Factors affecting the efficacy of cyclosporin A therapy for refractory ulcerative colitis
Abstract
Background and Aims: Cyclosporin A (CSA), an immunosuppressive agent, is highly efficacious in patients with refractory ulcerative colitis (UC). We retrospectively investigated patients with refractory UC treated with CSA therapy to elucidate the efficacy and the prognostic factors.
Methods: Forty-one patients (26 men and 15 women) were enrolled. The efficacy of CSA was assessed at three time points: short- and mid-term assessments took place 2 weeks and 1 year after CSA administration, respectively, and long-term assessments at the end of the observation period.
Results: The short-term response rate was 71%. Background analysis revealed risk factors for CSA unresponsiveness: (i) more than 10 000 mg of prednisolone used before CSA treatment; (ii) the presence of circulating (C7-HRP); and (iii) disease duration more than 4 years. The mid-term relapse-free survival rate was 51.0%. The addition of azathioprine (AZA) after CSA treatment significantly suppressed the incidence of relapse at 1 year (72.5% vs 26.7%, P = 0.0237). The overall colectomy-free survival rate was 46.4%, and the induction of AZA after CSA treatment significantly reduced the colectomy rate (66.7% vs 30.5%, P = 0.0419). Among CSA responders, AZA naïve patients had significant lower-probabilities for colectomies compared to patients with prior AZA treatment (22.5% vs 56.7%, P = 0.0309). The administration of CSA was discontinued in five cases.
Conclusion: Our results revealed factors affecting the efficacy of CSA therapy for patients with refractory UC. AZA is an important agent that maintains disease quiescence once one responds to CSA. However, refractory patients despite AZA treatment are more likely to have consequent colectomies.
Introduction
Ulcerative colitis (UC) is characterized by a long-standing chronic course with remissions and exacerbations. Approximately 15% of patients have severe attacks requiring hospitalization at some time during their disease course. These patients are traditionally treated with i.v. corticosteroids, with a response rate of approximately 60%. Patients that do not respond to 5-aminosalicylic acid compounds and corticosteroids are usually considered for colectomies. Few alternative treatments exist for severe UC; immunosuppressive medications (such as azathioprine [AZA]) have a slow onset of action and are therefore usually ineffective in acute disease flare-ups. Infliximab is a newly developed biological agent, and factors affecting its efficacy remain to be established.1,2
The induction of cyclosporine A (CSA) for severe, steroid-refractory UC has provided an effective medical alternative to patients previously faced with only surgical options. Uncontrolled trials3,4 and controlled trials5 established the efficacy of short-term CSA use as “rescue therapy” in severe UC. Lichtiger et al. reported i.v. CSA followed by oral therapy showed an initial response rate of 82% within a mean of 7 days versus 0% in a group that received steroids alone. Quality of life analyses comparing UC patients treated with CSA to those who underwent colectomies have shown that CSA patients consistently score as well as, or better than, their surgical counterparts.6 However, factors affecting CSA remain unclear. In this study, we investigated the efficacy of CSA therapy on refractory UC patients and tried to define factors responsible for its efficacy.
Methods
Patients
We reviewed medical charts and the recent follow up of 41 patients (26 men and 15 women) who had been administrated CSA for disease flare-ups between December 1999 and March 2009 at the Shiga University of Medical Science Hospital (Table 1). Basically, CSA was administrated in patients resistant to systemic corticosteroids. Cytomegalovirus infections were validated by cytomegalovirus antigenemia (C7-HRP). Ten out of 41 patients received concurrent gancyclovir treatment due to cytomegalovirus infections. The median patient age was 33 years (17–62), and the median disease duration was 4.08 years (19 days to 15 years); the disease type included one attack in four cases, chronic continuous attacks in nine and relapse remitting attacks in 28. The disease extent was total colitis in 28 cases and left-sided colitis in 13.
| Responder | Non-responder | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| No. of patients (M/F) | 29 (17/12) | 8 (9/3) |
| Sex (male, 1; female, 0) | 0.59 ± 0.501 | 0.88 ± 0.354 |
| Age | 33.10 ± 12.8 | 31.38 ± 13.7 |
| Disease duration (days) | 1260 ± 1572 | 2329 ± 1429 |
| Seo's CIDAI | 213.4 ± 23.4 | 223.4 ± 21.6 |
| Undermining ulcer (present, 1; absent, 0) | 0.52 ± 0.509 | 0.62 ± 0.518 |
| Disease extent (pancolitis type, 1; left-sided, 0) | 0.69 ± 0.471 | 0.62 ± 0.518 |
| C7-HRP (positive, 1; negative, 0) | 0.24 ± 0.435 | 0.75 ± 0.463 |
| Total PSL† used before CSA treatment (mg) | 3825 ± 4779 | 14317 ± 11598 |
- † All corticosteroids used were converted to PSL.
- CIDAI, complex integrated disease activity index; CSA, cyclosporine A; PSL, prednisolone; SD, standard deviation.
CSA administration
Cyclosporin A was administrated by continuous infusions with starting doses of 2–4 mg/kg per day for a maximum of 14 days. Serum CSA levels were monitored three times a week during infusion therapy and the infusion dose was altered by aiming for 350–450 ng/mL. After successful continuous CSA infusions, we switched from continuous infusions to p.o. dosing. Total p.o. daily doses were double those of continuous daily infusions. Trough serum levels were monitored and the dose of CSA was adjusted to trough serum levels of 100–200 ng/mL. The average duration of p.o. CSA administration was 139.2 days.
Definitions
Disease activity was assessed by using Seo's complex integrated disease activity index (CIDAI).7 Scores below 150 were classified as ‘remission’. ‘CSA responders’ were defined as those with a 50-point decrease during continuous CSA infusions. Follow ups to CSA therapy were assessed at three time points. The first time point was 2 weeks after CSA administration, defined as ‘short-term’. Second, ‘mid-term’ follow ups occurred 1 year after CSA administration. ‘Long-term’ follow ups were defined as the overall period of observation. The average period of observation was 3.58 years. Response rates to CSA, relapse-free survival rates and colectomy-free survival rates were investigated at short-, mid- and long-term follow up, respectively. A ‘relapse’ was defined as a hospitalization after one successful response to CSA.
Statistical analyses
Patient backgrounds from responders were compared with those of non-responders to elucidate characteristics of responders. For evaluating prognostic factors of efficacy, categorical data analyses were conducted on sex, age (≥ 40 years, < 40 years), disease duration (≥ 4 years, < 4 years), Seo's CIDAI at starting CSA treatment (≥ 220 points, < 220 points), endoscopic findings of undermining ulcers, disease extent (total colitis type or left-sided colitis), C7-HRP, total prednisolone (PSL) used before CSA treatment (≥ 10 000 mg, < 10 000 mg). Overall relapse- and colectomy-free survival was calculated using the Kaplan–Meier method. The log–rank test was used to elucidate the statistical difference between groups. All statistical analyses were performed with StatView ver. 5.0 (SAS Institute, Cary, NC, USA), GraphPad Prism ver. 4.03 and SPSS ver. 16.0.1 (SPSS, Chicago, IL, USA).
Results
Short-term results
An overview of patients treated with CSA is shown in Figure 1. The short-term response rate was 71%. Fifty-five percent of CSA responders showed a 50-point decrease during the first week in Seo's CIDAI (Fig. 2). A background analysis was performed on 37 patients without early CSA discontinuation and revealed three prognostic factors: (i) more than 10 000 mg of PSL used before starting CSA; (ii) positivity for C7-HRP; and (iii) disease duration of more than 4 years (Table 2). Response rates were significantly reduced in patients with large amounts of PSL prior to CSA therapy or C7-HRP positivity.
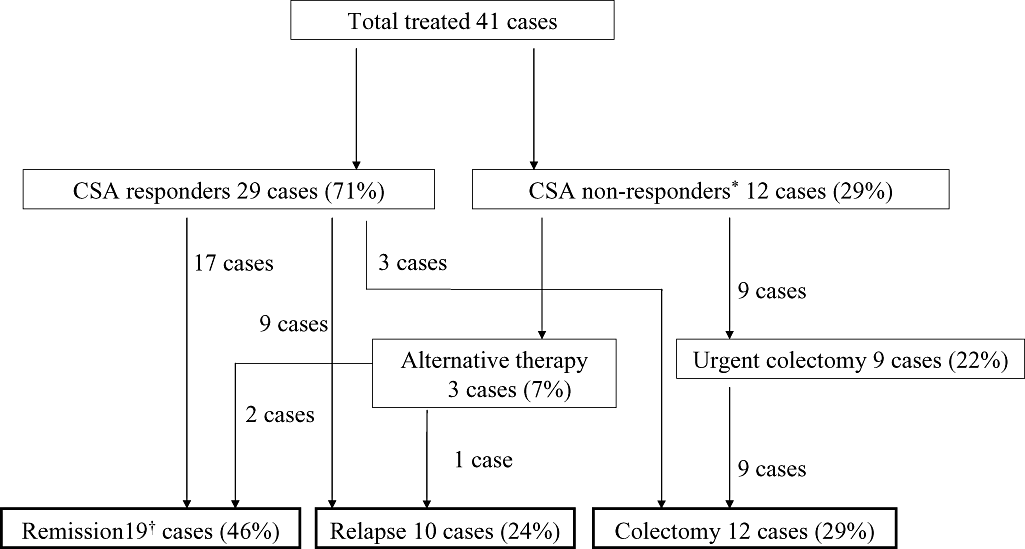
Flow-chart illustrating the outcome of all patients treated with cyclosporine A (CSA). Middle-term outcomes are exhibited in bold squares. The middle-term colectomy rate was 29%. Nine non-responders and three CSA responders received surgical procedures. Forty-six percent of the study participants remained in remission. *Including four cases with discontinued CSA administration: two cases due to liver dysfunction, one due to renal dysfunction and one due to a concomitant mental disorder. †Including four cases with a follow-up period of < 1 year.
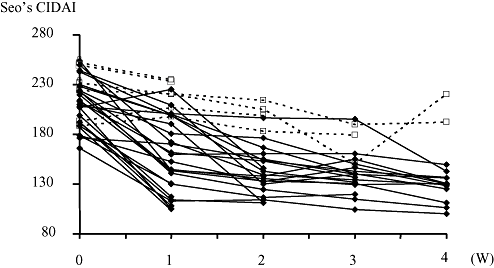
Clinical courses after staring continuous cyclosporine A (CSA) infusion therapies. Seo's complex integrated disease activity indexes (CIDAI) were recorded weekly, post-CSA administration. Sixteen out of 29 responders showed a 50-point decrease in Seo's CIDAI within 1 week.  , Responders;
, Responders; 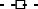 , non-responders.
, non-responders.
| Odds ratio | 95% Confidence Interval | ||
|---|---|---|---|
| Lower | Upper | ||
| Male gender | 0.202 | 0.022 | 1.867 |
| Age ≥ 40 | 0.875 | 0.145 | 5.270 |
| Disease duration ≥ 4 years | 9.429 | 1.540 | 57.744 |
| Seo's CIDAI ≥ 220 points | 3.214 | 0.554 | 18.650 |
| Prior AZA treatment | 0.271 | 0.029 | 2.526 |
| Undermining ulcer present | 1.556 | 0.312 | 7.751 |
| C7-HRP positive | 9.429 | 1.540 | 57.744 |
| PSL ≥ 10,000 mg | 18.750 | 2.757 | 127.513 |
Mid- and long-term results
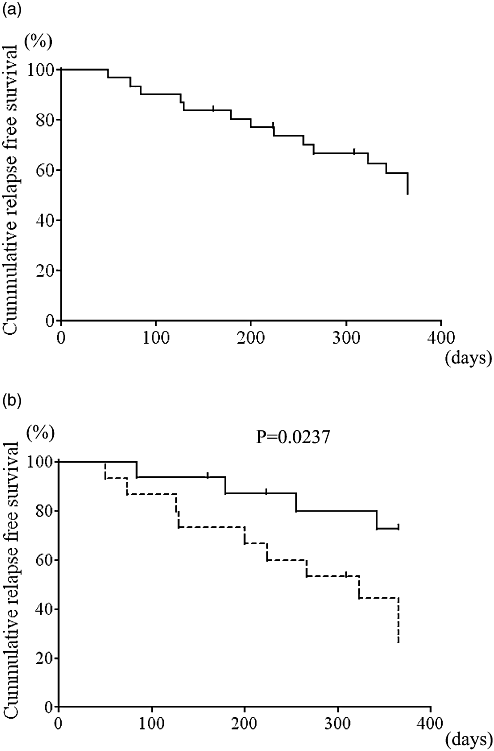
Mid- and long-term results were analyzed on 29 CSA short-term responders. Relapse-free survival at 1 year was 51.0% (Fig. 3a). The administration of AZA after CSA treatment significantly suppressed disease-relapse 1 year after CSA treatment (Fig. 3b). Overall colectomy-free survival was 46.4% (Fig. 4a). Similarly, the addition of AZA after CSA treatment significantly reduced the colectomy rate (Fig. 4b). Among CSA responders, AZA naïve patients (patients who did not receive AZA prior to CSA treatment) had a significantly lower probability of a colectomy than patients with prior AZA treatment (Fig. 4c).
Mid-term results. (a) Cumulative relapse-free survival at 1 year after CSA administration was 51.0% in all responders (n = 29). (b) Stratification analyses were conducted in patients receiving additional azathioprine (AZA) treatment (AZA added group, n = 15) and patients receiving continuous AZA administration and no AZA treatment (AZA unchanged group, n = 14). Addition of AZA significantly suppressed disease relapse (72.5% vs 26.7%, P = 0.0237, log–rank test).  , AZA added group;
, AZA added group;  , AZA unchanged group.
, AZA unchanged group.
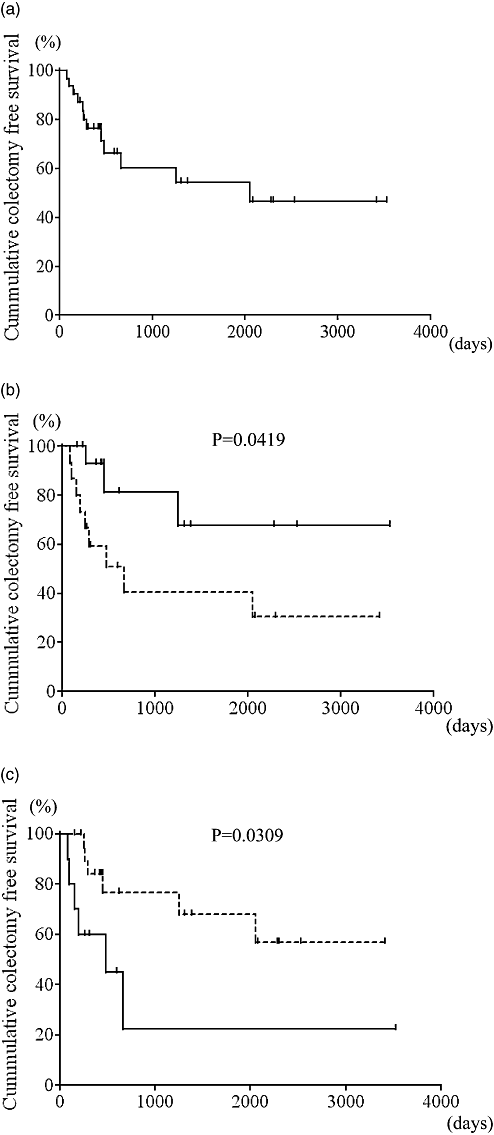
Long-term results. (a) Overall results of cumulative colectomy-free survival in all responders (n = 29). The colectomy-free survival rate was 46.4%. (b) Stratification analysis onto azathioprine (AZA) added group (n = 15) and AZA unchanged group (n = 14). Addition of AZA significantly reduced the rate of colectomy (66.7% vs 30.5%, P = 0.0419, log–rank test).  , AZA added group;
, AZA added group;  , AZA unchanged group. (c) Patients with prior AZA treatment, the AZA pretreated group (n = 10), had earlier inductions of colectomies compared to patients with no prior AZA treatment, the AZA naïve group (n = 19). Prior AZA administration is a poor prognostic factor for colectomies (22.5% vs 56.7%, P = 0.0309, log–rank test).
, AZA unchanged group. (c) Patients with prior AZA treatment, the AZA pretreated group (n = 10), had earlier inductions of colectomies compared to patients with no prior AZA treatment, the AZA naïve group (n = 19). Prior AZA administration is a poor prognostic factor for colectomies (22.5% vs 56.7%, P = 0.0309, log–rank test).  , AZA pretreated group;
, AZA pretreated group;  , AZA naïve group.
, AZA naïve group.
Optimal dosage of CSA
Among patients treated with a starting dose of 4 mg/kg per day of CSA, the first concentration of serum CSA was often over 600 ng/mL. A starting dose of 3 mg/kg per day significantly reduced initial serum CSA levels (463.0 ± 82.3 vs 611.0 ± 90.8, P < 0.0001). The rate of CSA effectiveness and adverse events was not significantly different between a starting dose of 3 mg/kg per day and 4 mg/kg/day (data not shown).
Adverse events
There were no serious bacterial infections that occurred during CSA treatment. The administration of CSA was discontinued in four cases: two cases due to liver dysfunction, one due to renal dysfunction and one due to a concomitant mental disorder. An antihypertensive drug was started in one case during CSA treatment. A magnesium agent was administrated in four cases due to hypomagnesemia.
Discussion
This retrospective study describes the experience of CSA therapy and the efficacy of an immunomodulator (AZA) as a bridging therapy. In our results, the short-term efficacy rate of CSA was approximately 70%, which is similar to previous reports from Western countries. Short-term efficacy was affected by three factors: (i) more than 10 000 mg of PSL prior to CSA treatment; (ii) positivity for C7-HRP; and (iii) disease duration more than 4 years. High amounts of PSL and long disease duration might indicate the disease severity and refractoriness and these two factors might confound with each other. Although C7-HRP-positive patients were treated with concomitant gancyclovir treatment, CSA-related immunosuppression might stimulate cytomegalovirus activity,8 resulting in a decrease in the response rate.
The administration of AZA after successful CSA treatments was the sole element that prolonged relapse-free survival at 1 year and colectomy-free survival. On the other hand, CSA responders with prior AZA treatment demonstrated poor responses and received colectomies. Our observations suggest a need for surgical intervention in patients with AZA administration prior to CSA treatment.
Initially, we followed the regimen of Lichtiger et al. (starting dose of 4 mg/kg per day) at the beginning of the CSA treatment.5 However, 3 mg/kg per day was sufficient to keep serum CSA levels at 350–450 ng/mL. CSA is primarily eliminated through biotransformation by cytochrome P450 (CYP)3A in the intestinal wall and liver, excreted to bile juice. However, the clearance of CSA differs between Japanese and white populations. Japanese populations have lower CSA clearance compared to white.9 Thus, we recommend a starting dose of 3 mg/kg per day CSA.
There were no serious adverse events in terms of CSA administration. In our hospital, serum CSA levels were reported on the same day as sample retrieval. Careful monitoring is necessary for the safe use of CSA.
In conclusion, our results showed the use of large amounts of PSL before staring CSA treatment and C7-HRP positivity were important factors responsible for the efficacy of CSA therapy. Although AZA is considered a key agent to maintain disease quiescence once one responds to CSA, refractory patients were more likely to have consequent colectomies despite AZA treatment.




