 and that the MT responses to static body images were greater than to other object images.30,31 From these findings it is suggested that face and body figural information might project to MT.26,32 The present findings of enhanced activations in MT along with EBA may support this view, although several studies have reported substantial overlapping between EBA and MT.14,30,31
In conclusion, EBA might be located at the entry of human MNS through which dynamic aspects of human motions are represented and contribute to the understanding of others' actions. The present results merit further investigation of the function of EBA in neuropsychiatric disorders such as schizophrenia and autism.
ACKNOWLEDGMENTS
This study was supported by a consignment expense for Molecular Imaging Program on Research Base for PET Diagnosis from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japanese Government, a Grant-in-Aid for Scientific Research from MEXT (15390438), a research grant for nervous and mental disorders (14B-3), and a Health and Labor Sciences Research Grant for Research on Psychiatric and Neurological Diseases and Mental Health (H19-KOKORO-004) from the Japanese Ministry of Health, Labor and Welfare.




