Long-term treatment of localized gastric marginal zone B-cell mucosa associated lymphoid tissue lymphoma including incidence of metachronous gastric cancer
Competing interests: None.
Abstract
Background and Aim: According to a few recent reports on the long-term clinical outcome of gastric marginal zone B-cell mucosa associated lymphoid tissue lymphoma (MALT lymphoma); localized gastric MALT lymphoma generally has a favorable prognosis. However, the risk of metachronous gastric cancer has not been evaluated. In this study, we analyzed long-term outcomes of localized gastric MALT lymphoma including the incidence of metachronous gastric cancer.
Methods: Between April 1996 and May 2008, 60 patients (31 men and 29 women; mean age 58.1 years) with localized gastric MALT lymphoma (stage I and II1 according to Lugano classification) were analyzed retrospectively.
Results: Forty-eight patients (82.6%) achieved complete remission by eradication therapy. Radiation therapy was conducted on eight patients as second-line treatment, and all of them achieved remission. The median follow-up period was 76 months (range, 12–157 months). One patient had local relapse after remission for 5 years and three patients developed early gastric cancer without recurrence of lymphoma (5%). All of the three gastric cancers appeared in the same areas where MALT lymphoma had been eradicated.
Conclusion: Eradication therapy and radiation therapy for localized gastric MALT lymphoma have a favorable long-term outcome, though regular follow-up endoscopy should be performed for detecting metachronous early gastric cancer.
Introduction
Recently, there have been some reports on long-term clinical outcome of gastric marginal zone B-cell mucosa associated lymphoid tissue lymphoma (MALT lymphoma).1–4 Localized gastric MALT lymphoma generally has a favorable prognosis, and the majority of Helicobacter pylori (H. pylori)-positive patients achieve complete remission (CR) of MALT lymphoma by eradication therapy.2,3 Radiation therapy is effective for H. pylori-positive patients who have not responded to antibiotics and for H. pylori-negative patients with gastric MALT lymphoma.4
Both gastric MALT lymphoma and gastric cancer are strongly associated with H. pylori infection.5–7 There have been some case reports on synchronous or metachronous gastric cancer with gastric MALT lymphoma,8–12 and it has recently been reported that gastric cancer risk in Dutch patients with gastric MALT lymphoma is six times higher than that in people without lymphoma.13 However, there have been few reports on metachronous gastric cancer in patients after remission of gastric MALT lymphoma,9 leaving its risk factor unclear.
The aim of this study was to clarify the long-term treatment outcomes and prognosis of localized gastric MALT lymphoma including the incidence of metachronous gastric cancer.
Methods
Patients
We reviewed 70 consecutive patients with histopathologically confirmed localized gastric MALT lymphoma (i.e. disease confined to the stomach with or without involvement of the paragastric lymph nodes and with no distant lymph node involvement) between April 1996 and May 2008 in our hospital. Three patients who were treated at other hospitals and seven patients who were observed for less than one year were excluded. In total, 60 patients (31 men and 29 women; mean age 58.1 years) were analyzed.
Staging procedure
Standardized stage work-up included a detailed physical examination, chest and abdomen computed tomography, endoscopic ultrasonography (EUS), and bone marrow examination, all of which were performed according to the Lugano staging system.14
H. pylori status
H. pylori infection status was determined when the results of at least one of the following were positive: rapid urease test (Helicocheck kit, Otsuka Pharmaceutical Co., Tokyo, Japan), 13C-urea breath test, H. pylori culture, histopathologic examination, and H. pylori antibodies using the E plate test (Eiken Kagaku, Tokyo, Japan).15
Treatment
Our standard treatment protocol for localized gastric MALT lymphoma is as follows. Stage I and II1 patients with or without H. pylori infection are assigned to a standard 7-day course regimen of lansoprazole, amoxicillin, and clarithromycin or metronidazole. As second-line treatment, radiation therapy (total dose of 30 Gy and fraction size of 1.5 Gy for 4 weeks) is performed.15 However, until 2001, chemotherapy, radiation therapy, and surgery were used either alone or in combination according to the patient's status.
Endoscopic and histological assessment
The location of lymphoma and endoscopic appearances were evaluated by endoscopists. Endoscopic appearances were divided into superficial type (including ulcer, erosion, gastritis, discolored areas, early cancer-like, cobblestone) and others (including mass-forming and submucosal tumors [SMT] and others).
Complete remission (CR) was defined as a complete disappearance of clinical evidence of lymphoma and an absence of histological evidence of lymphoma in biopsy specimens. Progressive disease (PD) was defined as endoscopic increase of the tumor and/or pathological large cell transformation. Patients who did not have CR or PD were defined as having stable disease (SD).
Pathological assessment was conducted by pathologists according to the Wotherspoon grading system, with Grade 4 or 5 indicating positive lymphoma.16
Follow up
Response assessment was performed every 3 months by endoscopy and examination of biopsy specimens until CR was achieved. In cases with CR, follow-up endoscopy with biopsy was repeated every 3 months for one year and every 6 months until the 2nd year, and yearly endoscopy was repeated after the 3rd year. Patients with PD and patients with SD who desired treatment or were H. pylori-negative underwent radiation therapy.
Statistical analysis
Statistical comparisons of the data for patients were performed using Fisher's test for categorical data and Student's t-test for numerical data. Probabilities of event-free survival for patients were calculated by the Kaplan–Meier method, and the values were compared by using the log-rank test. Significance of difference was determined by P-values of less than 0.05.
The current study was carried out in accordance with the Helsinki Declaration as revised in 1989.
Results
Staging and H. pylori infection status
Fifty-three patients were positive and seven patients were negative for H. pylori infection (Fig. 1). One H. pylori-negative patient developed gastric MALT lymphoma 4 years after H. pylori eradication for a gastric ulcer. All H. pylori-negative patients were tested for the API2-MALT1 gene and three patients were positive for it.17 Performance status was normal in the majority of patients, and no B symptoms were observed.
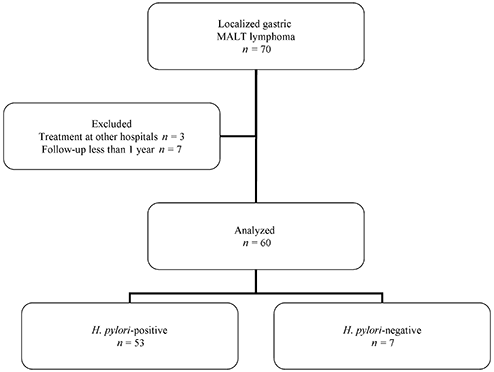
Flow diagram of the participants in this study. Data from 60 out of 70 patients diagnosed with localized gastric mucosa associated lymphoid tissue (MALT) lymphoma (including 53 Helicobacter pylori-positive patients and seven H. pylori-negative patients) were used for analysis.
Forty-nine patients including four H. pylori-negative patients were superficial type and 12 patients were in stage II1.
Outcome of first-line therapy
Endoscopic resection and surgery were performed as first-line therapy for two patients with H. pylori infection. H. pylori was eradicated in all of the 51 H. pylori-positive patients, but second-line antibiotic therapy was required for two of them.
Forty-eight patients (82.6%) who underwent eradication therapy achieved CR, and 10 patients (17.4%) did not respond to antibiotics (Fig. 2). These groups were compared (Table 1). The median time to CR was 3 months. One patient, who did not respond to eradication was evaluated as having SD and is in follow-up.
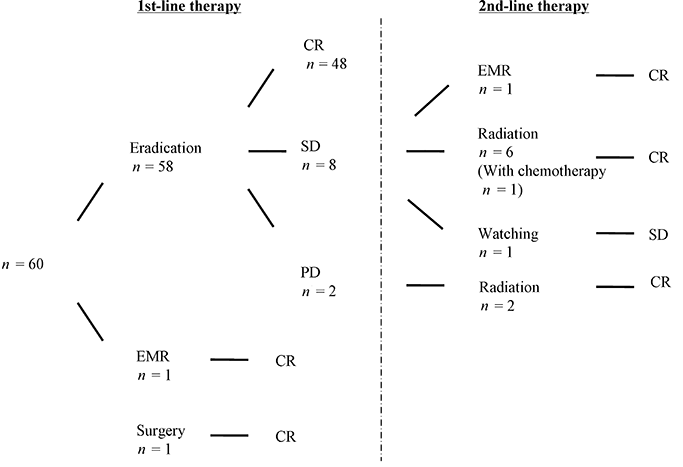
Flowchart showing treatment outcomes. Forty-eight patients achieved complete remssion (CR) by eradication as first-line therapy, and nine patients who did not respond to antibiotics received 2nd-line therapy. EMR, endoscopic mucosal resection; PD, progressive disease; SD, stable disease.
| Responders | Non-responders | P | |
|---|---|---|---|
| n | 48 | 10 | |
| Age (range) | 58.9 (39–78) | 57.6 (16–77) | n.s. |
| Male : female | 22:26 | −8:2 | n.s. |
| Endoscopic appearance: superficial† | 43 | 5 | P < 0.05 |
| Location (L : M : U : Mixed) | 17:26:3:2 | 2:5:1:2 | n.s. |
| Depth: SM : MP,SS | 43:5 | 7:3 | n.s. |
| Large cell transformation | 2 | 1 | n.s. |
| Lugano stage: I : II1 | 42:6 | 5:5 | P < 0.05 |
| H. pylori-positive | 48 | 2 | P < 0.05 |
- † Including ulcer, erosion, gastritis, discolored areas, early cancer-like, cobblestone. L, lower; M, middle; U, upper third of the stomach; n.s., not significant. There are significant differences in endoscopic appearance, Lugano stage and Helicobacter pylori infection between responders and non-responders to eradication therapy.
Outcome of second-line therapy
Radiation therapy was conducted for eight patients, including two patients with successful eradication, and all of them achieved CR. The median time to CR was 2 months. The only complication of radiation therapy was mild nausea in one patient. One patient who received radiation therapy also underwent chemotherapy (Fig. 2).
One hundred percent of the patients who received 1st-line and 2nd-line treatment achieved CR.
Survival analysis
The median follow-up period from diagnosis was 76 months (range, 12–157 months). There was no death associated with gastric MALT lymphoma. The median CR duration period for the 59 patients was 65 months with 25th and 75th percentiles of 33 and 100 months, respectively.
Comparisons of event-free survival according to the response to eradication therapy and the H. pylori infection status are shown in Figure 3. Only one patient had local relapse without large cell transformation and H. pylori re-infection after remission for 5 years, and that patient received radiation therapy.
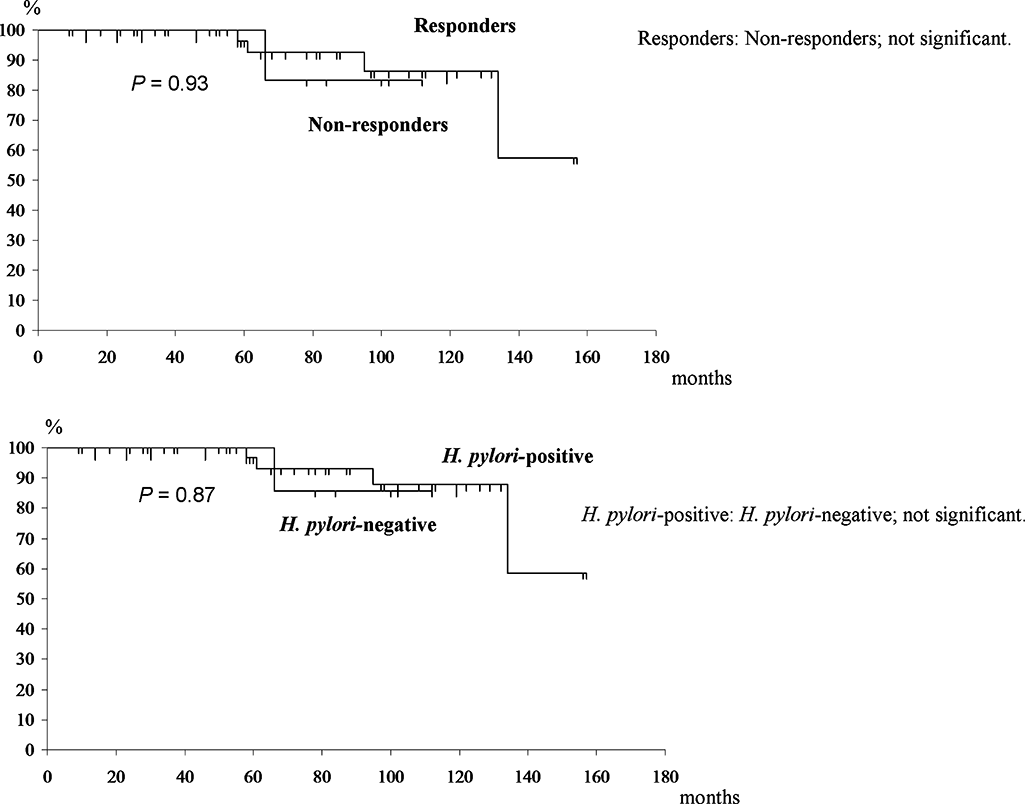
Event-free survival. Kaplan–Meier curves showing event-free survival. In the upper section, the comparisons according to response to eradication therapy are shown. In the lower section, comparisons according to Helicobacter pylori infection status are shown. There are no significant differences.
Incidence of metachronous gastric cancer
One synchronous gastric cancer was detected and treated by endoscopic surgery. Three cases of early gastric cancer were diagnosed during the follow-up period. Two patients had been eradicated of H. pylori and one patient was H. pylori-negative since she had been diagnosed with MALT lymphoma 11 years ago, and had achieved CR by radiation therapy. The features of the three cases are shown in Table 2 and the case of H. pylori-negative is shown in Figure 4.
| Age/sex | Location | Endoscopic appearance | Depth | Lugano stage | Helicobacter pylori status | CR duration | Treatment |
|---|---|---|---|---|---|---|---|
| 58/male | M | Ulcer | M | II1 | (+) | 55 | Eradication |
| Cancer | M | IIc 18 mm | M | (−) | EMR, surgery | ||
| 50/male | L,U | SMT | SM | I | (+) | 93 | Eradication |
| Cancer | L | IIc 6 mm | M | (−) | ESD | ||
| 53/female | M | Thickened | SS | II1 | (−) | 61 | Radiation |
| Cancer | M | IIc 10 mm | M | (−) | ESD |
- CR, complete remission; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; L, lower; M, middle; U, upper third of the stomach; SMT, submucosal tumor.
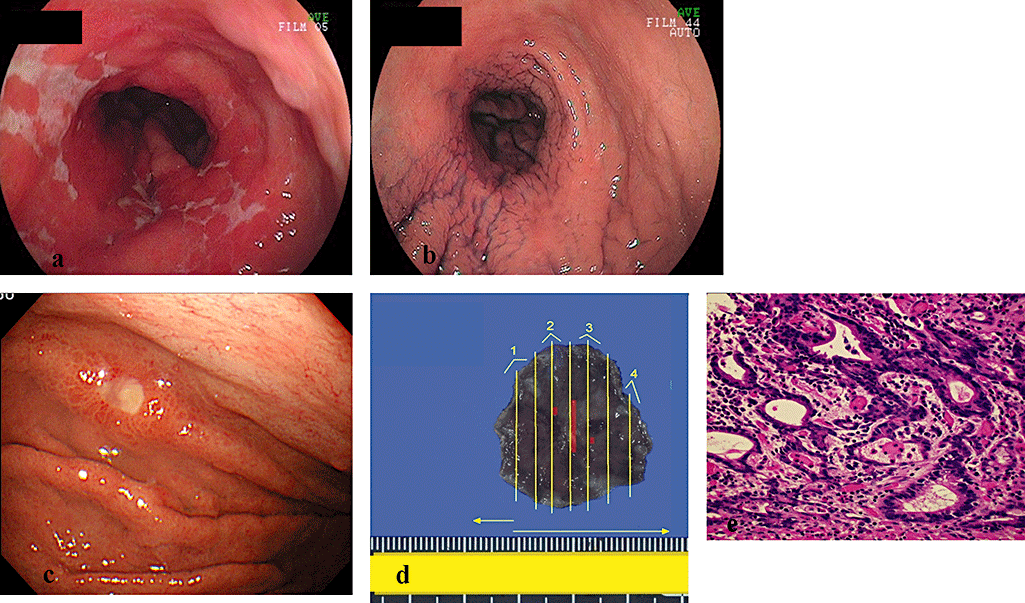
A case of metachronous gastric cancer after complete remission of gastric mucosa associated lymphoid tissue (MALT) lymphoma. (a) Conventional endoscopic image of gastric MALT lymphoma shows stenosis of stomach and gastric wall thickness with multiple erosions. (b) MALT lymphoma was eradicated by radiation therapy and complete remission has been maintained for 5 years. (c) At the 5th year after radiation therapy, regular endoscopy revealed an early intestinal-type gastric cancer in the corpus. (d) The lesion was resected en bloc by endoscopic submucosal dissection (ESD), and the lesion was 7 × 10 mm in diameter (red line). (e) Microscopic view of the resected carcinoma (hematoxylin-eosin, original magnification ×400). The histopathologic diagnosis was well differentiated adenocarcinoma (m, ly0, v0).
All of the three cases maintained a CR state of gastric MALT lymphoma and developed well-differentiated adenocarcinoma in the same areas of the lymphoma. Two patients with corpus carcinoma had severe corpus-dominant atrophy and the patient with antral carcinoma had mild antral atrophy. Intestinal metaplasia was pathologically proven in their gastric mucosa around the carcinomas.
Endoscopic resection was performed for these cases, but one patient received surgery after the recurrence of resected cancer. Pathologically, residual MALT lymphoma was not observed in the mucosal layer of all the resected specimens. All of the three patients are alive without recurrence.
Discussion
The clinical course of localized gastric MALT lymphoma is indolent with high response to therapeutic modalities; however, long-term prognosis has not been clarified. We could observe endoscopically for a median of 76 months from the diagnosis. Both eradication therapy and radiation therapy provided a favorable long-term remission (the median CR duration; 68 months, 65 months). However, long-term outcome of more than 5 years of stomach-conserving therapy in localized gastric MALT lymphoma has not been evaluated.
Our results showed that all H. pylori-positive patients with endoscopic superficial type achieved complete remission by antibiotic treatment, but that none of the H. pylori-negative patients responded. Although diagnosis of H. pylori infection is important as a predictive factor for antibiotics, false-negative or false-positive results may be obtained,18,19 and eradication therapy is generally accepted as a first-line treatment for localized gastric MALT lymphoma for both H. pylori-positive and H. pylori-negative patients. However, further studies on second-line therapy for patients without response to eradication and without H. pylori infection are required.
Although both gastric cancer and gastric MALT lymphoma are long-term complications of chronic H. pylori infection, it is controversial whether gastric cancer risk is increased in patients with metachronous gastric MALT lymphoma. According to Japanese mass screening for gastric cancer, the detection rate of gastric cancer was 0.094% in 2004 and Uemura et al. have reported that gastric cancer developed in 2.9% of H. pylori-positive patients during a mean follow-up of 7.8 years.6,20 Our percentage of metachronous gastric cancer after remission of MALT lymphoma was 5% and it was higher than in these reports about both the Japanese population as well as previous reports about gastric MALT lymphoma.9,13
Most patients with metachronous gastric cancer were H. pylori-positive patients with gastric MALT lymphoma and there have been few reports in H. pylori-negative patients.8–12 However, the gastric mucosa after remission of MALT lymphoma without H. pylori infection changed to partially atrophic mucosa, and intestinal metaplasia could develop after severe damage.
In our three cancer patients including an H. pylori-negative patient, carcinomas developed in the areas of atrophic gastritis and intestinal metaplasia after remission of lymphoma. MALT lymphoma may lead to the development of chronic atrophic gastritis and intestinal metaplasia and could be a cause of gastric cancer as well as H. pylori infection. In addition, radiation therapy and chemotherapy of MALT lymphoma might influence the development of metachronous gastric cancer.
Finally, our results must be interpreted in consideration of the following limitations: the sample size was small and only 12 patients were observed for more than 10 years, and gene abnormality was not considered enough in our series.
In conclusion, our strategy for the treatment of localized gastric MALT lymphoma provides a favorable long-term outcome, though regular follow-up endoscopy after remission is recommended for detection of metachronous gastric cancer at an early stage.
Acknowledgments
We wish to thank Professor Hiroshi Inagaki (Department of Pathology, Nagoya City University Medical School, Nagoya, Japan) for detecting API2-MALT1 gene.
Funding: None.




