Left cerebellar hemispheric tumor in an 80-year old man
CLINICAL COURSE
An 80-year-old man visited our clinic complaining of appetite loss and general fatigue over the previous month. Neurological examination at admission revealed clumsiness in the left finger–nose test. MRI revealed a 3 × 3 × 3 cm mass lesion in the left cerebellar hemisphere. The lesion was “open-ring” enhanced and characterized as thick and nom-uniform (Fig. 1). The preoperative diagnosis was a high-grade glioma or a metastatic brain tumor. Gross total removal of the tumor was successfully accomplished. After the operation, radiotherapy and chemotherapy were scheduled. Initially, several radiation therapies were performed. However, these treatments were canceled because of deterioration of his general condition. He died 4 months after the operation, due to intestinal pneumonia.
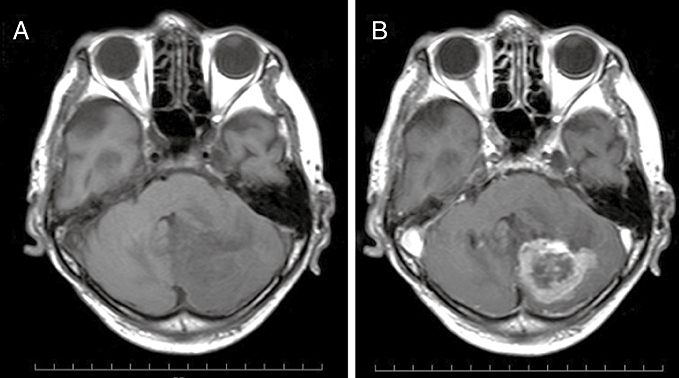
T1-weighted MRI (a) showing a 3 × 3 × 3 cm low-signal mass lesion in the left cerebellar hemisphere and (b) after intravenous administration on gadolinium showing “open-ring” enhancement of the lesion.
PATHOLOGICAL FINDINGS
Microscopic examination of the tumor showed diffuse proliferation of large lymphoid cells with an admixture of small lymphocytes, plasma cells and histiocytes, which were often accompanied by necrosis and an angiocentric pattern (Fig. 2a). These tumor cells were characterized by a broad range of B-cell differentiation, containing centroblasts, immunoblasts and Reed-Sternberg-like giant cells (Fig. 2b). Immunohistochemically, tumor cells were positive for B-cell markers (CD20 and CD79a) (Fig. 3a), and negative for T-cell markers (CD3 and CD45RO) and GFAP. Epstein-Barr nuclear antigen-2 (EBNA2) was seen in the nuclei of tumor cells (Fig. 3b). With regard to latent gene products, large cells within the tumor cells were latent membrane protein-1 (LMP-1-positive (Fig. 3c). Lymphoma cells showed positive signals for EBV-encoded small RNAs (EBERs) upon in situ hybridization (Fig. 3d).
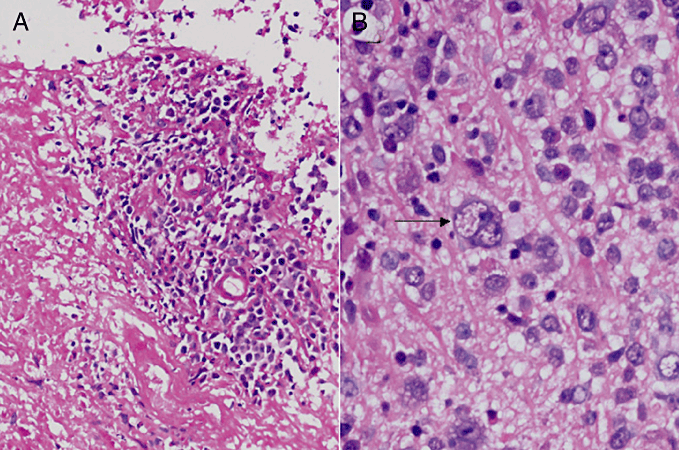
Histopathologic findings. (a) Section showing angiocentric pattern with extensive necrosis (HE stain; original magnification, ×100). (b) Diffuse mixed small-cleaved and large-cell lymphoma showing polymorphous features. A Reed-Sternberg-like giant cell is indicated by an arrow (HE staining; original magnification, ×400).
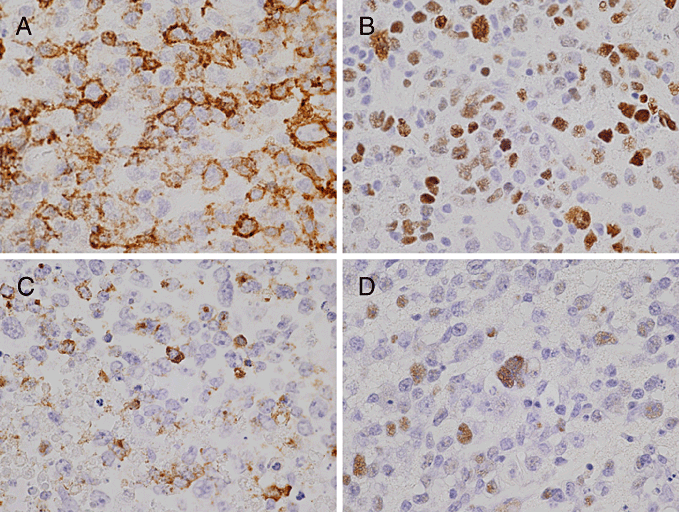
Immunohistochemical and in situ hybridization findings. (a) Lymphoma cells showing positive staining for CD20 (original magnification, ×400). (b) Lymphoma cells showing positive staining for Epstein-Barr nuclear antigen-2 (EBNA2) (original magnification, ×400). (c) Lymphoma cells showing positive staining for latent membrane protein-1 (LMP-1) (original magnification, ×400). (d) Lymphoma cells showing positive signals for EBV-encoded small RNAs (EBERs) in situ hybridization (original magnification, ×400).
DIAGNOSIS
Epstein-Barr virus-associated primary CNS lymphoma in an immunocompetent elderly patient.
DISCUSSION
Epstein-Barr virus-associated primary CNS lymphomas (PCNSLs) are well-recognized complications of congenital and acquired immunodeficiencies. These include deficiencies associated with ataxia teleangiectasia, Wiskott-Aldrich syndrome, renal transplantation, chronic alcoholism, several collagen vascular diseases, and immunosuppressive therapy for other diseases.1,2 Recently, O'Neill et al. and Kleinschmidt-DeMasters et al. have pointed out that EBV-associated PCNSLs can occur even in elderly patients receiving a variety of immunosuppresive drugs (i.e., azathioprine, methotrexate, steroids and mycophenolate mofetil), although these patients were not considered classically immunosuppressed.3,4 However, in terms of the incidence of EBV-associated PCNSL in immunocompetent patients, Kitai et al. studied 22 cases in their hospital and observed EBER-1 expression in 3/22 (13.6%) immunocompetent patients by in situ hybridization.5 Recently, four patterns of EBV latency have been recognized: type 1, which is observed in Burkitt's lymphoma, shows exclusive expression of EBV-encoded nuclear antigen 1 small non-polyadenylated RNA (EBER) molecules and EBNA-1; type II, which is observed in Hodgkin's lymphoma, shows additional expression of LMP; type III, which is observed in lymphoproliferative disorder (LPD) in immunocompromised patients, shows expression of all six EBNA, all three LMP and the two EBER; and type IV latency is less strictly defined and pertains to infectious mononucleosis and post-transplantation LPD.6 It was shown by Sugita et al. that EBV-associated PCNSLs without predisposing immunodeficiencies expressed EBV EBNA-1, EBNA-2 and LMP-1.7 In addition, histopathologically, their cases showed a broad range of B-cell maturation, from immunoblasts to plasma cells, with inflammatory and necrotic backgrounds.
They therefore considered that the pathological characteristics of EBV-associated PCNSLs in immunocompetent hosts were similar to those of latency phenotype III, with EBV LPDs arising in the setting of immunodeficiency. Interestingly, their cases of EBV-positive PCNLs developed in immunocompetent elderly patients (age ≥60 years) and tended to show various clinical courses. In contrast, with regard to systemic lymphomas, Oyama et al. recently documented 22 Japanese cases of senile EBV-associated B-cell lymphoproliferative disorders arising in elderly patients (age ≥60 years) without predisposing immunodeficiencies, and suggested that this condition is related to immunologic deterioration derived from the aging process.8 Since younger patients are rarely seen with this disease, the new entity was designated age-related EBV-associated B-cell lymphoproliferative disorders (AELDs).9 The latest version of the WHO classification of tumours of hematopoietic and lymphoid tissues (WHO 2008), describes AELDs as EBV-positive diffuse large B-cell lymphomas of the elderly.9 AELDs have distinctive histopathology, showing polymorphic tumor cells with extensive necrosis and reactive inflammatory cells.
In subsequent descriptions of AELDs, nodal and extranodal sites, such as the skin, stomach and lung, may be affected, but the CNS is not.8,9 However, EBV-positive PCNSLs occurred in immunocompetent patients, arising in elderly patients (age ≥60 years) without predisposing immunodeficiencies, thus suggesting a relationship of this disease with immunologic deterioration derived from the aging process. Based on these additional common clinicopathological features, some cases of EBV-positive PCNSLs in immunocompetent hosts may overlap with the latter group of AELDs.
Lymphomatoid granulomatosis (LG) is also another important differential diagnosis, as it is histopathologically characterized by angiocentric polymorphous lymphoid infiltrates and necrosis. LG is an EBV-driven lymphoproliferative disorder and is classified into three grades based on the proportion of EBV-positive B-cells relative to the reactive lymphocyte backgrund.10 A uniform population of large atypical EBV-positive B-cells such as that observed in the present case is beyond the spectrum of LG as currently defined. However, grade 3 lesions still show an inflammatory background, but contain large atypical B cells, and from a clinical aspect, patients with grade 3 lesions are approached as having diffuse large B-cell lymphoma. Therefore, EBV-positive PCNSLs may overlap with some grade 3 LGs. The epidemiology and prognosis of EBV-positive PCNSLs in elderly immunocompetent patients are enigmatic. EBV is widespread throughout the world, and > 90% of adults in any country are seropositive against the EBV viral capsid antigen.11
The rate of EBV seropositivity is much higher among children in Asia than those in Western countries. Differences in socioeconomic level, hygiene and varying cultural practices have been pointed out as the main causes of such differences between developing and developed communities.12 Based on these findings, Sugita et al. speculated that a delay in infection with EBV might decrease the incidence of late-onset EBV-associated malignancies, including EBV-associated PCNSLs in immunocompetent elderly patients in Western countries. Kitai et al. also reported that the incidence of EBV-positive PCNSLs in Japan was higher than that reported in Western countries.7 In addition, they commented that immunocompetent patients in their series of studies who had EBV-positive PCNSLs showed shorter survival times.
With the advent of MRI, MRI imaging features of PCNSLs have been established. Küker et al. reported imaging findings that should be regarded as suggestive of PCNSLs.13 First, almost all patients had at least one lesion of the PCNSL in contact with a CSF space. Second, PCNSL lesions nearly always display contrast enhancement. Third, necrotic lesions may be more frequent in HIV patients suffering from PCNSL, but are rare in the immunocompetent population. However, Sugita et al. histologically demonstrated that EBV-associated PCNSLs in immunocompetent elderly patients showed polymorphic tumor cells with extensive necrosis and were similar to those of latency phenotype III, with EBV LPDs arising in the setting of immunodeficiency.7 In fact, the preoperative diagnosis of the present case was a high-grade glioma or a metastatic brain tumor that was not considered as a PCNSL. Thus, differential diagnoses of “open-ring” enhancement of the lesion in elderly immunocompetent patients on MRI should not only include malignant gliomas, metastases and inflammatory disorders, but also EBV-positive PCNSLs.




