Asymmetric dimethylarginine may be a missing link between cardiovascular disease and chronic kidney disease (Review Article)
Abstract
SUMMARY: Decreased nitric oxide (NO) production and/or impaired NO bioavailability may occur in patients with chronic kidney disease (CKD), and could contribute to the elevation of blood pressure, cardiovascular disease (CVD) and the progression of renal injury in these patients. However, the underlying molecular mechanisms for reduced NO action in patients with CKD remains to be elucidated. Asymmetric dimethylarginine (ADMA) is a naturally occurring l-arginine analogue found in plasma and various types of tissues, acting as an endogenous NO synthase inhibitor in vivo. Further, plasma level of ADMA is elevated in patients with CKD and found to be a strong biomarker or predictor for future cardiovascular events. In addition, plasma level of ADMA could predict the progression of renal injury in these patients as well. These findings suggest that elevation of ADMA may be a missing link between CVD and CKD. In this review, we discuss the molecular mechanisms for the elevation of ADMA and its pathophysiological role for CVD in high-risk patients, especially focusing on patients with CKD.
In recent years, there has been a growing body of evidence that even minor renal dysfunction is associated with high risks of cardiovascular events.1,2 Now chronic kidney disease (CKD) is generally thought to be one of the risk factors for cardiovascular disease (CVD).3 Because endothelial dysfunction is an initial step to atherosclerosis in patients with hypertension, diabetes and CKD,2,4–7 reduced generation and/or bioavailability of nitric oxide (NO) may link these risk factors to the events of CVD. However, the underlying molecular mechanisms for the reduced action of NO in these high-risk patients remain to be elucidated.
Nitric oxide is synthesized by stereospecific oxidation of the terminal guanidine nitrogen of l-arginine by the action of the NO synthase (NOS). The synthesis of NO can be blocked by inhibition of the NOS active site with guanidino-substituted analogues of l-arginine, such as asymmetric dimethylarginine (ADMA).5,7 We, along with others, have demonstrated that plasma concentrations of ADMA are elevated in patients with cardiovascular risk factors such as hypertension,8 diabetes9 and hyperlipidaemia,10 and are one of the useful biomarkers for future cardiovascular events.9,11,12 Further, recently, ADMA level was also found to be elevated in patients with CKD (even in CKD stage I)13–115 and associated with atherosclerotic vascular complications.16 In addition, plasma ADMA level predicts future cardiovascular events17 and the progression of renal injury in patients with CKD.18,19 These findings suggest that elevation of ADMA may be a missing link between CVD and CKD. In this review, we discuss the molecular mechanisms for the elevation of ADMA and its pathophysiological role for CVD in high-risk patients, especially focusing on patients with CKD.
BIOLOGICAL ACTIONS OF ADMA
There are three types of methylated arginines (ADMA, NG-monomethyl-l-arginine (l-NMMA) and symmetric dimethylarginine (SDMA), an inert isomer of ADMA) in vivo (Fig. 1).5,7 The biological action of methylated arginine was first shown by Hibbs et al. They reported in their paper that l-NMMA inhibited macrophage activation in vitro.20 Further, Vallance et al. reported in 1992 that ADMA and l-NMMA were found in human plasma and urine, and acted as endogenous competitive inhibitors of NOS.13 Because only minor amounts of l-NMMA are found in human plasma and SDMA has no effect on NOS activity, ADMA is now thought to be a major type of endogenously generated methylated arginines that possess the inhibitory activity of NOS.5
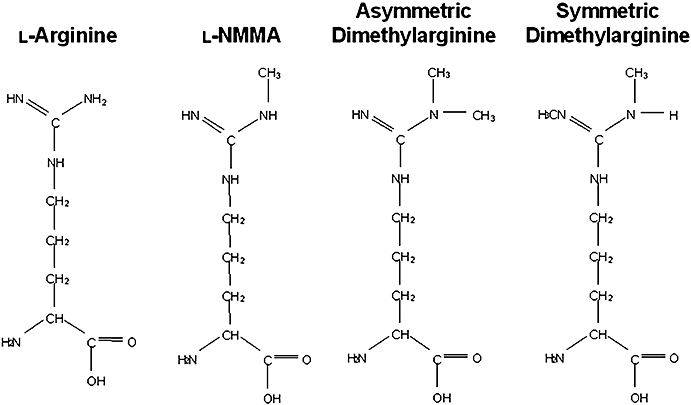
Structure of l-arginine and endogenous methylarginines.
Asymmetric dimethylarginine inhibits all three isoforms of NOS, which is reversed by excess l-arginine supplementation, and its inhibitory activity of the three isoforms of NOS is approximately equipotent with that of l-NMMA.5,13 The IC50 is dependent on the prevailing l-arginine concentration21 and is approximately 2–10 μM22In vitro studies have shown that physiologically relevant levels of ADMA significantly inhibit NOS and subsequently reduce NO generation in cultured endothelial cells (EC) and in isolated human blood vessels.23–25 Administration of ADMA to normal rats causes an increase in renal vascular resistance and blood pressure (BP).26,27 Further, plasma from patients with renal failure, in whom plasma ADMA concentrations are markedly elevated, significantly inhibits NO production in cultured EC.28 These observations suggest a biologically relevant action of ADMA in vivo. However, because it has been reported that circulating l-arginine levels in human plasma are in the range of 40–100 μM and that l-arginine concentration as low as 3 μM is sufficient to induce half-maximal activity of NOS,29,30 it is still questioned that 2–10 μM of circulating ADMA could actually compete with l-arginine for the NOS activity. However, Cardounel et al. recently showed that intracellular level of ADMA was greatly higher than extracellular one, which was sufficient to inhibit endothelial NOS in cultured EC.31 Further, they showed that ADMA levels in balloon-injured artery were elevated by fourfold, which caused prominent impairment of vascular relaxation. Taken together, these observations suggest that endogenous ADMA is pathophysiologically relevant in CVD. Recently, chronic infusion of ADMA was found to induce vascular lesions in endothelial NOS knockout mice,32 thus suggesting some additional unrecognized action (NO/NOS-independent action) of ADMA in vivo.
In addition, all three methylarginines are reported to interfere with l-arginine transport mediated by plasma membrane cationic amino acid transporter (y+).33 Therefore, it is also possible that SDMA could inhibit NO generation by limiting intracellular l-arginine availability,33 although it has no direct effects on NOS activity. Indeed, Bode-Boger et al. recently showed that SDMA dose-dependently blocked NO synthesis by cultured EC and that plasma levels of SDMA were positively associated with extent of coronary artery disease (CAD) in patients with CAD.34
SYNTHESIS OF ADMA
Humans generate approximately 300 μmol of ADMA per day.35 Various types of cells can generate ADMA, l-NMMA and SDMA.5,7 These dimethylarginines are derived from the degradation products of methylated proteins, which have been produced by protein methyltranferases (PRMT) (Fig. 2).36 S-adenosylmethionine works as a methyl donor in the reactions mediated by PRMT.36 After proteolysis of arginine-methylated proteins, free dimethylarginines are released from the cells. To date, nine PRMT genes, PRMT1–9, are reported.37–39 There are two types of PRMT: type I (PRMT1–4, 6 and 8) catalyses asymmetrical dimethylation and monomethylation of arginine residues and produces ADMA and l-NMMA, whereas type II (PRMT5, 7 and 9) catalyses symmetrical dimethylation and monomethylation of arginine residues and produces SDMA and l-NMMA.37 Thus, type-I PRMT is thought to be mainly involved in the generation of ADMA in vivo. Type-I PRMT are found in various organs, including vessels, heart and kidney.40–42 Recently, oxidized low-density lipoprotein (LDL) was reported to increase the generation of ADMA by EC via upregulation of type-I PRMT genes.36 Further, ADMA secretion from EC is also enhanced by shear stress through the induction of PRMT genes.43 These observations suggest that generation of ADMA may be regulated partly by type-I PRMT in vivo.

Synthesis and metabolism of asymmetric dimethylarginine (ADMA). DDAH, dimethylarginine dimethylaminohydrolase; PRMT, protein methyltransferase; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine.
DEGRADATION OF ADMA
It is known that more than 90% of the circulating ADMA is metabolized by the action of dimethylarginine dimethylaminohydrolase (DDAH) in rats.44 ADMA and l-NMMA, but not SDMA, are metabolized into citrulline and dimethylamine by the action of DDAH (Fig. 2).5,7 DDAH is widely distributed throughout the body45–48 and there are two isoforms of DDAH (DDAH-I and DDAH-II).49 DDAH-I is located in tissues expressing neuronal NOS, whereas DDAH-II predominates in tissues containing the endothelial NOS.49 Recently, DDAH activity in DDAH-I+/– mice was found to be decreased to approximately 50% of that in control mice.50 As DDAH-II gene expression was not disturbed in these mice, DDAH-I, but not DDAH-II, may be a predominant isoform for ADMA degradation.
It has been demonstrated that 4124W, an inhibitor of DDAH, inhibits methylarginine metabolism and increases ADMA sufficiently enough to inhibit endothelial NOS.51 In addition, we have previously reported that DDAH overexpression decreases the production of ADMA by cultured vascular smooth muscle cells, thus resulting in the enhancement of NO generation via inducible NOS.52 Collectively, the endogenous ADMA–DDAH systems may also be involved in the regulation of NO synthesis.5,7,52 Furthermore, it has been reported that the impaired metabolism of ADMA by DDAH is associated with elevated ADMA levels in animal models of hypercholesterolaemia and diabetes.10,45 Taken together, these observations suggest that decreased DDAH action may be one of the main mechanisms for the elevation of ADMA levels in patients with risk factors for CVD.
ADMA AND DISEASES
Hypertension
There is a growing body of evidence to show that NO plays an important role in the regulation of vascular tonus and BP.53,54 There are two possible mechanisms by which endogenous ADMA is involved in the pathogenesis of hypertension: (i) ADMA may exert vasoconstrictor/pressor effects by inhibiting endothelial NOS activity35,55; and (ii) ADMA may inhibit renal sodium excretion by reducing NO synthesis in the kidney.56–58 Indeed, increased urinary levels of ADMA were observed in Dahl salt-sensitive rats, which were associated with an increase in BP.59 Further, BP levels were significantly lower in DDAH-transgenic mice than those in wild-type mice,60 whereas the levels of BP were higher in DDAH-I+/– mice than in control mice.50 In humans, we have recently found that the plasma levels of ADMA are associated with mean BP levels in Japanese subjects without coronary or peripheral arterial disease.8
Blood pressure itself may elevate plasma ADMA levels through upregulation of PRMT. In cultured EC, shear stress enhanced mRNA levels of PRMT via activation of nuclear factor-κB pathway, thus increasing the production of ADMA levels.43 Angiotensin II (Ang II)-elicited reactive oxygen species (ROS) generation may also be involved in the elevated ADMA levels in hypertension on the basis of the following observations: (i) DDAH enzymatic activity in EC is decreased under oxidative stress conditions45,61,62; (ii) Ang II elicits ROS generation and subsequent activation of nuclear factor-κB in vascular wall cells63–66; and (iii) plasma ADMA levels in hypertensive patients are decreased by the treatment with inhibitors of the renin–angiotensin system.67 These observations suggest that the Ang II-elicited ROS generation in vascular wall cells may impair the DDAH activity, thus resulting in the increase in ADMA levels in patients with hypertension.
Diabetes
Cardiovascular disease is responsible for approximately 70% of all deaths in patients with type 2 diabetes.68 In the Framingham study, the incidence of CVD was 2–4 times greater in diabetic patients than in the general population.69 The levels of conventional risk factors for atherosclerosis were certainly increased in diabetic patients, but not enough to explain the exaggerated risk for macrovascular complications in this population.70 Plasma ADMA levels were elevated in animal models of diabetes45,71 and in patients with impaired glucose tolerance,8 insulin resistance72 or diabetes.9,73 It has been also reported that elevated plasma levels of ADMA are associated with increased risks of non-fatal stroke and myocardial infarction in patients with early diabetic nephropathy.9 These findings suggest that the elevated ADMA in diabetes could contribute to accelerated atherosclerosis in this population.
The mechanism by which insulin resistance or diabetes may increase ADMA is not fully understood. However, high glucose reduced DDAH activity associated with an increase in ADMA in cultured EC.45 Decreased aortic DDAH activity is also associated with an increase in plasma levels of ADMA in an animal model of diabetes.45 More recently, advanced glycation end products (AGE) were found to decrease DDAH activity and subsequently inhibit endothelium-dependent relaxation of rat aortic rings.74 Therefore, impairment of DDAH actions by high glucose and/or AGE could be one possible molecular mechanism of ADMA elevation in diabetic patients.
Recently, rosiglitazone, a ligand for peroxisome proliferator-activated receptor-γ (PPAR-γ), was shown to enhance insulin sensitivity and reduce ADMA levels in insulin-resistant subjects with hypertension.72 These findings have extended the previous observations showing that PPAR-γ ligands increased NO production in various types of tissues.75,76 As there is a PPAR-γ/retinoid X-receptor-responsive element in the promoter region of DDAH-II,77,78 rosiglitazone may increase NO synthesis by reducing ADMA levels via upregulation of the DDAH-II gene.
Hypercholesterolaemia
Plasma ADMA levels were also elevated in hyperlipidaemic animals and patients.10,79In vitro experiments have suggested the involvement of ROS in the elevation of ADMA levels in hypercholesterolaemia. Indeed, oxidized LDL was shown not only to stimulate the expression of PRMT, but also to suppress the activity of DDAH through ROS generation.36,61 Therefore, although it is still controversial, lipid lowering agents, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), could decrease the ADMA levels in hypercholesterolaemic patients, at least in part, through their antioxidative properties.80–84
Heart failure
We, along with others, have previously shown that ADMA levels were elevated in patients or animal models with heart failure.85–87 ADMA levels were associated with left ventricular hypertrophy and dysfunction in patients with end-stage renal disease (ESRD) as well.88 As an intravenous injection of ADMA significantly reduced heart rates and cardiac output in healthy subjects,35 ADMA may be one of the causative factors for heart failure in humans.
Whether heart failure itself may affect synthesis or metabolism of ADMA remains to be clarified. However, the reduction in renal clearance or metabolism of ADMA may account for the elevation of ADMA in patients with heart failure because renal excretory function deteriorates as heart failure progresses.89 Moreover, recently, DDAH activity was shown to be reduced in coronary endothelium, smooth muscles and cardiac myocytes in an animal model of heart failure.90 A positive feedback loop may exist between heart failure and ADMA levels.
CKD
Mechanisms for accumulation of ADMA in CKD
Recently, the potential role of ADMA in CKD was comprehensively reviewed by Zoccali C et al.91 According to their theory, there are at least four possible mechanisms that may explain the accumulation of ADMA in CKD: (i) increased methylation of proteins; (ii) increased protein turnover; (iii) decreased metabolism by DDAH; and (iv) impaired renal excretion. As dimethylarginines are excreted in urine,92 impaired renal clearance may, at least in part, account for the elevation of ADMA levels in patients with CKD. However, recently, evidence against this assumption has been accumulated. First, when dimethylarginines such as SDMA and ADMA are injected into rats intravenously, 66% of the injected SDMA is recovered in urine whereas only 5% of the ADMA is excreted in the urine.93 Second, plasma ADMA levels are elevated even in incipient renal disease patients with normal renal function.14 These observations suggest that only a small portion of circulating ADMA is excreted in the urine and that the contribution of renal clearance of ADMA to its circulating levels may be very small. In support of this, plasma levels of ADMA in patients with ESRD are markedly lower than those of SDMA.94 Further, plasma ADMA levels are elevated in proportion to the degree of nephrectomy in rats despite marked increases in renal clearance of ADMA.95 As renal clearance of SDMA is decreased and its plasma levels are increased in subnephrectomized rats, an experimental model of CKD,95 mechanisms other than impaired renal clearance are responsible for the elevation of ADMA in CKD. We have recently found that renal and liver DDAH-I and DDAH-II protein expression levels are significantly decreased in subnephrectomized rats,95 thus suggesting that impaired degradation of ADMA due to reduced DDAH levels may be a causative factor for the elevation of ADMA in this model. In addition, we, along with others, have demonstrated that the expression levels of PRMT is increased in subtotal nephrectomy rats as well.95,96 These observations suggest that enhancement of ADMA production could also be one possible mechanism for the elevation of ADMA in CKD. Okubo et al. showed that inhibition of PRMT decreased plasma ADMA levels in an animal experimental model of renal failure.97
Although the molecular mechanisms of upregulation of PRMT and/or downregulation of DDAH in CKD are still unclear, oxidative stress may be involved in the dysregulation of PRMT and DDAH given the following evidence: (i) gene expressions of type-I PRMT were increased by oxidized LDL in cultured ECs through a redox-regulated mechanism36; (ii) DDAH activities in vascular cells were reduced under high glucose conditions, which were prevented by an antioxidant, polyethylene glycol-conjugated superoxide dismutase45; and (iii) it is well known that oxidative stress generation is increased in patients with CKD.98,99 Uraemia-related oxidative stress as well as uraemic toxins such as homocysteine and AGE, which decrease DDAH activity,62,74 may have contributed to dysregulation of these enzymes as well.
CVD in CKD
Plasma levels of ADMA is elevated in patients with ESRD13 as well as mild to moderate CKD.14 Elevation of plasma ADMA levels may be a missing link between CVD and CKD.100 In fact, it has been reported that plasma ADMA was strongly associated with carotid intima-media thickness,16 left ventricular hypertrophy,88 cardiovascular complications17 and mortality17 in patients with ESRD. Furthermore, short-term reduction of circulating ADMA by haemodialysis was associated with increased endothelial function in ESRD patients.101 These observations suggest that ADMA may have an active participation in the development and progression of atherosclerosis in patients with ESRD. In addition, an important role of the ADMA–DDAH axis in angiogenic responses has been recently shown. Transfection of DDAH into cultured EC enhances vascular endothelial growth factor mRNA expression and stimulates tube formation of these cell types.102 In a murine model of hindlimb ischaemia, enhanced neovascularization and limb perfusion were observed in DDAH-transgenic mice, which were associated with reduced plasma levels of ADMA.103 Further, it has also been reported that there is an inverse correlation between plasma levels of ADMA and circulating endothelial progenitor cells.104 These observations suggest that the ADMA–DDAH axis may regulate angiogenic responses and/or endothelial repair in patients with CKD.
Hypertension in CKD
Hypertension is the most common complication in patients with CKD and is not only a predictor of CVD mortality, but also an independent determinant for the progression of renal disease.106 As mentioned earlier, ADMA could play a pathophysiological role in hypertension. Accumulated ADMA in CKD seems to be involved in the development of hypertension as well. Indeed, we have recently reported that plasma levels of ADMA are strongly correlated to BP levels in subnephrectomized rats.95 Further, DDAH overexpression was found to decrease plasma levels of ADMA and subsequently prevent the elevation of BP levels in this model.95 These observations suggest the pathological relevance of the increased plasma ADMA levels in hypertension in patients with CKD.
Proteinuria
Proteinuria, even microalbuminuria, is a strong and independent indicator of CVD among individuals with or without diabetes or hypertension.106–109 Yet the precise mechanisms of this link remain controversial and poorly understood. However, there is an increasing body of evidence that endothelial dysfunction is linked to proteinuria.110–112 Impaired NO production is the characteristic feature of endothelial dysfunction. Further, ADMA levels were related to endothelial dysfunction in an animal model and patients with CKD.97,101 Therefore, it is conceivable that endogenously accumulated ADMA in CKD may play a role in the development of proteinuria. Indeed, a positive correlation between ADMA and proteinuria was observed in patients with CKD.15,18 In addition, we have recently found that ADMA reduction by DDAH overexpression significantly decreases proteinuria in subtotal nephrectomized rats.113 Further, proteinuria per se may affect ADMA levels. The greater part of filtered proteins is reabsorbed by proximal tubular cells, in which they are degraded by lysosomes, indicating that proteinuria is a state of increased protein turnover. It has been demonstrated that the release of free dimethylarginines is increased under the condition of high protein turnover.114 These observations suggest that proteinuria itself may enhance ADMA production. In addition, DDAH is abundantly expressed in tubular cells.115 Therefore, DDAH could also play some role in the degradation of proteinuria-deriven ADMA in tubular cells.
Progression of CKD
Both hypertension and proteinuria are well-recognized risk factors for the progression of CKD.116 As already mentioned, because ADMA plays an important role in the pathogenesis of hypertension and proteinuria, it is conceivable that ADMA may also be involved in CKD progression. In fact, the plasma level of ADMA is a strong predictor for the progression of renal dysfunction in patients with CKD.18,19 NOS inhibition may accelerate the progression of renal injury by impairing the angiogenic responses and subsequent loss of peritubular capillaries via suppression of NO.117–119 In addition, we have recently found that plasma levels of ADMA are associated with decreased number of peritubular capillaries, enhanced tubulointerstitial fibrosis and progressive loss of renal function in the remnant kidney model, all of which were prevented by DDAH overexpression-elicited ADMA reduction.113
CONCLUSIONS
Asymmetric dimethylarginine is a potent endogenous NOS inhibitor and its accumulation may play important roles in endothelial dysfunction, hypertension and renal dysfunction, thereby contributing to the development and progression of CVD in patients with CKD. Although a number of in vitro and in vivo reports discussed here suggest the pathophysiological role of NO and ADMA in CKD and CVD, some clinical studies using l-arginine supplementation have produced disappointing results.120,121 These observations suggest that suppression of ADMA by inhibition of PRMT activation and/or enhancement of DDAH activity may be better strategies than simple l-arginine supplementation for preventing the cardiorenal complications in patients with CKD.




