From marrow oedema to osteonecrosis: Common paths in the development of post-transplant bone pain (Review Article)
Abstract
Summary: Osteonecrosis, the calcineurin-inhibitor-induced pain syndrome and transient marrow oedema may occur after renal transplantation, are generally painful and can be diagnosed by X-ray, radionuclide scan or magnetic resonance imaging. They share features of increased intraosseous pressure, compromised vascular supply, marrow oedema and the development of a ‘bone compartment syndrome’. Glucocorticoid dosage is the most commonly implicated risk factor for osteonecrosis. Mechanisms may include the differentiation of mesenchymal stem cells to adipocytes causing increased intraosseous pressure and collapse of marrow sinusoids, as well as increased osteoblast and osteocyte apoptosis. Some of these effects may be ameliorated by lipid lowering drugs. Calcineurin-inhibitors, particularly cyclosporine, may increase the risk of osteonecrosis because of vasoconstrictive effects and sirolimus may influence the development of osteonecrosis by potentiating the effects of calcineurin inhibitors or by influencing the lipid profile. For osteonecrosis, early stages are generally managed conservatively or with core decompression sometimes accompanied by bone grafting and more recently the injection of bone morphogenic protein. The use of iloprost to improve blood flow and bisphosphonates and RANK-ligand inhibition to reduce osteoclastic resorption of remaining trabecular structures are as yet unproven strategies. Unfortunately, the rate of total hip arthroplasty remains high. For the calcineurin-inhibitor-induced pain syndrome and transient marrow oedema, calcium channel blockers, the reduction or withdrawal of calcineurin-inhibitors and core decompression have been used. Although a lack of randomized controlled trials makes management decisions difficult, early recognition of these bone pain syndromes affords the best opportunity for avoiding prolonged pain or joint replacement surgery.
Musculoskeletal pain is reported by 19–35% of patients after renal transplantation.1 While there are a number of possible causes, osteonecrosis and lower limb pain caused by calcineurin-inhibitors or transient marrow oedema are common enough to be encountered by most nephrologists. These conditions may share common pathophysiology, are relatively easily diagnosed and may be amenable to early treatment. However, some patients suffer prolonged pain, reduced mobility and may require joint replacement because of delayed management.
OSTEONECROSIS
Osteonecrosis, also known as avascular necrosis or aseptic necrosis, most commonly affects the femoral head followed by other weight bearing long bones. It is a particularly devastating condition because of a propensity to affect young individuals and because it often progresses despite treatment to fracture and bony collapse of the articular surface. The median time from transplantation until the onset of symptoms is around 18 months2 and patients generally complain of hip pain with limitation of weight bearing and movement. The incidence has been reported to vary from 2.8% to 16% of transplanted patients in series reported from 1998.2–5 It is likely that rates using newer immunosuppressive regimens are lower than they were previously.1,2
Risk factors
Risk factors remain unclear because many studies are retrospective with limited or incomplete patient data. Osteonecrosis has been positively associated with a higher daily dose of prednisolone, a higher cumulative methylprednisolone dose in the first post-transplant year, more frequent rejection episodes and greater post-transplant increases in body weight.2,5,6 Using the United States Renal Data System to access information on the hospital admissions of 42 906 transplant recipients, osteonecrosis was positively associated with Afro-American race and negatively associated with diabetes and prior peritoneal dialysis.6 However, the association with Afro-American race may relate to poorer donor–recipient human leukocyte antigen (HLA) matching and for patients with diabetes, more rapid steroid tapering and higher mortality may explain the negative association with osteonecrosis. Most series have not detected associations with the mode of dialysis, donor source, levels of parathyroid hormone, serum calcium or creatinine. Although the risk of osteonecrosis after renal transplantation may be influenced by pre-existing renal osteodystrophy, pretransplant bone histomorphometric data that would be needed to support this suggestion are not available.
Diagnosis and staging
Typically osteonecrosis of the femoral head progresses to collapse and joint damage, because necrotic bone has a breaking load approximately 50% that of viable bone. The outcome of treatment is directly related to the disease stage at diagnosis, so early diagnosis and accurate, reproducible staging are very important. A number of classification systems have been introduced, including those of Marcus (1973), Ficat and Arlet (1977, 1985), the Association Research Circulation Osseous (1993) and Steinberg (1995). In the system of Ficat and Arlet,7,8 stages 0 and 1 have normal or minor radiographic appearances and stage 2 has sclerotic, cystic or sclerocystic changes. A crescent sign, which is a line of diminished density beneath the subchondral bone indicating subchondral collapse, is seen in the transition stage. In addition to having diagnostic importance, this sign is considered a prognostic indicator of progression. Stage 3 is characterized by a broken contour of the femoral head and stage 4 is characterized by collapse of the femoral head and a decrease of the joint space. A combination of diagnostic investigations is used in the Association Research Circulation Osseous system of staging. In stage 0, bone biopsy results are consistent with osteonecrosis with normal findings on all other tests. In stage I, there is a positive bone scan, magnetic resonance imaging (MRI) or both. In stage II there are radiographic abnormalities but no sign of collapse of the femoral head, in stage III the crescent sign is present and in stage IV there are a flattened articular surface, narrow joint space and changes in the acetabulum. Unfortunately, there is marked inter- and intraobserver discrepancy in interpretation, particularly of the crescent sign and joint space narrowing, so an alternative, simpler Pittsburgh classification system incorporating the comparison of MRI and radiographic appearances has also been proposed.9
Magnetic resonance imaging is generally regarded as the gold standard for diagnosing and staging, although it remains limited in following the repair process because destruction and reconstructive repair show similar signals.10 Another accurate diagnosis modality is the radionuclide bone scan with single photon emission computed tomography (SPECT). The finding of a ‘cold’ area with surrounding hyperaemia has been reported to have 100% sensitivity for the diagnosis of osteonecrosis, but the specificity is lower because of a 38% false positive rate.11 On the other hand, these false positives may represent patients with early, reversible changes that do not progress to frank osteonecrosis. When MRI was used to assess the same patients, the sensitivity for osteonecrosis was 66% but with no false positives. At approximately 1 year from the onset of symptoms, both the radionuclide bone scan and MRI (showing ‘geographic’ areas of low signal intensity on T1 and T2-weighted images) were highly sensitive. MRI sensitivity may be further improved using dynamic gadolinium-enhanced subtraction to enhance the contrast of marrow spaces.12,13
MARROW OEDEMA AND THE CALCINEURIN-INDUCED PAIN SYNDROME
The calcineurin-inhibitor-induced pain syndrome (CIPS), also called the ‘symmetric bone pain syndrome’, occurs in the context of organ transplantation and is associated with the use of cyclosporine A, less frequently with tacrolimus and not with azathioprine. CIPS has not been reported when calcineurin-inhibitors are used to treat autoimmune disorders, possibly because higher doses are used for transplantation.14 After solid organ transplantation, 1–17% of patients report symptoms, which vary from deep, aching rest pain to the sudden onset of severe, symmetrical, periarticular bone pain.14,15 The onset of pain is generally within the first month of transplantation resolving within 3 months, but has occasionally been described to occur up to 14 months post transplant with resolution over periods up to 18 months.14,16 Osteonecrosis has been reported to develop following multiple episodes of CIPS, in which pain typical of CIPS responded initially to treatment with calcium channel blockers.15 Also, symptoms of lower limb pain with MRI features of marrow oedema affecting the metatarsals, knees and hips have been reported to progress to osteonecrosis despite withdrawal of cyclosporine.17
Diagnosis
The calcineurin-inhibitor-induced pain syndrome may sometimes be difficult to distinguish from conditions such as gout, hyperparathyroidism, osteoporosis with fragility fracture or osteonecrosis. Weight-bearing areas are affected, particularly the feet, ankles and knees. However, the pain may be unrelated to weight bearing and is generally bilateral and symmetrical, which distinguishes it from the usual pain of osteonecrosis. The diagnosis is generally confirmed by MRI or a radionuclide bone scan that identifies areas of hyperaemia and marrow oedema, often associated with soft tissue swelling and joint effusions,14 although in some cases radionuclide scans are reported to be normal.15 Pain may be associated with higher trough levels of cyclosporine or tacrolimus and improves as marrow oedema declines. In patients who are not on calcineurin-inhibitors, acute hip pain may be caused by transient marrow oedema, with bone scan and MRI features similar to those of CIPS.
PATHOPHYSIOLOGY
A number of pathophysiologic mechanisms have been suggested to underlie the development of osteonecrosis including fat embolism and abnormalities of coagulation (such as hypofibrinolysis and thrombophilia) leading to venous thrombosis, increased intraosseous venous pressure with reduced arterial perfusion and ‘intraosseous stress’.10 Certainly a number of conditions that predispose to vascular occlusion and thrombosis such as systemic lupus erythematosis and sickle cell anaemia are associated with an increased incidence of osteonecrosis. Although glucocorticoids are associated with up to 40% of cases of non-traumatic osteonecrosis, most patients do not develop this condition – so patients who have predisposing factors such as low levels of protein C or S may be particularly susceptible to the influence of glucocorticoids that are described below.
The role of immunosuppressive drugs: glucocorticoids
Immunosuppressive drugs play key roles in the development of syndromes of bone pain after transplantation. Glucocorticoids have been shown to modulate the differentiation of bone marrow cells, consistent with regulation at the level of a common precursor.18 Glucocorticoid treatment of cloned pluripotential cells from bone marrow increases the expression of fat-specific genes and favours cellular differentiation to adipocytes rather than to osteogenic or chondrogenic cells.19 This glucocorticoid effect is reported to occur in a dose-related fashion.20,21 Mechanisms by which glucocorticoids influence mesenchymal stem cell differentiation remain to be clarified, but one potential effect is by acting synergistically with endogenous inhibitors of the Wnt signalling pathway to increase adipogenesis. Wnts, particularly Wnt 10b, are considered to be important repressors of preadipocyte differentiation.22
In experimental animals, both the size of bone marrow adipocytes and the prevalence of osteonecrosis increase at higher glucocorticoid doses23 and autopsy studies of fat cell size in patients with glucocorticoid-induced osteonecrosis suggest a similar effect in humans.24 Glucocorticoids may also induce the transdifferentiation of osteoblast-like cells into mature adipocytes,25 but whether this contributes to the development of osteonecrosis is unknown. Figure 1 illustrates some ways by which glucocorticoids may influence bone marrow constituents.
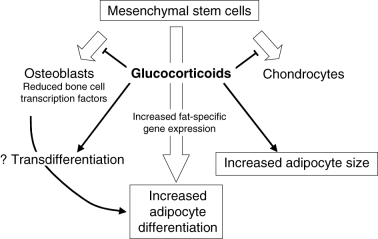
Possible effects of glucocorticoids on differentiation of mesenchymal stem cells and on adipocyte size.
In addition to increasing adipogenesis and depleting the pool of mesenchymal stem cells available for differentiation to osteoblasts, glucocorticoid use is associated with increased apoptosis of both osteocytes and osteoblasts. Apoptosis of these cells has been identified in femoral heads resected from patients with glucocorticoid-induced osteonecrosis, but not in femoral heads removed because of trauma,26 suggesting that this is a direct effect of glucocorticoid treatment rather than secondary to a reduction of vascular supply. Similar apoptosis of osteocytes and osteoblasts has been identified in iliac bone biopsies from patients with glucocorticoid-induced osteoporosis and it is speculated that a loss of mechanoreceptor function owing to osteocyte apoptosis may lead to an accumulation of microdamage and eventual fracture.26
Calcineurin-inhibitors and target of rapamycin (TOR) inhibitors
Although the mechanism is unconfirmed, calcineurin-inhibitors may cause bone pain and increase the risk of osteonecrosis by inducing vasoconstriction, leading to intraosseous venous thrombosis and marrow oedema. Compared with cyclosporine, there is a lower frequency of osteonecrosis with tacrolimus, which may be related to fewer episodes of acute rejection requiring high-dose steroids27 or less adverse effects on the lipid profile.28 Tacrolimus may also affect vascular perfusion to a lesser degree than cyclosporine. In the renal circulation perfusion has been reported to decrease within an hour of cyclosporine treatment but not after tacrolimus treatment.29 The reduced perfusion appeared to be caused by medium-sized arterial vasoconstriction and was abrogated by calcium channel blockade. Whether similar changes occur in bone is speculative, but a reduction of intraosseous pressure has been reported after IV nifedipine was given to two patients intraoperatively30 and in some case studies a rapid reduction of bone pain has corresponded to the commencement of treatment with calcium channel blockers. Use of the TOR-inhibitor sirolimus has also been associated with an increased incidence of bone pain and osteonecrosis in phase 3 trials.31 This may relate to a sirolimus-induced prothrombotic lipid profile or, when used concurrently with calcineurin-inhibitors, to potentiation of the effects of those drugs.
POSSIBLE COMMON PATHWAYS FOR OSTEONECROSIS, CIPS AND TRANSIENT MARROW OEDEMA
Figure 2 illustrates a hypothetical pathway for the development of osteonecrosis and the syndromes of transient marrow oedema and CIPS. Steps in this pathway that are common to various precipitants include reduced intraosseous perfusion followed by marrow oedema and the development of a bone compartment syndrome. When marrow oedema is severe and fibrinolysis inadequate, tissue anoxia is likely to result in osteonecrosis. On the other hand, transient marrow oedema or CIPS may result when hypoxia is followed by fibrinolysis, reperfusion and hyperaemia without osteonecrosis. Some recent studies lend support to these mechanisms. Kim and co-workers investigated MRI changes that accompany the development of transient marrow oedema after temporary ischaemia of the femoral head.32 The femoral head generally contains both haematopoietic and fatty marrow that produces a signal of intermediate intensity. However, after resolution of marrow oedema the authors reported high signal intensity at the proximal femoral metaphysis on T1-weighted sequences, which was consistent with an increase in metaphyseal fat. This MRI finding was supported by bone histology showing necrotic fat cells, depleted haematopoietic cells and viable trabecular bone. The proposed mechanism was of increased adipocyte volume causing increased intraosseous pressure, collapse of marrow sinusoids, reduced blood flow and marrow oedema. In a cross-sectional study of 29 females with glucocorticoid treated systemic lupus erythematosis, MRI indicated a higher percentage of fatty marrow in patients with lupus than in matched controls.33 In a small longitudinal extension of the same study, fatty conversion correlated positively with the daily prednisolone dose. Marrow fat was also greater in patients with ischaemic bone lesions. Interestingly, the same authors recently reported a longitudinal study which showed that femoral head osteonecrosis correlated with a high fat content in the proximal femur even before glucocorticoid therapy was commenced.34 Such patients may be particularly susceptible to the adverse impact of glucocorticoid therapy.
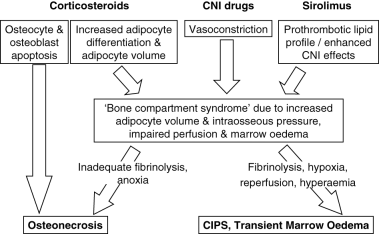
Factors that may mediate the development of osteonecrosis, calcineurin-inhibitor induced pain syndrome (CIPS) and transient marrow oedema. CNI, calcineurin-inhibitor.
SURGICAL MANAGEMENT OF OSTEONECROSIS
Before loss of femoral head sphericity management is generally conservative or by core decompression, a procedure aimed to normalize interstitial oedema, intraosseous venous hypertension and relieve pain. Core decompression is often supplemented by additional procedures such as the use of allograft bone or bone marrow cells. Overall, core decompression appears to offer better outcomes than conservative management in early stages of osteonecrosis. However, in two randomized controlled trials (Table 1) core decompression improved the Harris hip score (an index of pain, functional capacity, range of movement and absence of deformity) in one35 and reduced pain but not the likelihood of collapse in another.36 A literature review published in 1996 assessed 42 reports that included general surveys, prospective studies and multicentre studies of core decompression and non-operative management.39 Of 2025 hips with osteonecrosis, core decompression was used in 1206 and conservative management in 819. In 24 studies of core decompression, satisfactory outcomes were reported in 63.5% for all hips and 71% for precollapse hips. In 21 studies of non-operative management, results were satisfactory for 22.7% of all hips and for 34.5% of precollapse hips. A meta-analysis published in 2000 that assessed 22 studies using core decompression and eight studies in which patients were treated with conservative management reported that the success rate for core decompression exceeded that of non-operative management only in Steinberg stage 1 (84%vs 61%).40 Failure of core decompression (progression to collapse or need for a subsequent operation or both) is predicted by advanced preoperative radiographic stage, a shorter duration of symptoms and the use of glucocorticoids, but age, gender, alcohol intake and having a renal transplant do not appear to influence the outcome.41 Complications occur in 5–15% of cases and include subtrochanteric fracture, intertrochanteric fracture, femoral neck fracture, fracture of the femoral head and penetration into the joint space.
| Treatment | Author and year | No. patient | Stage of osteonecrosis | Randomization procedure | Follow up | Response | Comments | |
|---|---|---|---|---|---|---|---|---|
| Conservative treatment vs core decompression | Stulberg et al. 199135 | 36 (55 hips) | Ficat stages 0–IV but numbers for analysis only sufficient in stages I–III | 29 core decompression 26 conservative | Operated; Ficat stage and Harris hip score Stage I; 70% Stage II; 71% Stage III; 73% | Conservative; Ficat stage and Harris hip score Stage I; 20% Stage II; 0% Stage III; 10% | In stages I–III, scores better after surgery | |
| Conservative treatment vs core decompression | Koo et al. 199636 | 33 (37 hips) | Early stage | 18 core decompression 19 conservative | Pain 6 months Collapse 24+ months | Operated; Pain persisted in 1 of 10 painful hips; (1 of 18 patients; 6%) 78% femoral head collapse | Conservative; Pain persisted in 3 of 4 painful hips; (3 of 19 patients; 16%) 79% femoral head collapse | Surgery offers improved symptom relief |
| Alendronate vs no treatment to prevent femoral head collapse in non-traumatic osteonecrosis | Lai et al. 200537 | 40 | Steinberg stages II–III Necrotic area >30% (class C) | 20 patients treated with alendronate 70 mg/week for 25 weeks 20 control patients (non-placebo) | 24+ months | Alendronate; Collapse of 2/29 femoral heads (7%) 1/29 THA (3%) | No treatment; Collapse of 19/25 femoral heads (76%) 16/25 THA (64%) | Improved results with alendronate |
| Extracorporeal shock wave vs core decompression and non- vascularized fibular bone grafting | Wang et al. 200538 | 48 (57 hips) | Stages I, II, III | 23 (29 hips); Extracorporeal shock wave (single treatment of 6000 impulses of 28 kV to the affected hip25 (28 hips); Decompressed and non-vascularized fibular bone grafting | 25 months average | Decompression and fibular graft; 29% improved, 36% unchanged, 36% worse (based on pain and Harris hip scores). On imaging 79% progressed | Extracorporeal shock wave; 79% improved, 10% unchanged 10% worse (pain and Harris hip scores). On imaging 15% progressed | Shock wave therapy more effective |
- Studies assessing differing surgical techniques or prostheses excluded. THA, total hip arthroplasty.
Core decompression has been combined with bone grafting to replace necrotic bone at the femoral head and other sites with mixed results. In one study, 36% of 312 hips followed for at least 2 years after core decompression and bone grafting required total hip arthroplasty.42 Even in those cases that did not develop complications, a high rate of radiographic progression was reported with poorer results for later stages and larger lesions. Non-vascularized cancellous bone grafting has low morbidity and may provide structural support to prevent collapse during the repair process. On the other hand, vascularized bone grafting may offer better outcomes than non-vascularized grafts in patients with more advanced disease, and has been used with reported success in glucocorticoid-induced osteonecrosis.43,44 When a vascularized graft has been considered, the fibula has been widely used, but results for vascularized grafts from the iliac crest suggest that this might be an attractive alternative.45 New bone formation may also be enhanced by the injection of harvested autologous bone marrow containing stem cells, bone morphogenic protein-2 and angiogenic cytokines.46,47
Extracorporeal shock-wave treatment is a novel therapy reported in one randomized controlled trial to be more effective than core decompression and non-vascularized fibular grafting for patients with early stage osteonecrosis of the femoral head (Table 1).38 Longer-term results are needed to determine if this technique is effective, because current results only extend to around 25 months.
Low viscosity cement can be injected to restore the femoral head to its correct shape and delay progression to osteoarthritis, but joint replacement is required once severe joint collapse has occurred and osteoarthritis has developed. Total hip arthroplasty should be used judiciously, because the overall failure rate exceeds that of total hip arthroplasty done for causes other than osteonecrosis. It is also unlikely that prosthetic replacements will endure for the lifetime of relatively young patients.
The results of total hip arthroplasty have been assessed in a study of 32 renal transplant recipients who developed osteonecrosis.48 Preoperative symptoms were rated as poor or fair in 91%. The early complications of 48 total hip arthroplasties included phlebitis, haematoma, dislocation and deep infection. Reoperation was required in 29% for loosening or instability and when last assessed at a mean follow-up time of 5 years and 7 months, function was good to excellent in 75%.
NON-SURGICAL MANAGEMENTS
Osteonecrosis and bone marrow oedema
Lipid-lowering agents may have prophylactic and therapeutic potential in osteonecrosis. In 1983, clofibrate was reported to reduce the size of marrow fat cells and to decrease intrafemoral head pressure.49 More recently, in a retrospective study of 284 patients taking statins while being treated with glucocorticoids, 1% of patients developed MRI-proven osteonecrosis over a mean follow up of 7.5 years, compared with 3–20% of historical controls.50 In studies using chickens, lovastatin reduced the adipogenic effects of glucocorticoids and prevented the development of osteonecrosis.51 Lovastatin has been reported to reduce glucocorticoid-mediated adipogenesis by influencing fat-specific gene expression and also to counteract the inhibitory effects of glucocorticoids on osteoblast gene expression, so that uncommitted marrow progenitors may be shunted from adipocytic to osteoblastic differentiation.37,51 Because the potential of these drugs has not been proven in a clinical setting, a randomized controlled trial was commenced in 2002 to assess the value of atorvastatin for prevention of osteonecrosis in patients with systemic lupus erythematosis.52 This study is due to be completed in October 2006.
In established disease, the bisphosphonate ibandronate and RANK-ligand inhibition using recombinant osteoprotegerin-Fc have been reported to reduce femoral head deformity in animal models,53,54 possibly by reducing osteoclastic resorption of trabecular structures that may act as scaffolds for new bone formation if left intact. In a recent randomized controlled trial of alendronate 70 mg weekly or no treatment, collapse of the femoral head and total hip arthroplasty was less frequent in patients treated with alendronate than in those on no treatment (Table 1).55
Intravenous iloprost, a vasoactive prostacyclin derivative used in the management of pulmonary hypertension has also been used for treatment of osteonecrosis and bone marrow oedema. Improvements in symptoms, the Harris hip score and oedema assessed by MRI have been reported for both conditions,56 but there are no controlled studies that assess the efficacy of this therapy. In one report, the bone pain of established osteonecrosis diminished within 30–60 min of oral nifedipine.57
Calcineurin-inhibitor-induced pain syndrome
For CIPS, therapies that are reported to improve pain include elevation of the feet and dosage reduction or withdrawal of calcineurin-inhibitors, although responses are variable. After the commencement of calcium channel blockers, some patients have been reported to experience rapid relief of pain in small (2–15 patients), uncontrolled case reports.14,15 Effective doses of extended release nifedipine were in the range of 30–60 mg daily. If calcium channel blockers are tried, such dihydropyridine derivatives may be most appropriate because they do not inhibit the calcineurin-inhibitor degrading cytochrome p450. Unfortunately, there are no randomized controlled studies on the efficacy of calcium channel blockers in the treatment of CIPS.
Non-steroidal anti-inflammatory drugs may relieve symptoms but can adversely affect renal function, and the efficacy of vitamin D is unproven. Core decompression is reported to rapidly improve symptoms of transient marrow oedema, but when managed conservatively, CIPS and transient marrow oedema generally improve over 2–4 months.16,32
CONCLUSION
Thankfully, more attention is being focused on the prophylaxis of ‘silent’ bone disease after renal transplantation, particularly the development of osteoporosis. Symptomatic bone pain is also common, despite a possible reduction in rates of osteonecrosis because of drug regimens incorporating lower doses of glucocorticoids. Patient outcomes are likely to be significantly improved if these symptomatic conditions are recognized and diagnosed early and if access to advice by experienced physicians and orthopaedic surgeons is expedited. Further studies of cellular events that mediate the development of osteonecrosis, CIPS and transient marrow oedema are quite likely to result in therapies that could ameliorate or avert these conditions. For all stages of osteonecrosis, there is clearly a need for interventions to be assessed by randomized controlled trials, using terminology for diagnostic categories and responses to treatment that simplify comparisons and allow results to be included in meta-analyses. Once those results are in, it will be easier to formulate appropriate therapeutic strategies.
ACKNOWLEDGEMENT
The author wishes to thank Dr S Gruenewald, Department of Nuclear Medicine, and Dr P Vladica, Department of Radiology, Westmead Hospital, for their helpful comments.




