What determines the molecular composition of abnormal protein aggregates in neurodegenerative disease?
Abstract
Abnormal protein aggregates, in the form of either extracellular plaques or intracellular inclusions, are an important pathological feature of the majority of neurodegenerative disorders. The major molecular constituents of these lesions, viz., β-amyloid (Aβ), tau, and α-synuclein, have played a defining role in the diagnosis and classification of disease and in studies of pathogenesis. The molecular composition of a protein aggregate, however, is often complex and could be the direct or indirect consequence of a pathogenic gene mutation, be the result of cell degeneration, or reflect the acquisition of new substances by diffusion and molecular binding to existing proteins. This review examines the molecular composition of the major protein aggregates found in the neurodegenerative diseases including the Aβ and prion protein (PrP) plaques found in Alzheimer's disease (AD) and prion disease, respectively, and the cellular inclusions found in the tauopathies and synucleinopathies. The data suggest that the molecular constituents of a protein aggregate do not directly cause cell death but are largely the consequence of cell degeneration or are acquired during the disease process. These findings are discussed in relation to diagnosis and to studies of to disease pathogenesis.
INTRODUCTION
Since the earliest descriptions of neurodegenerative disease, pathological diagnosis has been based on the presence or absence of distinct brain lesions.1–4 These lesions are of two basic types. First, extracellular protein deposits that develop as senile plaques (SP) such as the β-amyloid (Aβ) deposits in Alzheimer's disease (AD) and Down's syndrome (DS),5 and deposits of the protease resistant form of prion protein (PrPSc) found in Creutzfeldt-Jakob disease (CJD).6 Second, intracellular protein aggregates form inclusions within the cell bodies, nuclei, and processes of neurons. These include the various neuronal cytoplasmic inclusions (NCI) such as neurofibrillary tangles (NFT) in AD, Lewy bodies (LB) in Parkinson's disease (PD) and dementia with Lewy bodies (DLB),7 Pick bodies (PB) in Pick's disease (PiD),8 and tau positive neurons in corticobasal degeneration (CBD).9 In addition, there are glial inclusions (GI) such as the glial cytoplasmic inclusions (GCI) (Papp-Lantos lesion) found specifically in multiple system atrophy (MSA)10 and in a variety of other disorders.
The major molecular constituents of brain lesions, e.g., Aβ, tau, and α-synuclein, have played a defining role in diagnosis and classification of disease. In some diseases, a direct link has been postulated between the presence of a specific gene mutation and the formation of brain lesions. Mutations of the amyloid precursor protein (APP)11,12 and presenilin (PSEN) genes,13,14 for example, have been linked to familial forms of AD (FAD), the tau gene (MAPT) to frontotemporal dementia (FTD) with parkinsonism linked to chromosome 17 (FTDP-17),15 and α-synuclein,16 leucine-rich repeat kinase 2 (LRRK2),17 and PARK7 (DJ-1)18 genes to familial forms of PD (FPD). Since the pathological phenotypes of familial disease are often similar, apart from age of onset, to those of sporadic forms of the same or related diseases,19,20 studies of gene mutation have had a major influence on the development of theories as to the pathogenesis of neurodegenerative disease in general. Hence, in familial disease, the major molecular constituent of a lesion is regarded as the residue of a direct or indirect effect of a pathogenic gene mutation that via the accumulation of an insoluble protein aggregate directly leads to cell death. This type of theory is best exemplified by the “amyloid cascade hypothesis” proposed to explain the pathogenesis of AD in which deposition of Aβ is the primary pathological event resulting in NFT, cell death, and eventually dementia.21
Chemical analysis of brain lesions shows them to have a complex and varied composition (Table 1). There may be at least three types of factor that could influence the molecular composition of such lesions (Fig. 1). First, a molecular constituent could be the residue of a pathogenic gene mutation and therefore directly reflect the primary etiology. Second, it could be the product of cellular degeneration and therefore, a consequence of the disease process rather than its cause.86 Third, a protein aggregate could acquire new substances as a result of diffusion within the brain and molecular binding to existing proteins.87,88 This review examines the importance of these three factors in determining the molecular composition of the major brain lesions in neurodegenerative disease.
| Lesion | Primary constituent | Associated constituents |
|---|---|---|
| Aβ deposit | Aβ22,23 | Apo E,24 Apo D,25 PrPsc,26 Tau,27 MAC,28 Amyloid-P,29,30 bFGF,31 Complement (C3d, C1q, C5, C4p),32α-ACT,33 IL-6, α2-macroglobulin,33 CAM1,33 Clusterin,33 Vibronectin,33 HSPG,33 Maillard reaction products,34,35 PHF antigens,27 NF protein,27 Ubiquitin,36 CgA,37 ACHE,38 Somatostatin,38 GABA,38 Neuropeptide-Y,38 Catecholamine positive neurites,38 Doppel,39 Parvalbumen37 |
| Prion protein deposits | PrPsc40,41 | Apo E,42 CgB,42 Aβ,43 Complement (C1q, C3b),42 Laminin,44 Clusterin43 |
| Neurofibrillary tangles | 3R/4R Tau45,46 | Apo E,47 HSPG,48 Ubiquitin,49 NF protein,50 Synaptophysin,50 Aβ,51 MAC,52 GFAP,51 bFGF,48 Amyloid-P,53 Ubiquitin34 |
| Lewy bodies | α-synuclein54,55 | Tau,56 Ubiquitin,57 Tubulin,58 MAP5, cdk5,59 IF proteins,60αB-crystallin,61 NF protein62 |
| Pick bodies | 3R Tau45,46 | Apo E,63 bFGF, Advanced glycation end-products,64 CgB,65 Complement (C1, C1q, C4, C2, C3, C5, C6, C8),66 Clusterin,67 CD59,67 Clathrin,68 NF protein,66 Synaptophysin,66 GFAP,69 Ubiquitin69 |
| CBD inclusions | 4R Tau70,71 | GFAP,72 Leu-773 |
| PSP inclusions | 4R Tau45,46 | GFAP,74 PHF antigens,74 Ubiquitin74 |
| Glial cytoplasmic inclusions | α-synuclein54,55 | Tau,75,76 MAP2,77αB-crystallin,78,79 tubulin,58 Ubiquitin,78,79 cdk5,68 NAPK,68 Rabaptin 577 |
| NIFID inclusions | α-internexin80–84 | Ubiquitin,80–84 IF proteins80–84 |
| Ubiquitin inclusions (FTLD-U, ALS) | TDP-4385 | Ubiquitin85 |
- αACT, Alpha antichymotrypsin; A β, β-amyloid; ACHE, Acetylcholinesterase; ALS, Amylotrophic lateral sclerosis; Apo E, D, Apolipoproteins E and D; bFGF, Basic fibroblast growth factor; CAM1, Cell adhesion molecule 1; CD59, Membrane complement inhibitor; cdk5, Cyclin-dependent kinase-5; CgA, CgB, Chromogranin A and B; FTLD-U, Frontotemporal lobar degeneration with ubiquitin inclusions; GABA, γ Aminobutyric acid; GFAP, Glial fibrillary acidic protein; HSPG, Heparan sulfate proteoglycan; IF, Intermediate filament; IL-6, Interleukin-6; Leu-7, Marker for killer lymphocytes; MAC, Membrane attack complex; MAP-2, MAP-5, Microtubule associated proteins; NAPK, Nitrogen-activated protein kinase; NF, neurofilament; NIFID, Neuronal intermediate filament inclusion disease; PHF, Paired helical filament; PrP, Prion protein; TDP-43, Product of the transcriptor repressor gene (TARDP).
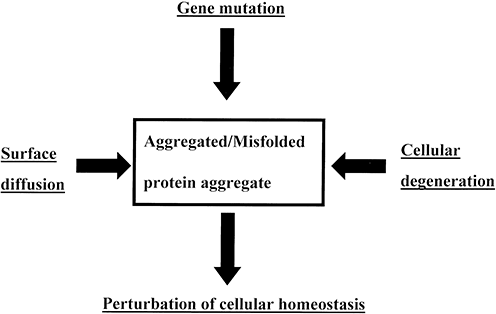
Factors that influence the molecular composition of a brain lesion in neurodegenerative disease.
THE MOLECULAR COMPOSITION OF BRAIN LESIONS
Extracellular protein deposits
A variety of Aβ peptides are present within Aβ deposits in AD and are formed as a result of secretase cleavage of APP (Table 1).89–91 The most common of these peptides is Aβ42/43 found largely in SP, whereas the more soluble Aβ40 is also found in association with blood vessels22,23 and may develop later in the disease.92 Aβ deposits also occur in some cases of DLB, but the ratio between the different types of peptide differs from AD. In DLB, the predominant form of Aβ is Aβ42/43, as in AD, but the level of Aβ40 is reduced compared with that in AD.93 In addition, SP have a variety of “secondary” constituents including acute-phase proteins such as α-antichymotrypsin and α2-macroglobulin,33 intercellular adhesion molecules such as cell adhesion molecule 1 (CAM1),33 apolipoprotein E (apo E)24 and D (apo D),25 the heterodimeric glycoprotein clusterin, vibronectin, the complement proteins C1q, C4 and C3,32 blood proteins such as amyloid-P and the sulfated glycosaminoglycans such as heparan sulfate proteoglycan (HSPG). In addition, the prion like protein DOPPEL encoded by the PRND gene occurs in the peripheral regions of SP in AD and DLB.39
Prion disease is characterized by the deposition of PrPSc an abnormal structural conformation of normal PrP (PrPc). There are various morphological types of PrPSc deposit,40,41 and these may contain complement proteins (C1q, C3b), apo E, chromogranin B,42 Aβ, clusterin,43 and laminin.44
Intracellular inclusions
There are two major groups of neurodegenerative diseases whose major pathological feature is the presence of intracellular inclusions, viz., the tauopathies: AD, PiD, argyrophilic grain disease (AGD),36 progressive supranuclear palsy (PSP), CBD, and FTDP-17; and the synucleinopathies: PD, DLB, and MSA.94 Recently, abnormal neuronal intermediate filament (IF) aggregates immunopositive for the IF protein α-internexin have been identified as the pathological hallmark of neuronal intermediate filament inclusion disease (NIFID).80–84 Moreover, frontotemporal lobar degeneration with ubiquitin positive, tau negative inclusions (FTLD-U) and amylotrophic lateral sclerosis (ALS) have been linked to the product of the transcriptor repressor gene (TARDP), viz. TDP-4385 suggesting these diseases should be combined in a single entity, viz., TDP-43 proteinopathy.
The molecular composition of tau varies in the different tauopathies. For example, PiD is characterized by tau with three microtubule repeats (3R tau) while PSP and CBD are composed of four-repeat (4R) tau.45,46 In AD, the molecular composition of the inclusions varies markedly depending whether they are intracellular NFT (I-NFT) or extracellular NFT (E-NFT). Hence, unlike I-NFT, E-NFT are glial fibrillary acidic protein (GFAP) and Aβ positive,51 and also contain significant amounts of amyloid-P53 and ubiquitin.49 PB contain apo E, neurofilament (NF) protein, synaptophysin, and basic fibrous growth factor (bFGF) as well as advanced glycation end-products,64 complement proteins, and synaptophysin.66 Moreover the NCI in CBD are GFAP and Leu-7 (CD57) positive73 while the NFT of PSP contain GFAP, paired helical filament (PHF)-antigens, and ubiquitin.74
In the synucleinopathies, LB in DLB may contain, in addition to α-synuclein,54,55 IF60 and NF proteins,62 cyclin dependent kinase-5 (cdk5),59 the heat shock protein alpha-B crystallin (αB-c),61 and polyubiquinated chains.57 The GCI characteristic of MSA are immunoreactive for α-synuclein54,95,96 while tau, microtubule associated protein 2 (MAP2), αB-c, and cdk5 may also be present.68
PATHOGENIC GENE MUTATIONS
Alzheimer's disease (AD)
The first demonstration of a direct association between a protein aggregate and a gene mutation was made in AD.11,12 A primary pathogenic role for the APP gene was suggested by the discovery of missense mutations in a small number of families, mutations in exons 16 and 17 being the first established genetic link with FAD.97 There are three isoforms of APP, viz., APP695, APP751, and APP770, all of which are cell surface glycoproteins with a single membrane-spanning region.98 Aberrant degradation of APP is believed to result in Aβ formation, especially the peptide Aβ42/43, the major constituent of the SP. APP has a large extracellular N-terminal domain and a short intracellular C-terminal domain while the Aβ sequence itself has 15 amino acids lying within the membrane and 28 extracellular amino acids. The metabolism of APP is mediated by α-, β-, and γ-secretase. In cultured cells that overexpress APP, there are two catabolic pathways. First, the “nonamyloid” pathway in which APP is cleaved within the Aβ sequence by α-secretase and second, the “amyloid” pathway in which APP is cleaved by β- and γ-secretase after endocytosis of the trans-membrane portion.99 It was originally believed that soluble Aβ was non-toxic but became extremely toxic once fibrils were formed.99 More recent evidence suggests that Aβ oligomer intermediates are the dominant toxic species.100 Hence, altered proteolytic processing of APP and the accumulation of excess Aβ is assumed to be an early pathological event in FAD, a process which also occurs to a limited extent in aged humans.101 Following Aβ deposition, the formation of SP, microglial activation, astrocytosis, and neuritic dystrophy presumably lead to the formation of NFT, cell death, and dementia as proposed by the “amyloid cascade hypothesis”.21
Subsequently, the most common subtypes of FAD were linked to mutations of the PSEN genes PSEN113 and PSEN2,14 and the effect of these mutations were also assumed to lead, albeit more indirectly, to the enhanced deposition of amyloidogenic Aβ42/43.90 The normal function of the PSEN genes, and how gene mutations result in Aβ deposition is unclear. PSEN1 may be involved in notch signaling102 and may therefore be important in cell differentiation. PSEN1/2 genes may also be implicated via the perturbation of cellular calcium homeostasis103 or in interactions with the transcriptional coactivator cAMP-response element binding (CREB-binding) protein which plays a key role in regulating gene expression.104 In addition, PSEN genes are part of the γ-secretase complex which are important in generating Aβ from APP. Hence, mutant PSEN1 could enhance 42-specific-γ-secretase cleavage of normal APP resulting in increased deposition of Aβ42/43.105
Creutzfeldt-Jakob disease (CJD)
PrPc is a small protein of unknown function comprising predominantly α-helices whereas PrPSc is highly aggregated with an extensive β-sheet configuration. Familial forms of CJD (fCJD) are linked to germline mutations in the coding region of the PrP gene located on chromosome 20.106 Peptides homologous to mutated regions of PrP also exhibit enhanced fibrillogenic properties especially if mixed with PrPc and therefore, deposition of insoluble fragments of PrPSc could result in the development of vacuolation (“spongiform change”) and neuronal loss.107,108 Furthermore, in sporadic CJD (sCJD), different patterns of pathology are present depending on a polymorphism at codon 129 of the PrP gene.109 For example, patients homozygous for valine at this codon (V/V) develop pathology in the deep gray matter while methionine homozygotes (M/M) exhibit a largely cortical pathology110 suggesting a close link between the genetic polymorphism and brain pathology.
Tauopathies
Dysfunction of the cytoskeletal protein tau is implicated most directly in the hereditary tauopathy FTDP-17.15 Multiple mutations in or near the microtubule binding domain of MAPT have been identified and affect all six of the major tau isoforms. The majority of the tau amyloid fibrils formed are composed of the entire tau protein. As a consequence, there is extensive tau pathology in neurons (NCI) and glial cells (GI) suggesting that the disease stems from early, primary alterations in tau function.111 Exon 10 mutations perturb the normal 1:1 ratio of 4–3 microtubule binding repeat tau,112 the increase in 4R tau promoting aggregation to form fibrillary inclusions.113 In the Chinese hamster, different tau mutants result in distinct pathological phenotypes suggesting that there may be multiple mechanisms involved in the tauopathies.114 This heterogeneity is also present in human patients since the pathological phenotype appears to be determined by the location of the tau mutations, those in the coding region resulting in a reduced ability of tau to interact with microtubules. In addition, several tau mutations promote glycosaminoglycan induced assembly of tau into filaments115 while those in intron and coding regions cause an increase in splicing of exon 10 resulting in the overexpression of 4R tau isoforms. Furthermore, CBD, a disease of 4R tau, has a phenotype similar to FTDP-17 with tau mutation at exon 10.70,71 Changes in the tau gene have also been observed in PSP, especially in exons 1, 4 A, and 8 but their functional significance is unclear.116
Synucleinopathies
α-Synuclein, a small presynaptic protein without a well-defined function, is the major component of the LB found in PD, DLB, the Lewy body variant of AD (DLB/AD) and the GCI found in MSA.10,95,117 As in tau pathology, the entire molecule undergoes conformational change to result in insoluble protein aggregates. Missense mutations of the α-synuclein gene have been linked to FPD and therefore could directly cause this disorder by increasing the probability that α-synuclein will fibrillize.16 This hypothesis is supported by experiments that suggest first, that low concentrations of α-synuclein added to neuronal cultures are toxic to dopamine cells118 and second, that transgenic animals that express α-synuclein mutations develop fibrillary inclusions accompanied by the degeneration of dopamine nerve cells.119 Mutations in the PARK7 gene DJ-1 are associated with recessive FPD and DJ-1 is abundantly expressed in reactive astrocytes.85 Moreover, mutations of LRRK2 are associated with FPD with dopaminergic neuronal degeneration and accumulation of either synuclein or tau, neither protein, or in some patients both proteins.120 Some forms of parkinsonism have also been linked to a heterozygous missense mutation together with a heterozygous deletion in the “parkin” gene (PARK2) believed to function in the ubiquitination of proteins.121 This results in a parkinsonism with a mild gait ataxia, spinocerebellar syndrome, and tau pathology but without the presence of distinct LB or NFT.
These studies collectively suggest a close relationship between a specific gene mutation and familial disease. In each case, a pathogenic process is hypothesized in which the mutation results in the formation of an insoluble and/or misfolded protein aggregate leading to cell death. In the cases of APP, PrP, MAPT and α-synuclein, there is a direct link between the gene and the molecular composition of the resulting lesion. In other familial cases, e.g., those linked to PSEN, LRRK2, PARK2 genes, the connection between the gene mutation and the accumulation of pathological protein is more indirect. These processes, however, account for only a small proportion of cases of neurodegenerative disease. For example, in AD, the APP and PSEN1/2 genes together account for less than 5% of cases.122 In addition, various questions remain, e.g., how do misfolded and aggregated proteins cause cell death in the various diseases, why is the density of pathological aggregates so low in some disorders,123 and why are familial and sporadic forms of the same disease so similar?
STRUCTURAL DEGENERATION WITHIN THE CELL
How do gene mutations result in pathology?
That a less direct relationship may exist between a gene mutation, protein aggregation, and neurodegenerative disease was first suggested as early as 1993.124 Amino acid changes associated with the codon 717 mutation of the APP gene (APP717) appeared to shed little light on the pathogenic mechanism of AD. In addition, it was concluded that the existing data from neurotoxicity experiments did not establish a primary role for Aβ in disease pathogenesis.124 Furthermore, generation of the pathological Aβ sequence requires cleavage by β- and γ-secretase at the N and C-terminal sites.125,126 A possibility is that membrane damage, resulting from cell degeneration, may be underway before the Aβ fragment is generated and therefore, structural degeneration of the cell membrane may precede the formation of the aggregated protein.
Extracellular protein deposits
Animal models also suggest that Aβ aggregates are often generated in reponse to cellular degeneration. Lesions of the nucleus basalis of Meynert (nBM) in the rat elevate APP synthesis in cortical neurons;127 the production of excess APP being a response to loss of functional innervation. Similarly, subacute and prolonged neuronal damage in humans can induce the formation of APP.128 In the rat, injury to an area of brain results, four to seven days later, in the presence of APP in axonal swellings, cell bodies, and dystrophic neurites.129 Lesions of the fimbria-fornix pathway in the rat also result in a marked accumulation of APP in regions of the hippocampus associated with degenerating cholinergic fibres.130 Injections of toxins into the brain produce very similar results, e.g., there are changes in the expression or induction of APP in brain cells after intrathecal or intraparenchymal injections of various toxins while administration of chloroquine results in the production and accumulation of C-terminal fragments of APP in the cell bodies of pyramidal cells. Furthermore, APP shares structural features with precursors of epidermal growth factor suggesting that APP is an endogenous protectant activated by injury to brain cells.131 These observations suggest alternative schemes of the pathogenesis of AD in which the amyloid peptide is not the primary cause. Hence, theories involving perturbations of vesicular trafficking, the cytoskeletal network, and the distribution of membrane cholesterol are increasingly being explored.132
Many other SP constituents could be a consequence of structural degeneration. Approximately 40% of diffuse plaques contain degenerating neuronal perikarya133–135 and many contain the processes of astrocytes. Acetylcholinesterase rich neurites have been found in SP and may be the degenerating axonal terminals of neurons originating in the nBM.38 Cholinergic neurites, and neurites positive for somatostatin, γ-amino butyric acid (GABA), neuropeptide Y, and the catecholamines have all been recorded in SP.38 As a consequence of neuritic degeneration, PHF antigens, tau, A68 protein, ubiquitin, and NF epitopes have all been recorded within SP.27 In addition, the presence of neuronal markers such as parvalbumin suggests the preferential incorporation of processes of pyramidal neurons into SP.37 The presence of chromagranin A, a soluble protein in dense-core synaptic vesicles within the dystrophic neurites of the “coronas” of classic plaques, may also be the result of cellular degeneration.136 Similarly, chromagranin B, but not A, is associated with the PrPSc plaques in CJD.42
Intracellular inclusions
The presence of tau and α-synuclein in inclusions could also be a consequence of cell injury and degeneration. Hence, the formation of NFT could be a part of the neurons limited response to injury125 as neurons will often respond to degeneration by increasing the synthesis of tau.137 Dopamine denervation and septal lesions affecting cholinergic and GABA neurons projecting to the dentate gyrus result in the loss of dendritic MAP2 and tau immunostaining.138 Hence, transynaptic changes affecting dentate gyrus neurons may result in the precursor stages of NFT. Axonal injury can also lead to the accumulation of α-synuclein within the cytoplasm of cells.139 Primates treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) develop inclusion bodies, but not true LB, which may result from the redistribution of α-synuclein from its normal synaptic location to the cell body.140 In a chronic rat model of PD, infusion by osmotic pump of a toxin 1-methyl-4-phenylpyridinium (MPP) for 28 days caused ipsilateral loss of nigral perikarya and the formation of LB like structures in adjacent neurons.141
Inclusions may also contain additional constituents that result from cellular degeneration. Hence, NFT may be positive for NF proteins and synaptophysin50 while PB express chromogranin B,142 clathrin,66 and synaptophysin.66 In CBD, tau positive glial cells express the oligodendrocyte marker Leu-7 which is likely to be a consequence of glial cell degeneration. In addition, proline directed protein kinases are associated with inclusions in various disorders.77 Hence, in the GCI of MSA there is aberrant expression of MAP2 and Rabaptin-5, the latter being involved in the process of endocytosis in neurons.77
Secondary acquisition of proteins
The major constituents of a protein aggregate can bind additional substances, e.g., tubulin is an α-synuclein associated protein58 and PHF and amyloid fibres can “decorate” themselves with various proteins.143 Furthermore, during aging, long-lived proteins accumulate post-translational modifications (“Maillard reaction products”) such as cross-linking, decreased solubility, and increased protease resistance. These changes may alter the chemical composition of a lesion with time and significantly change its binding properties.34,35
Extracellular deposits
Apo E labels a proportion of Aβ deposits in AD144 suggesting that it is not a prerequisite for Aβ plaque formation even in individuals expressing allele ε4 but is acquired secondarily. Apo E binds to several proteins including Aβ and in the cell targets lipoprotein particles.145 Apo E also accumulates in some PrPSc deposits in CJD.47,65 Several acute-phase proteins and proteins associated with the immune system accumulate in SP. The membrane attack complex (MAC) has been identified in the dystrophic neurites of SP,28 and whereas immunoglobulin G (ImG) has not been identified in AD lesions, SP are associated with a variety of complement proteins, acute phase proteins, CAM, and blood proteins,33 many of which may have been acquired secondarily. The presence of C3 and antichymotrypsin suggest that the classic pathway is activated in association with diffuse plaques but it is unclear whether the process proceeds beyond C3. Amyloid-P is a complex glycoprotein made in the liver and present in blood serum29 and is found in both SP and NFT suggesting that the substance accumulates following impairment of the blood brain barrier.146 Approximately 90% of Aβ positive SP contain amyloid-P.30 The staining pattern of amyloid-P parallels that of the complement proteins suggesting that it may assist microglia during phagocytosis.29
SP also contain basic fibroblast growth factor (bFGF), a substance that appears to attract neurites into the plaque.31 As a consequence, SP acquire various markers associated with the processes of neurons and glial cells. In addition, PrPSc may accumulate at the periphery of Aβ positive plaques and may further influence plaque morphology.26 PrPSc is a high affinity receptor for laminin44 and interactions between PrPSc and laminin may affect the development of the deposit and influence its ability to acquire additional proteins.87,88
Inclusions
The maturation of NFT is associated with several changes in molecular composition. Hence, E-NFT contain many SP constituents including Aβ, HSPG, amyloid-P, and various serpins.53 I-NFT are less compact, silver positive, and eosinophilic compared with E-NFT.51 E-NFT are also immunoreactive for GFAP and Aβ, both of which are likely to be deposited after cell death. The acquisition of Aβ by E-NFT suggests either that Aβ is inaccessible to I-NFT or that there are conformation changes of the proteins in the extracellular space that facilitate binding of Aβ.34 A major constituent of inclusions is ubiquitin, which is found either as a free molecule or as a protein-ubiquitin conjugate. Ubiquitin may contribute to the polymerization of abnormal fibriller structures in an attempt to eliminate them.49 As I-NFT develop into E-NFT, they lose the N/C termini of tau and two-thirds of the N-terminus of ubiquitin.34 NFT are also immunopositive for apo E.47 In AD, all apo E positive neurons are positive for PHF proteins but not all PHF, tau-2 positive neurons exhibit apo E immunoreactivity.147 These results suggest that apo E plays a secondary role in NFT formation and is accumulated within neurons in response to repair processes induced by NFT. In the presence of calcium ions, HSPG will bind to the free carboxyl groups of NFT proteins and this binding may play a role in increasing the insolubility of PHF.48 In addition, bFGF will bind to heparinase sensitive sites in NFT due to the presence of HSPG.48 The MAC has also been identified in association with NFT.52 Neurons remove membrane inserted MAC fragments by endocytosis and hence, retrograde transport to cell bodies may result in the attachment of MAC to abnormal cytoskeletal proteins such as tau.52
PB accumulate apo E, especially in the limbic system,63 advanced glycation end-products,30 and complement proteins of the classical cascade, although there is no evidence to suggest the formation of C9 or MAC.67 In addition, PB often contain the regulatory proteins S-protein, clusterin, and the membrane complement inhibitor CD59. As they age, PB become discharged into the neuropil and become “ghost” PB.69 At this stage, they lose various markers and become tau and ubiquitin negative but acquire weak GFAP activity.69 Both the NFT in PSP74 and tau positive neurons in CBD72 also acquire GFAP activity as they age.
Changes in the molecular composition of α-synuclein inclusions occur with age. In DLB, intracellular LB (I-LB) and some extracellular LB (E-LB) are α-synuclein positive but at a later stage, E-LB lose various filamentous components and acquire astrocytic markers.148 The presence of tau in association with α-synuclein positive GCI is also likely to be secondary and not of pathogenic significance.75 In particular, there is colocalization of tau and α-synuclein in MSA cases of long duration because some α-synuclein filaments become “decorated” with phosphorylated 4R tau.76 Similarly, tau is incorporated into some LB in DLB, which may reflect the collapse of the intraneuronal organization of the microtubules and subsequent aggregation of hyperphosphorylated tau.56 Several other proteins accumulate in α-synuclein lesions in MSA including αB-c, tubulin, and ubiquitin but their role in the development and maturation of GCI remains to be elucidated.78,79
DISCUSSION
What determines the composition of a protein aggregate?
This review examined three factors that may contribute to the molecular composition of a brain lesion, viz. (i) pathogenic gene mutations, (ii) cellular degeneration, and (iii) secondary acquisition.
Evidence for the importance of specific genetic mutation in determining the molecular composition of lesions comes from studies of familial disease in which a specific mutation results, directly or indirectly, in the accumulation of an abnormal protein aggregate. The degree to which these processes “directly” promote cell death, however, is more controversial. First, the phenotypes of familial disease are often similar, apart from age at onset, to those of related sporadic diseases19,20 and hypotheses such as the “amyloid cascade hypothesis” do not specify what initiates the common late-onset form of AD.149 Second, it is difficult to establish a mechanism that clearly and unambiguously links a specific gene mutation to cell death. A variety of mechanisms have been proposed by which misfolded proteins may directly affect cellular homeostasis including disruption of the ubiquitin degredation system, axonal transport, synaptic function, and protein sequestration and these are reviewed in detail by Forman et al.123 Third, the presence of various aggregated and misfolded proteins within lesions may obscure the primary etiology because of secondary toxicity effects. Fourth, the molecular constituents themselves may be formed as a consequence of cellular degeneration.86,127,131,139
Aggregated lesions also contain constituents that are clearly the result of cellular degeneration. Hence, synaptic disconnection, neuritic degeneration, and invasion by the processes of glia add constituents to developing plaques. These processes may explain the presence of tau, PHF antigens, synaptic proteins, and specific neurotransmitter-positive neurites within the SP. Subsequently, as lesions age, the activity of some constituents is lost and new compounds acquired. Many of these newly acquired proteins may be made by glial cells or originate in blood serum as a result of breakdown of the blood brain barrier. Hence, GFAP, complement proteins, and acute phase proteins become incorporated into protein aggregates. Surface diffusion of substances into aggregated protein deposits may cause changes in plaque morphology and in the ability of the plaque to bind yet further proteins.87
Relationship of protein aggregation to neuronal loss
A critical question raised by this review is the relationship between protein aggregation and neuronal loss, it often being assumed that the formation of abnormal proteins causes neuronal death. On the balance of evidence, however, it is likely that the in majority of disorders described to date, the formation of brain lesions may not the primary cause of neuronal death. Many of the molecular determinants of brain lesions, such as Aβ and α-synuclein can be shown to be neurotoxic, but the effects of these constituents could be secondary. To varying degrees, brain lesions, whether extracellular protein deposits or changes in neuronal morphology appear to arise as the consequence of earlier degenerative processes. Both AD and CJD are characterized by the formation of extracellular protein deposits, the main constituents being Aβ and PrP, respectively. In these disorders, it is possible that neuronal degeneration could be initiated by processes related to the presence of intracellular PrP and APP, respectively, but that the formation of the characteristic plaques is a later reaction to the degeneration resulting primarily from synaptic disconnection. Hence, Aβ and PrP deposits could be seen as reactive changes but directly related to the primary cause of the disorder. The balance of evidence also suggests that inclusion bodies represent degenerative changes in cell bodies in response to neuronal degeneration. In AD, these changes may be directly related to the primary cause of the disease (“Amyloid Cascade Hypothesis”) but in other disorders, the formation of inclusions may be purely a reactive change and unrelated to cell death. In some disorders, most notably in FTDP-17 and some of the disorders related to α-synuclein, the formation of lesions may be more directly responsible for the neuronal degeneration.
Implications for disease diagnosis
Aβ, tau and α-synuclein are currently the most important molecular constituents of lesions that define neurodegenerative disease. Nevertheless, the molecular complexity of brain lesions raises questions about the reliability of using individual markers in pathological diagnosis. First, when many chemical constituents are present, there is the problem of distinguishing the primary “pathological” protein from the breakdown products of the cell, and the compounds acquired by surface diffusion. Studies of familial disease have often been the most important in identifying the “primary” pathogenic proteins, the results then being extrapolated to sporadic disease of similar phenotype. Many of these “primary” proteins, however, may themselves be the consequences of cellular degeneration, especially in sporadic disease, rather than their cause. Second, the chemical composition of a lesion changes with age and, in some cases, activity of the primary protein may decrease or become substantially altered, and this may cause potential problems in diagnosis especially of longer duration cases.
Relationship between genetics and disease
Examination of the factors influencing the molecular composition of brain lesions raises the question as to the precise relationship between genetic mutation and disease phenotype. In the conventional view (Fig. 2), a causal pathway is hypothesized linking a specific genetic change to cell injury and death mediated by a variety of changes in cellular homeostasis123 and caused by the accumulation of an aggregated misfolded protein and is best exemplified by the amyloid cascade hypothesis.21
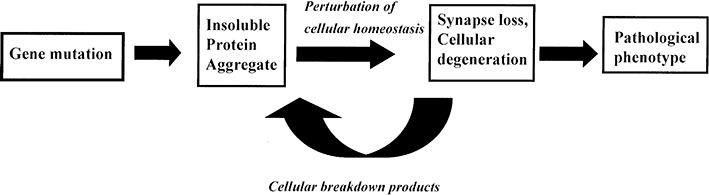
“Conventional” hypothesis to explain the pathogenesis of familial neurodegenerative disease.
The effect of a genetic mutation is likely to be greatly amplified by neuronal degeneration as many of the major proteins are up-regulated as a consequence of cell damage.127,128,131,150 Hence, we propose a modified scheme (Fig. 3) in which the most important factor is the age-dependent breakdown of anatomical systems and pathways within the brain and the consequence loss of synapses.151 The extent of this aging effect, which begins early in life, is mediated by the degree of lifetime stress (the “allostatic load”). The brain is the ultimate mediator of stress-related mortality through hormonal changes resulting in hypertension, glucose intolerance, cardiovascular disease, and immunological problems.151 The consequence is gradual synaptic disconnection, neuronal degeneration, and the up-regulation of various reactive and breakdown products. Second, in small numbers of families, specific mutations or allelic polymorphisms influence the outcome of this age-related degeneration by determining the solubility and/or toxicity of the molecular products. Cells have mechanisms to protect against the accumulation of misfolded and aggregated proteins including the ubiquitin system and the phagosome-lysosome system. In individuals with specific gene mutations, however, neuronal degeneration often results in the accelerated formation of an insoluble misfolded protein which overwhelms the protection systems. Early onset familial disease is the consequence of this process. By contrast, in individuals without a specific genetic mutation, but where more complex genetic and environmental risk factors are present, the outcome of age-related loss of synapses is mainly soluble and smaller quantities of insoluble proteins which are degraded by the cellular protection systems and do not significantly accumulate to form lesions. With advancing age, however, the protective systems become less efficient resulting in slowly accumulating quantities of insoluble proteins. The result of these insidious processes is that the cellular protection systems do not become overwhelmed until much later in life, the consequence being late-onset sporadic forms of disease similar in phenotype to their familial counterparts.
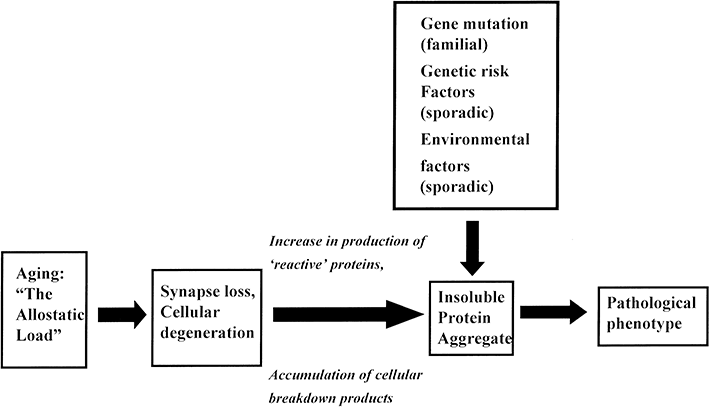
Modified hypothesis to explain the pathogenesis of familial and sporadic forms of neurodegenerative disease.
Future research
The process described above may be common to the majority of neurodegenerative diseases. A major future challenge is to describe and understand all of the possible variants of disease likely to result from such a process, i.e., what determines the diversity of disease phenotypes? Moreover, what is the best conceptual system for describing this diversity? Should a classificatory system be employed or does neurodegenerative disease reflect a continuum of pathological change?4 New systems may need to be devised in future to provide a framework for the description of all the molecular variants of neurodegenerative disease.
To help in this endeavour, future research should be directed to several further questions. First, what are the factors that determine the morphology and molecular determinants of lesions? The data suggest that particular types of lesion reflect specific forms of neurodegeneration, e.g., protein deposits are a consequence of synaptic disconnection affecting axonal terminals and ballooned neurons reflect axonal degeneration. Variation in morphology of a lesion may depend on the brain area and specific cell types involved. In addition, the molecular determinants of a brain lesion could reflect the genes expressed in specific types of cell in which degenerative processes occur and therefore on host genotype. Second, does the degeneration of specific anatomical systems generate particular types of lesion? The various neurodegenerative disorders are characterized by the degeneration of particular anatomical systems, and it is possible that variations in the lesions observed in these disorders are determined by differences in the anatomical pathways involved. Third, to what extent do the genes expressed in the cells associated with a particular anatomical pathway influence the pattern of neurodegeneration? If brain lesions are largely the consequence of neurodegeneration, then the genes expressed in particular cells could influence the molecular determinants of resulting lesions but would not necessarily be an important factor in the primary degenerative process. The molecular constituents of lesions could, however, determine the degree of toxicity of the resulting lesions and therefore, could influence the extent of the secondary degeneration. Fourth, should the presence, distribution, and molecular determinants of brain lesions continue to play such a significant role in the pathological description and classification of disorders? If lesions represent particular types of neurodegeneration rather than being characteristic of specific disorders then there are likely to be cases that show combinations of pathological features and appear to be intermediate between existing disorders, i.e., there will be a considerable degree of overlap between existing disorders.4 As a consequence, it may be more accurate to consider neurodegenerative disorders as representing a “continuum” of pathological change.




