Protein kinase C activation and its role in kidney disease (Review Article)
Abstract
SUMMARY: Protein kinase C (PKC) comprises a superfamily of isoenzymes, many of which are activated by cofactors such as diacylglycerol and phosphatidylserine. In order to be capable of activation, PKC must first undergo a series of phosphorylations. In turn, activated PKC phosphorylates a wide variety of intracellular target proteins and has multiple functions in signal transduced cellular regulation. A role for PKC activation had been noted in several renal diseases, but two that have had most investigation are diabetic nephropathy and kidney cancer. In diabetic nephropathy, an elevation in diacylglycerol and/or other cofactor stimulants leads to an increase in activity of certain PKC isoforms, changes that are linked to the development of dysfunctional vasculature. The ability of isoform-specific PKC inhibitors to antagonize diabetes-induced vascular disease is a new avenue for treatment of this disorder. In the development and progressive invasiveness of kidney cancer, increased activity of several specific isoforms of PKC has been noted. It is thought that this may promote the kidney cancer’s inherent resistance to apoptosis, in natural regression or after treatments, or it may promote the invasiveness of renal cancers via cellular differentiation pathways. In general, however, a more complete understanding of the functions of individual PKC isoforms in the kidney, and development or recognition of specific inhibitors or promoters of their activation, will be necessary to apply this knowledge for treatment of cellular dysregulation in renal disease.
Protein kinase C (PKC) participates in signal transduction pathways, cell proliferation, differentiation, cell cycle and apoptosis. However, the role of PKC in diseases of the kidney has not yet been fully defined. Most research to date has concentrated on its role in vascular dysfunction of diabetes and progressive chronic renal disease, and includes patient studies as well as experimental models. The activation of PKC induced by hyperglycaemia appears to be due to an increase in 1,2-diacylglycerol (DAG) levels, a physiological activator of PKC, although other cofactors such as phosphatidylserine (PS) or phorbol ester (PE) are also known stimulants. Inactivation of PKC isoforms by its specific inhibitors has been found to reverse many diabetes- or hyperglycaemia-associated vascular dysfunctions in the kidney, as well as in the retina and cardiovascular systems.1 Another key area of research is the role of PKC in the development and progression of kidney cancers.2 In kidney cancers, and in particular in renal cell carcinoma (RCC), there is altered expression or activation of several specific isoforms of PKC, compared with normal renal tissue expression. Different PKC inhibitors have been found to decrease invasiveness of aggressive human RCC cell lines, suggesting an invasion-promoting role for PKC. Although there are other examples of PKC acting in the pathogenesis of renal diseases, for example IgA nephropathy, the present review will give an overview of the general role of PKC and its activation, and report on recent research into the role and modulation of PKC in diabetic nephropathy and RCC.
SUBFAMILIES AND ISOFORMS OF PKC
Protein kinase C was first characterized by Takai and colleagues in the late 1980s (reviewed in the study by Newton3) and it is now known to constitute a superfamily of serine-threonine kinases that catalyse numerous biochemical reactions critical to cellular function. Currently, the PKC family incorporates 13 isoforms, which can be classified into three subfamilies on the basis of their mode of activation4 (Table 1, Fig. 1). The group of classical or conventional PKC (cPKC) consists of the α, βI, βII and γ isoforms, all of which depend on calcium (Ca++), DAG or its analogue phorbol 12-myristate 13-acetate, and in most cases PS for activation. The isoforms that are independent of Ca++, but require DAG and PS, are classified as the new PKC (nPKC), and include the subtypes δ, ɛ, η and θ. The third group of isoforms is that of the atypical PKC (aPKC) ξ, ι/λ, ν and µ. Their activation does not require Ca++ or DAG, but only PS. More recently, a fourth group of structurally distinct aPKC, called PKC-related kinases (PRK) and having isoforms 1, 2 and 3, has been identified. However, their assignment as a true PKC subfamily, as demonstrated in Figure 1, is controversial. The tissue distribution of the PKC isoforms, including for the kidney, is listed in Table 2.
| PKC subfamily | Isoforms |
|---|---|
| cPKC | α, βI, βII, γ |
| nPKC | Δ, ɛ, η, θ, ζ |
| aPKC | ξ, ι/λ, ν, µ |
- aPKC, atypical PKC; cPKC, classical or conventional PKC; nPKC, new PKC.
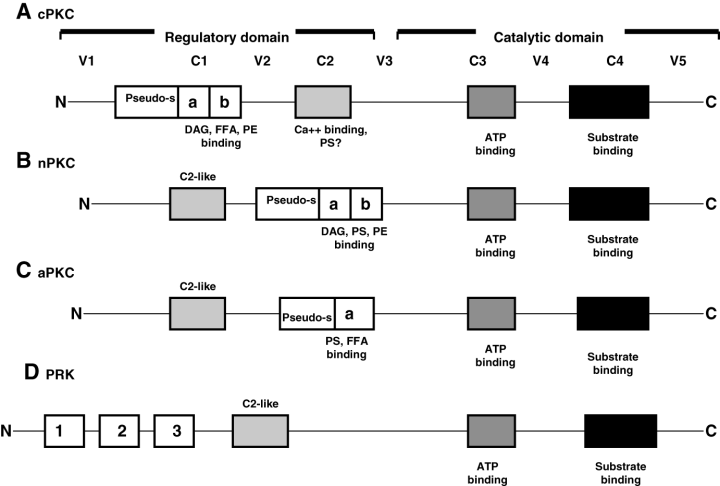
Protein architecture and cofactor requirements of various subfamilies of protein kinase C (PKC): all PKC subfamilies, and so all isoforms, contain a regulatory and a catalytic domain. These consist of four conserved regions (C1–C4) and five variable regions (V1–V5). As can be seen by the differences in protein architecture and cofactor requirements, the inclusion of the PRK (D) as a true subfamily of PKC is controversial. cPKC, conventional PKC; nPKC, new PKC; aPKC, atypical PKC; PRK, protein kinase C regulatory kinase; DAG, diacylglycerol; FFA, free fatty acids; PS, phosphatidyl serine; PE, phorbol ester; pseudo-s, pseudo-substrate; Ca++, calcium; ATP, adenosine triphosphate. The cysteine-rich zinc finger regions C1a and C1b are indicated with a and b. (Figure adapted from the study by da Rocha et al.5)
| Cell/tissue† | PKCα | PKCβI | PKCβµ | PKCγ | PKCδ | PKCɛ | PKCη | PKCξ | PKCθ | PKCλ/1 | PKCµ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kidney | + | + | + | – | + | + | + | + | – | + | + |
| Central nervous system | + | + | + | + | + | + | + | + | + | + | ? |
| Heart | + | + | + | – | + | + | + | + | + | + | ? |
| Small intestine | ? | ? | ? | – | + | ? | ? | ? | ? | + | ? |
| Liver | + | + | + | – | + | + | ? | + | + | + | ? |
| Airways smooth muscle | + | + | + | – | + | + | + | + | + | + | + |
| Lung | + | + | + | – | + | + | + | + | + | + | + |
| Neutrophil | + | + | + | – | + | ? | ? | + | ? | ? | ? |
| Monocyte | + | + | + | – | + | + | + | + | – | – | + |
| Macrophage | + | + | + | – | + | + | + | + | – | – | + |
| Eosinophil | + | + | + | – | + | + | – | – | – | + | + |
| Platelet | + | + | + | – | + | + | ? | + | + | ? | ? |
| T-lymphocyte | + | + | + | – | + | + | + | + | ? | ? | ? |
| B-lymphocyte | + | + | + | – | + | + | + | + | ? | ? | ? |
| Vascular smooth muscle | + | + | + | – | + | + | + | ? | ? | + | ? |
| Retina | + | + | + | – | + | + | ? | + | ? | ? | ? |
| Spleen | + | + | + | – | + | + | + | ? | ? | + | ? |
| Testis | + | + | + | – | + | + | ? | + | ? | + | ? |
| Ovary | + | + | + | – | + | + | ? | + | ? | + | ? |
| Pancreas | + | + | + | – | ? | + | ? | ? | ? | + | ? |
| Thymus | + | + | + | – | ? | – | ? | ? | ? | ? | ? |
- † Distribution may vary between species. +, Isoform detected at the protein and/or mRNA level; –, isoform absent; ?, expression unknown.
Since the discovery of PKC, its diverse functions in signal transduction, cell and tissue development, differentiation, proliferation, apoptosis and carcinogenesis have been described. All PKC isoforms contain a regulatory and a catalytic domain,5 each of which consists of four conserved regions (C1–C4) and five variable regions (V1–V5) (Fig. 1). The C1 and C2 regions are within the regulatory domain, and the C3 and C4 regions are within the catalytic domain of the enzyme. The C1 region of the cPKC and the nPKC contains two cysteine-rich zinc fingers referred to as C1a and C1b, the latter being involved in the binding of the cofactor DAG and PE. The aPKC contain a C1 domain with only one zinc finger, and the PRK do not possess this structure at all, explaining why these isoforms do not require DAG for activation, and do not respond to treatment with PKC-activating DAG analogues such as phorbol 12-myristate 13-acetate. The function of the zinc finger in the aPKC is not known. A ‘pseudo-substrate’ structure is situated close to the C1 region of the c, n and aPKC. It interacts with the catalytic region, maintaining the enzyme in its inactive form. Conversion into the active form occurs when the affinity of the pseudo-substrate for the catalytic region decreases in response to the binding of DAG to the C1 region, a reaction that takes place upon the interaction of Ca++ with the C2 domain, the introduction of critical conformational changes in the enzyme, and the association of membrane-bound PS with the C2 domain.5 This mechanism of activation – involving PS and Ca++– only applies to the cPKC. Whether these events also occur during activation of the non-Ca++-dependent nPKC, aPKC and PRK remains to be determined.
REGULATION OF PKC
Unless it is post-translationally or cotranslationally phosphorylated, PKC is incapable of being activated by DAG and other cofactors. PKC goes through three functional phosphorylations to take on the mature form.7 First, trans-phosphorylation at a residue in the activation loop renders the kinase competent to conduct auto-phosphorylation. Second, auto-phosphorylation of Thr641 enables the enzyme to adopt a catalytically competent conformation. This seems to stabilize the enzyme, so a later dephosphorylation near the catalytic site is incapable of affecting its activity. The final phosphorylation, on Ser660, allows the enzyme to be released into the cytosol. Both mature and underphosphorylated forms may exist in a single cell, localized to different subcellular sites, making their analysis very difficult unless specific antibodies to the different activation states are used.
Protein kinase C is regulated by two sequential, and equally critical, mechanisms: phosphorylation triggered by 3-phosphoinositide-dependent kinase (PDK)-1, and binding to DAG and/or other cofactors. Each mechanism regulates the structure, subcellular localization and function of PKC. Newly synthesized PKC associates with a membrane compartment in the cell8 in an ‘open’ conformation in which the auto-inhibitory pseudo-substrate sequence is removed from the catalytic domain.9 The open conformation is critical to the subsequent processing of PKC, because the activation loop sequence is unmasked. In this ‘open’ conformation, the unphosphorylated C-terminus of newly synthesized PKC is also exposed, and provides a docking site for PDK-1. Specifically, PDK-1 binds the unphosphorylated hydrophobic motif of newly synthesized PKC, positioning it to phosphorylate the exposed activation loop sequence.10 The turn motif and hydrophobic motif are then phosphorylated, an event that proceeds via an intramolecular auto-phosphorylation mechanism in the case of cPKC.11 As more information comes to hand regarding activation of PKC isoforms, it is likely that other mechanisms of phosphorylation and activation of specific isoforms will be found.
CELL PROLIFERATION, DIFFERENTIATION AND PKC
A direct link between cell proliferation, cell cycling and activation of some PKC isoforms has been identified. Paradoxically, these proteins may also induce cell differentiation, cell cycle arrest or cell death. There are many variables that contribute to these disparate outcomes, including the cell type, its genetic background and its microenvironment.12 For cell proliferation and cell differentiation, PE co-stimulation appears to have a key role. For example, PE can partially mimic the mitogenic effects of growth factors such as interleukin-3 or insulin, and do this in part by activating DAG and, finally, PKC.13 However, PKC activity has also been implicated in mediating some of the effects of growth inhibitory cytokines such as the interferons.14 Several of the PKC isoforms are upregulated in tumour progression and invasion, indicating a role in cellular differentiation. In contrast, expression of some PKC isoforms is lost during the same process. The disparate nature of the role of PKC in cell proliferation, differentiation and cell death may be explained, in part, by considering the relationships between PKC and individual signal transduction pathways.
SIGNAL TRANSDUCTION PATHWAYS AND PKC
Protein kinase C participate in signal transduction pathways by reversibly activating certain downstream proteins after the PKC have been activated by upstream signalling events. Their activation serves to amplify signals generated by the binding, for example, of a growth factor to its own cell surface-associated receptor.15,16 Once activated, PKC can transmit signals to the nucleus via different signal transduction pathways. For example, the mitogen-activated protein kinase (MAPK) cascade may be activated. Such diverse functions as cell proliferation, differentiation and cell death are outcomes of particular MAPK members: Raf-1 can be activated by Ras, in some cases after crosstalk with PKC; activated extracellular signal-regulated kinases can in turn activate transcription factors such as myc, myb, fos and jun, enabling the expression of genes encoding for enzymes required for key metabolic functions such as cell proliferation and invasion; and the JNK pathway often initiates cell death and in some cases this is stimulated by PKC isoforms. Another gene that is activated by PKC is ornithine decarboxylase, the rate-limiting enzyme in the biosynthesis of spermine, spermidine and putrescine. These polyamines have been implicated in cell transformation, the accelerated G1-to-S phase passage of cancer cells, as well as processes related to excessive extracellular matrix (ECM) degradation. These changes explain, in part, PKC involvement in cancer development and progression.5
APOPTOSIS AND PKC
Tissue homeostasis is dependent on the balance between cell proliferation and cell death. An imbalance can result in diseases linked with unwanted apoptosis or unwanted cell growth. PKC appears to also have a role in both processes, not only by stimulating cell cycling and proliferation but also by stimulating apoptosis.4 The cPKC and aPKC are predominantly anti-apoptotic, being principally involved in promoting cell survival and proliferation. The nPKCs, however, generally have a tumour suppressor function and are regarded as pro-apoptotic proteins. Again, as for cell proliferation, there are many conflicting data for these generalizations.
PKCα, one of the cPKC, is perhaps the best characterized isoform with respect to its important function in preventing or promoting apoptosis. The disparate roles may be cell- or tissue-specific.17 The mechanism by which PKCα prevents apoptosis may be via an interaction with the anti-apoptotic Bcl-2 protein, perhaps at the mitochondrial membrane,18 or via phosphorylation and stabilization of Bcl-2.19 Another possible target for PKCα is the serine/threonine protein kinase Raf-1. Raf-1 has been shown to mediate the anti-apoptotic function of protein kinase B/Akt in haematopoietic cells, through a mechanism that is PKC-dependent.20 In contrast, in human prostate cancer cell lines, the presence of PKCα in the mitochondrial membrane was associated with apoptosis, perhaps via action of pro-apoptotic Bax. Its absence, in this case, corresponded with resistance to apoptosis.21 PKCα may mediate activation of caspase-3, the apoptosis effector molecule, downstream of cytochrome c release in renal proximal tubule cells treated with the DNA-damaging agent cisplatin.22
Other PKC isoforms associated with apoptosis modulation are the nPKC ɛ, δ and θ. Growth factors such as insulin-like growth factor-1 can protect cells via pathways that include activation of PKCɛ and MAPK.23 In contrast, the activation of PKCδ occurs in response to a variety of stimuli, such as signals initiated by the cell’s death receptor,24 ultraviolet radiation25 and etoposide,26 all of which induce apoptosis. PKCδ is also a substrate for caspase-3,27 and so it is understandable that its loss can be linked to tumour development via a lack of promotion of apoptosis.28
PKC AND KIDNEY DISEASE – ROLE IN VASCULAR DYSFUNCTION IN DIABETES
Although there have been several publications that detail a role for several PKC isoforms in renal development, via initiation of the metanephros in nephron development,29 or renal diseases involving acute inflammation,30 the majority of publications refer to its role in diabetic nephropathy and end-stage kidney disease. Hyperglycaemia is believed to be the major cause of diabetic vascular complications involving both microvessels and arteries in the retina, renal glomeruli and aorta. Diabetic nephropathy is characterized by excessive accumulation of ECM in the kidney. Reactive oxygen species (ROS) play a central role in the ECM synthesis and degradation in the glomeruli and tubulointerstitium leading to renal fibrosis. High glucose induces cellular ROS through PKC-dependent activation of NAD(P)H oxidase and through altered mitochondrial metabolism.31,32 ROS are further generated from injured mitochondria and activate a signal transduction cascade involving the MAPK and the janus kinase/signal transducers and activators of transcription. There is then subsequent upregulation of the pro-fibrotic cytokine transforming growth factor-beta1, along with other pro-fibrotic factors such as angiotensin II that further promote formation of advanced glycation end-products and enhanced ECM synthesis.15
The activation of PKC has been described in diabetic nephropathy33–35 and this information, especially regarding specific isotopes, forms the basis for novel treatments for the disease. PKCβI and βII isoforms are commonly activated in diabetes-related renal and vascular injury, acting via stimulation of multiple signalling pathways.36 Tissue-specific regulation of several isoforms has been described.37 For example, PKCα, δ, ɛ and µ isoforms, found in pre-glomerular vessels, were upregulated by captopril and insulin treatments in the streptozotocin (STZ) rat model of diabetes, whereas no such regulation occurred in glomeruli. The authors concluded that angiotensin II receptors and PKC isoforms, on pre-glomerular vessels versus glomeruli, were differentially regulated by these treatments. In vascular disease, in general, protection of the vascular smooth muscle cells from apoptosis by insulin-like growth factor-1 requires PKC∈ and activation of the MAPK pathways.23 This information may also be applicable in the pathogenesis and reversal of vascular dysfunction in diabetic nephropathy as well as other vascular diseases of diabetes.
PKC AND KIDNEY DISEASE – ROLE IN KIDNEY CANCER
Protein kinase C participates in cellular signal transduction pathways, proliferation, differentiation, cell cycle and apoptosis. All of these cellular characteristics are involved in tumour development and progression. Although aberrations in PKC expression and localization have been implicated in the development of multiple human diseases, by far the most prominent association of PKC with disease has been in the promotion and progression of cancer.38 This will be briefly reviewed specifically with respect to RCC.
In both clear cell RCC and normal renal cells, all PKC isoforms apart from PKC-γ and PKC-θ are detectable. PKC-α, recorded to have tumour-suppressor properties, was found to be decreased in RCC versus normal tissue.39 Enger and colleagues investigated four different human RCC cell lines of the clear cell type in vitro.2 All cell lines expressed PKCα, ɛ, ζ, µ and ι/λ, but no PKC-δ. Different generalized PKC inhibitors markedly inhibited invasiveness of the aggressive cell lines, suggesting that PKC promotes invasion of RCC. PKCɛ expression levels correlated positively with a high proliferative activity. However, no obvious correlation between expression levels of specific PKC isoenzymes and in vitro invasiveness was observed. Membrane localization of PKCα and PKCɛ, reflecting activation of the enzymes, was associated with a highly invasive potential.
Protein kinase C may interact directly and indirectly with other factors in the development and progression of RCC. For example, vascular permeability factor/vascular endothelial growth factor (VPF/VEGF),40 intercellular adhesion molecule-141 and wild-type von Hippel–Lindau (VHL)42 are all factors in RCC by which PKC regulates the cellular activity, including signal transduction, proliferation, apoptosis and tumour angiogenesis. PKCs are reversibly activated by upstream signalling elements such as growth factor receptors and are able to reversibly activate downstream signalling modules such as the Raf-1 and the Bcl-2 cascade. The Raf-1/MAPK cascade is one of the main pathways for the transduction of signals through the cytoplasm. Its overstimulation by a hyperactive PKC may therefore contribute to the erroneous expression of many genes, including those that participate in cell proliferation and invasion. The involvement of PKC in the activation of the Bcl-2 protein is thought to represent an important cytoprotective device against lethal stimuli. This mechanism, presumably in conjunction with a diminished availability of cell death-mediating substances, such as neutral sphingomyelinase and ceramide, may further contribute to the reduced propensity of neoplastic cells to undergo apoptosis in response to cytotoxic agents.5
One of the reasons that RCC does not respond well to treatment is its inherent multidrug resistance (MDR). Developments in PKC targeting in experimental RCC therapeutics often focus on two RCC characteristics: one is apoptosis resistance, the other is MDR. These may not be mutually exclusive in RCC development. Drug-induced MDR has been linked to decreased expression of PKCα and β, increased expression of nPKC without any alteration to aPKC, depending on cancer cell type and drug type.43 There are other reports of increased PKC activity in drug-selected cell lines with MDR in comparison with drug-sensitive parent cell lines.44 PKC activation can also induce expression of the P-glycoprotein (Pgp) message mdr1 in mammalian cells45 and also activate the protein via phosphorylation. Treatment of MDR RCC with PKC activators increased phosphorylation of the Pgp and decreased drug accumulation and drug sensitivity. Conversely, treatment of MDR cells with PKC inhibitors decreased phosphorylation of the Pgp, drug-efflux activity and Pgp drug binding.40
MODULATION OF PKC AND REVERSAL OF KIDNEY DISEASE
As mentioned previously, diabetic nephropathy is one of the most common outcomes of the vascular complications of diabetes mellitus. In addition to the current treatments of glycaemic control, blood pressure control (with special emphasis on agents targeting the renin-angiotensin system), a low-protein (0.6–0.8 g/kg) diet and the use of hypolipidaemic agents, other therapeutic agents are needed. Inhibition of PKCβ, a common signalling molecule in diabetes-related renal and vascular injury, holds promise as a novel strategy to improve micro- and macrovascular outcomes in diabetes.36 Ruboxistaurin (LY333531) mesylate is a bisindolylmaleimide that shows a high degree of specificity within the PKC superfamily for inhibiting PKCβ isoforms.35 In animal models of diabetes, including the STZ rat, Lepr(db)/Lepr(db) mouse and STZ-Ren2 rat models, LY333531 normalized glomerular hyperfiltration, decreased urinary albumin excretion and reduced glomerular transforming growth factor-beta1 and ECM protein production. As a result, improvements were noted in mesangial expansion, glomerulosclerosis, tubulointerstitial fibrosis and renal function. Phase II studies have now been initiated. To determine whether clinical outcomes (mortality, renal and cardiovascular events) are improved beyond the current standard of care, phase III trials are planned.1,46
Another key mechanism in the pathogenesis of vascular dysfunction in diabetic nephropathy is ROS formation followed by the activation of specific isoforms of PKC. De novo formation of DAG during hyperglycaemia activates PKC. Acute hyperglycaemia may cause rapid endothelial impairment. In the STZ rat model, blockade of the PKCβII isoform with LY333531 prior to hyperglycaemia protected nitric oxide formation within the arteriolar wall. As well, oxygen radical scavenging and cyclo-oxygenase blockade prior to bouts of hyperglycaemia minimized endothelial impairment with limited side-effects. Therefore, a multi-targeted treatment strategy may prove most beneficial.47
Sporadic RCC is known to be well vascularized and characteristically overexpresses VPF/VEGF. These cancers are often associated with a mutation or loss of function of the VHL tumour suppressor gene. Pal and colleagues demonstrated that the wild-type VHL gene product neutralized PKC isoforms δ and ζ, thereby inhibiting MAPK activation.42 Aberrant VPF/VEGF overexpression, and the angiogenesis that results from such overexpression, were prevented. More recently, Brenner and colleagues reported on modulation of PKC and integrins, to minimize the migratory cell phenotype central to RCC invasion and secondary cancer development.48 The influence of PKC inhibitors RO31-8220, GF109203X and GO6976 on β1, β2 and β3 integrin expression, and cell proliferation and motility of RCC cells, was investigated, at this stage in cell culture. RO31-8220, a PKCɛ inhibitor, reduced β1 integrin expression by 92% and inhibited proliferation by 42% of untreated RCC cells. It did not influence cell migration. The other inhibitors were not as effective.
Protein kinase C remains an attractive cancer therapeutic target because of its regulation of cellular processes central to tumour initiation and progression, and response of the cancers to anti-tumour treatments. In non-RCC cancers, single agent activity for PKC modulation has had limited success; however, some potential synergies have been found between PCK modulation and conventional cytotoxic drugs. For example, the PKCα inhibitor ISIS 3521/LY900003 has been trialled with bryostatin for non-Hodgkin’s lymphoma and ovarian cancer in phase I studies. A randomized phase III study has commenced of ISIS 3521 in combination with carboplatin and paclitaxel, compared with the chemotherapy alone, in advanced non-small-cell lung cancer.49 The PKCβ inhibitor LY379196 was found singly to suppress the growth of three neuroblastoma cell lines and it also augmented the growth-suppressive effect of doxorubicin, etoposide, paclitaxel and vincristine, but not carboplatin.50 These findings, and those reported previously for in vitro studies with RCC, provide a conceptual basis for treatment of human RCC. However, further research needs to be completed to bring these ideas to clinical trial.
CONCLUSION
Most isoforms of PKC are found in the normal kidney. Expression of specific isoforms is altered, sometimes by deletion but often by activation, in diseases of the kidney. The two kidney diseases that have had most investigation are diabetic nephropathy and kidney cancer. In diabetic nephropathy, an elevation in DAG and PKCβ isoforms leads to dysfunctions in the micro- and macrovasculature of the kidney and progressive chronic kidney disease. Mutations, deletions or altered activity of several of the isoforms of PKC has been noted in the development and progressive invasiveness of kidney cancer. The ability of isoform-specific PKC inhibitors to antagonize the development and progression of these diseases is providing new avenues for their treatment. A more complete understanding of the functions of individual PKC isoforms in the kidney, and further development of isoform-specific inhibitors or gene therapy that otherwise modulates expression of specific isoforms will be necessary to apply this knowledge for treatment of cellular dysregulation in renal disease.




