The epidemiology of hepatitis C in Australia: Notifications, treatment uptake and liver transplantations, 1997–2006
Abstract
Background and Aim: Regular monitoring of hepatitis C (HCV)-related surveillance data is essential to inform and evaluate strategies to reduce the expanding HCV burden. The aim of this study was to examine trends in the epidemiology and treatment of HCV in Australia.
Methods: We reviewed data about HCV notifications, treatment of HCV infection through the Highly Specialised Drugs (s100) Program, and liver transplants (Australia and New Zealand Liver Transplant Registry) for the period 1997–2006.
Results: HCV case notification rates declined by almost 50% between 1999 and 2006, with the greatest reductions between 2001 and 2002 and amongst young adults. For newly acquired HCV cases, 89% were Australian-born and 90% reported injecting drug use as a risk factor for infection. Overall, 30% of liver transplant recipients had HCV-related cirrhosis, but the number and proportion of HCV diagnoses increased between 1997 and 2006. HCV treatment also increased over the review period. However, only 1.4% of the 202 400 people estimated to be living with chronic HCV at the end of 2006 received treatment that year.
Conclusion: The decline in HCV notifications is consistent with a decline in HCV incidence in Australia. However, the burden of advanced HCV disease continues to expand. To reduce this burden, treatment uptake needs to increase. Consistent and sensitive surveillance mechanisms are required to detect newly acquired cases together with an expansion of surveillance for chronic HCV infections.
Introduction
Hepatitis C virus (HCV) infection is a significant public health burden worldwide. In Australia, there were an estimated 264 000 people who were HCV antibody positive in 2005 (population prevalence 1.3%), including 5300 with HCV-related cirrhosis, and 105 with hepatocellular carcinoma (HCC).1 Under current treatment practices, the burden of HCV-related cirrhosis and HCC is projected to double over the next 20 years.2
Regular review of HCV-related surveillance data is essential to inform and evaluate strategies aimed at reducing the expanding burden of HCV and can also identify areas where surveillance needs to be strengthened. In this report, we examine HCV case notifications, liver transplant data (from the Australia and New Zealand Liver Transplant Registry [ANZLTR]), and treatment prescriptions (from the Highly Specialised Drugs [s100] Program), for the ten-year period 1997–2006. These data provide a comprehensive picture of recent and longer term epidemiological trends in HCV prevalence and patterns of transmission, the health care burden of chronic infection, and treatment uptake among people living with HCV, respectively.
Methods
Case notifications
Australia is a federation of states and territories and disease surveillance is governed by their public health legislation. As in most developed countries, surveillance for HCV relies on information about notified cases. Notifications of HCV infection are made to local health authorities and de-identified information such as the case's age, gender, postcode of residence, and year of diagnosis are forwarded to the National Notifiable Diseases Surveillance System (NNDSS). Serological screening tests for HCV have been available since 1990 and HCV became notifiable in most states and territories in 1991.3 The separation of newly acquired cases from cases with an unspecified date of infection began in some states in 19934 and by 1997, had occurred in all states and territories except Queensland (all years) and the Northern Territory (pre 2003).5 The national case definition for notification was: detection of anti-hepatitis C antibodies (in a person aged at least 18 months) or detection of HCV RNA. The case was classified as newly acquired (incident) if the person seroconverted or had acute hepatitis in the previous 24 months (12 months prior to 2002).6
Enhanced notification data for newly acquired cases
Enhanced surveillance for newly acquired HCV infections has been conducted in all states and territories except Queensland (all years), Tasmania (pre 1998), the Northern Territory (pre 2005), and Western Australia (in 2004). Enhanced data include the most likely source of infection, reasons for testing and country of birth (COB). These data were requested from state and territory surveillance registers as they were not collated nationally during the review.
Liver transplants
Liver transplant data were provided by the ANZLTR.7 The Registry collects data from all transplant units in Australia on all patients listed for transplants at their unit. Each of these patients is routinely tested for hepatitis B (HBV) and HCV infection. Data received for each recipient included year of transplant, primary and up to three secondary indications (reasons) for transplant, recipient's age and gender.
Treatment
Prescriptions for the treatment of HCV are monitored and delivered through the government-subsidized Highly Specialised Drugs (HSD) Program under Section 100 (s100) of the National Health Act, 1953. The drugs can only be prescribed by medical practitioners affiliated with specialized hospital units and the subsidy is not available for hospital in-patients. Numbers of prescriptions dispensed to public patients were available by quarter for the period July 1997–December 2006. To estimate annual numbers it was assumed that, for each quarter, 50% of patients were receiving treatment for 6 months and the remaining 50% for 12 months, consistent with the HCV genotype distribution in Australia of approximately equal proportions of genotype 1 and non genotype 1.8
Analyses
HCV case notification rates by age group, gender and year of diagnosis were calculated using mid-year population estimates provided by the Australian Bureau of Statistics as the denominator. National rates for newly acquired cases were computed using population estimates adjusted to exclude states/territories not reporting. Continuous variables were compared using the Mann–Whitney U-test and categorical variables using the χ2-test.
Results
Case notification data
Unspecified and newly acquired cases
There were 164 416 HCV notifications between 1997 and 2006. Notifications with an unspecified date of infection declined from a peak of 19 967 (rate 105.5/100 000) in 1999 to 11 934 (rate 57.6/100 000) in 2006 (Fig. 1). Notifications for newly acquired infections peaked in 2001 (691 cases; rate 4.4/100 000). Newly acquired cases made up 2.8% of all notifications, but the proportion generally increased over the 10 years to above 3% in 2003–2006.
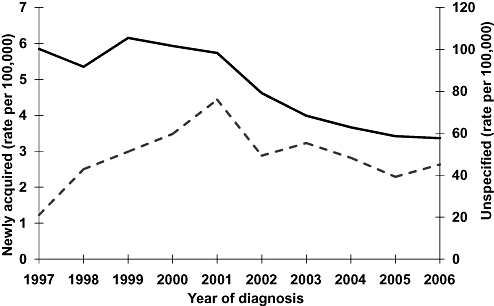
Hepatitis C notification rates by year of diagnosis and disease notification category. 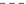 Newly acquired cases;
Newly acquired cases;  Unspecified cases.
Unspecified cases.
Total cases
The male to female ratio for HCV notifications remained constant over time at 1.7 : 1 but varied with age (Fig. 2). The median age was stable at 34 to 35 years in 1997–2001 but from 2002 increased progressively each year to 37 years in 2006. This trend was observed for both male and female cases and also when examining newly acquired and unspecified cases separately.
Mean hepatitis C notification rates by age group and gender, 1997–2006.  Males;
Males;  Females.
Females.
Notification rates for all five-year age groups between 15 and 44 years declined by at least 1.8-fold between 1999 and 2006. In contrast, rates in older age groups remained relatively constant or increased slightly over the same time period (Fig. 3). The greatest rate reductions for 1999–2006 were in the 15–19 (4.2-fold) and 20–24 year age groups (2.7-fold) and between 2001 and 2002 (1.3-fold).
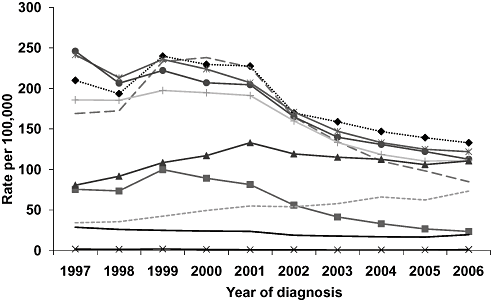
Hepatitis C notification rates by age group and year of diagnosis.  0–14 yrs;
0–14 yrs;  15–19 yrs;
15–19 yrs;  20–24 yrs;
20–24 yrs;  25–29 yrs;
25–29 yrs;  30–34 yrs;
30–34 yrs;  35–39 yrs;
35–39 yrs;  40–44 yrs;
40–44 yrs;  45–49 yrs;
45–49 yrs;  50–54 yrs;
50–54 yrs;  55+ yrs.
55+ yrs.
Enhanced notification data for newly acquired cases
COB was missing for 38% (1721/4584) of the newly acquired cases with enhanced data, although data completeness improved from 45% in 1997 to 83% in 2006. For cases where COB was reported, 89% were Australian-born, which is slightly higher than would be expected based on the population distribution by country/region of birth using the 2001 Australian Bureau of Statistics census (77%).
Overall, 25% (1147/4584) had no reported risk factors, although this proportion declined from 38% in 1998 to 17% in 2006. Of those cases where risk factor information was reported (n = 3437), injecting drug use (IDU) was by far the most common exposure (Table 1). The proportion with IDU recorded remained constant over the period 1997–2006, but decreased with increasing age in adults: from 94% in 15–29-year-olds to 86% in 30–39-year-olds, 77% in 40–49-year-olds and 46% in ages 50 years and over (P < 0.001). Of cases reporting IDU, 31% also reported at least one other risk factor. IDU was reported in at least half of the cases reporting other risk factors, except where mother to child transmission or ‘other’ risk was specified. Sexual contact was reported in 13% of cases, but only 3.8% (131/3437) did not also report IDU.
| Risk | Total with riskn (%†) | Non IDU risk‡n (%†) |
|---|---|---|
| Injecting drug use | 3082 (89.7) | NA |
| Imprisonment | 496 (14.4) | 73 (2.1) |
| Sexual contact§ | 449 (13.1) | 131 (3.8) |
| Skin penetration procedure¶ | 428 (12.5) | 76 (2.2) |
| Household contact†† | 139 (4.0) | 29 (0.8) |
| Healthcare exposure (patient)‡‡ | 107 (3.1) | 31 (0.9) |
| Needlestick other§§ | 58 (1.7) | 23 (0.7) |
| Blood/tissue recipient | 40 (1.2) | 14 (0.4) |
| Other | 35 (1.0) | 26 (0.8) |
| Mother to child transmission¶¶ | 16 (0.5) | 16 (0.5) |
| Healthcare worker | 15 (0.4) | 7 (0.2) |
| Needlestick injury in HCW | 8 (0.2) | 4 (0.1) |
| Total | 3437 (100) | 354 (10.3) |
- † Multiple risk factors were reported so column percentages add to more than 100%.
- ‡ Non IDU risk: cases where injecting drug use was not also recorded and per cent of total with risk (n = 3437).
- § Sexual contact includes: sex worker, high risk sex, ‘sex’, HCV positive partner.
- ¶ Skin penetration procedure includes: ‘piercing’, body piercing, tattoos, acupuncture, but not ‘ear piercing’.
- †† Household contact includes: sharing clippers or razor when not in prison.
- ‡‡ Healthcare exposure (patient) includes: any hospital or dental procedure, needlestick injury in patient.
- §§ Needlestick other includes: community acquired, unspecified, or other occupation besides HCW.
- ¶¶ MTCT includes: only cases specifying MTCT and aged <2 years. Cases >2 years old were coded as a household contact. Two cases reported both household contact and MTCT.
- HCV, hepatitis C virus; HCW, healthcare worker; IDU, injecting drug use; MTCT, mother to child transmission; NA, not applicable.
Reason for testing was reported in 63% (2881/4584), however data completeness improved from 42% in 1998 to 83% in 2006. Overall, 30% of cases with data available were recorded as being investigated for a symptomatic illness, although this proportion declined from 41% in 1997–2000 to 27% in 2001–2006. Over the same periods, the proportion with screening recorded as a reason for testing increased from 47% to 74%. IDU was less frequently recorded for cases with a symptomatic illness than cases detected through screening (87% vs 92%; P = 0.001).
Liver transplants
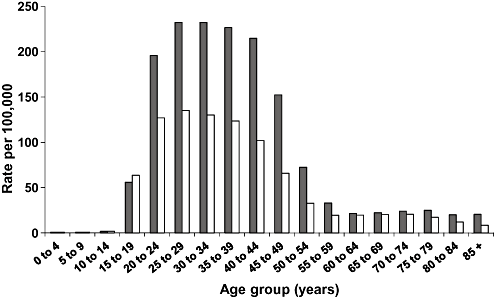
The number of liver transplant recipients with HCV-related cirrhosis as the primary indication increased over the period 1997–2006 (Fig. 4), and overall HCV-related cirrhosis was the most commonly reported primary indication (25%, 292/1189). An additional 65 transplant recipients (5.5%) had HCV-related cirrhosis recorded as one of up to three secondary indications.
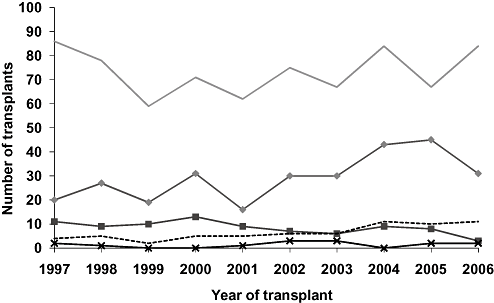
Number of liver transplants by primary indication for transplant and year of transplant, 1997–2006. HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.  HBV;
HBV;  HBV/HCV;
HBV/HCV;  HCC;
HCC;  HCV;
HCV;  Other diseases.
Other diseases.
The median age of recipients with HCV-related cirrhosis was 49 years (range 18–70 years) and there were significantly more male recipients than female (85% vs 15%; P < 0.001). Most (66%) had HCV-related cirrhosis without HBV or HCC. Twenty-seven percent also had HCC, 5% HBV, and 2% had both HCC and HBV. Of recipients with a primary indication of HCC, 52% (34/65) also had HCV-related cirrhosis, 31% (20/65) had HBV-related cirrhosis, and two had HBC/HCV-related cirrhosis. The number and proportion of transplant recipients with HCV-related cirrhosis (with and without HCC and HBV) showed an upward trend over the review period as did figures for HCC with non HBV/HCV causes (Table 2). In contrast, the number and proportion of transplant recipients with HBV-related cirrhosis (without HCC or HCV) declined in 1997–2006.
| Year | HCVn (%) | HCV + HCCn (%) | HBVn (%) | HBV + HCCn (%) | HBV + HCV‡n (%) | HCC-non HBV/HCVn (%) | Other diseasesn (%) | Totaln (%) |
|---|---|---|---|---|---|---|---|---|
| 1997 | 20 (16.3) | 6 (4.9) | 9 (7.3) | 3 (2.4) | 2 (1.6) | 4 (3.3) | 79 (64.2) | 123 (100) |
| 1998 | 23 (19.2) | 9 (7.5) | 5 (4.2) | 5 (4.2) | 1 (0.8) | 4 (3.3) | 73 (60.8) | 120 (100) |
| 1999 | 17 (18.9) | 3 (3.3) | 4 (4.4) | 8 (8.9) | 0 (0) | 2 (2.2) | 56 (62.2) | 90 (100) |
| 2000 | 25 (20.8) | 8 (6.7) | 10 (8.3) | 5 (4.2) | 0 (0) | 5 (4.2) | 67 (55.8) | 120 (100) |
| 2001 | 9 (9.7) | 9 (9.7) | 7 (7.5) | 6 (6.5) | 1 (1.1) | 3 (3.2) | 58 (62.4) | 93 (100) |
| 2002 | 26 (21.5) | 10 (8.3) | 6 (5) | 2 (1.7) | 3 (2.5) | 5 (4.1) | 69 (57) | 121 (100) |
| 2003 | 26 (23.2) | 11 (9.8) | 4 (3.6) | 3 (2.7) | 3 (2.7) | 5 (4.5) | 60 (53.6) | 112 (100) |
| 2004 | 34 (23.1) | 13 (8.8) | 2 (1.4) | 6 (4.1) | 6 (4.0) | 8 (5.4) | 78 (53.1) | 147 (100) |
| 2005 | 31 (23.5) | 17 (12.9) | 5 (3.8) | 7 (5.3) | 4 (3.1) | 7 (5.3) | 61 (46.2) | 132 (100) |
| 2006 | 24 (18.3) | 11 (8.4) | 2 (1.5) | 5 (3.8) | 5 (3.8) | 12 (9.2) | 72 (55) | 131 (100) |
| Total | 235 (19.8) | 97 (8.2) | 54 (4.5) | 50 (4.2) | 25 (2.1) | 55 (4.6) | 673 (56.6) | 1189 (100) |
- † Reported as one of up to four indications for transplant.
- ‡ Includes transplant recipients with HBV/HCV-related cirrhosis with and without HCC.
- HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Treatment
Prior to the availability of interferon α-2b and ribavirin combination therapy in October 1999 there were between 1000 and 1500 public patients dispensed treatment for HCV each quarter and all prescriptions were for interferon α-2a or 2b (Fig. 5). Interferon α-2b and ribavirin replaced interferon α-2a/b as the predominant drug during 2000 and 2001 but the number of patients on treatment overall declined from the third quarter of 2001. Following the availability of pegylated interferon α-2a/b and ribavirin in November 2003, the number of patients who were dispensed treatment again increased, with a further sharp increase following the removal of the requirement for biopsy-proven liver damage in April 2006, to a record high of 2578 in the last quarter of 2006. It was estimated that approximately 2900 public patients received treatment in 2006, which equates to the treatment of 1.4% of the 202 400 people estimated to be living with chronic HCV infection at the end of 2006.9
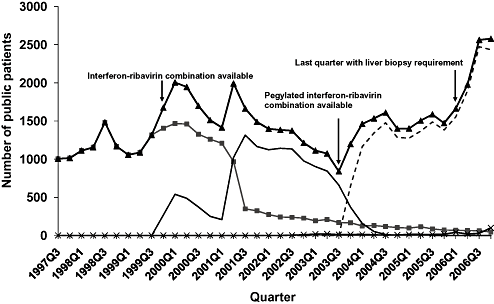
Numbers of public patients who were dispensed drugs for hepatitis C infection through the Highly Specialised Drugs program by quarter.  Interferon alpha-2a/b;
Interferon alpha-2a/b;  Pegylated interferon alpha-2a/b;
Pegylated interferon alpha-2a/b;  Interferon alpha-2b & ribavirin;
Interferon alpha-2b & ribavirin;  Pegylated interferon alpha-2a/b & ribavirin;
Pegylated interferon alpha-2a/b & ribavirin;  All treatments.
All treatments.
Discussion
Although the surveillance data presented here have been included in annual surveillance reports,7,9 this is the first time they have been interpreted together for a ten-year period and reported in the peer-reviewed literature. The review demonstrates several important trends in the epidemiology of HCV in Australia. HCV case notification rates declined by almost 50% between 1999 and 2006, with the greatest reductions between 2001 and 2002 and amongst young adults, consistent with a decline in HCV incidence. Enhanced surveillance data for newly acquired HCV cases demonstrate that HCV transmission occurs predominantly among Australian-born (89%) and injecting-drug-user (90%) populations. Despite indications of a reduction in HCV incidence, the burden of HCV-related advanced liver disease has expanded, as evidenced by the increased number and proportion of liver transplants with an HCV diagnosis between 1997 and 2006. Although the number of patients prescribed HCV treatment increased over the review period, uptake remains low.
Evidence of a decline in HCV incidence
Optimally, notifications of newly acquired cases would be used to monitor HCV incidence. Currently, however, trends in notifications of newly acquired cases in Australia mostly reflect changes in surveillance mechanisms (see data limitations). The 50% reduction in total notifications between 1999 and 2006 provides some evidence of a decline in HCV incidence, although a proportion of this decline is probably due to a reduction in the pool of prevalent cases not previously tested. Notifications from young adults provide the best measure of HCV incidence during 1997–2006 as a high proportion of infections in this age group are likely to be recently acquired. A recent study in Victoria of all newly notified cases aged 16–19 years old, found that 27% were new infections.10 Our review of notification data for newly acquired cases found that a high proportion (94%) of young adults acquire their infection through IDU. Given the median age of initiating injecting in Australia is 18–19 years11,12 and the average time to infection is estimated to be 1.6 years,11 it is expected that a high proportion of cases among young adults would be newly acquired. Therefore, the more than 2.5-fold reduction in notifications from 15–24-year-olds between 1999 and 2006 is likely to reflect a true reduction in HCV incidence.
The most likely explanation for the decline in HCV incidence comes from data describing the IDU population.1,13,14 The greatest decline in notifications was between 2001 and 2002, coincident with a dramatic reduction in the availability of heroin in Australia.15,16 While the injection of other drugs increased in some groups of IDU as a consequence,14,17,18 multiple data sources suggest a decline in the overall prevalence of IDU from 2001 onward1,13,14 and that most of this decline was due to a reduction in the number of young adults initiating injecting.12,19,20 These findings are consistent with both the decline in HCV notification rates, especially for 15–24-year-olds, and a reduction in HCV incidence, although reductions in risk behavior related to drug injecting may have also contributed to the decline in HCV incidence.
Evidence of expanding burden of HCV-related liver disease
Although the incidence of HCV infection appears to have declined from a peak in 2000, there are still an estimated 10 000 new infections occurring each year1 and 202 400 people were already chronically infected by the end of 2006.9 A minority of these cases will progress to advanced liver disease, but due to the large and increasing number of people infected, the burden of HCV-related cirrhosis and HCC is expected to continue to expand. The liver transplant data presented here demonstrate that HCV-related cirrhosis was the most commonly reported primary indication for liver transplant (25% of transplants) and 50% of recipients with a primary indication of HCC also had HCV. The increasing number and proportion of transplant recipients with HCV, and HCV with HCC, over the review period provides evidence that this burden is expanding. This is in contrast to the downward trend in transplant recipients with HBV-related cirrhosis, which is probably due to the recent availability of improved antiviral treatments for advanced HBV-related liver disease.21
Treatment uptake remains low
Advances in HCV treatment, both in terms of improved response rates22 and decreased rates of liver disease progression in patients who respond to treatment23 mean that the burden of advanced liver disease could be reduced by expanding treatment uptake. It is therefore promising to see an increase in the number of patients receiving treatment between 1997 and 2006. However, treatment uptake remained low, with only 2900 (1.4%) of the estimated 202 400 people living with chronic HCV at the end of 2006 receiving treatment that year. Modeling suggests that close to 10 000 people would need to be treated each year to reduce the rate of HCV-related advanced liver disease.2 This would require targeted programs designed to increase treatment uptake in the IDU population, including provision of treatment in opioid pharmacotherapy clinic settings.24
Data limitations
HCV case surveillance relies on a case being tested (detected) and then notified. As the majority of HCV infections are mild or asymptomatic, notified cases are likely to be an underestimate of the true number of infections and may be biased if certain population groups are preferentially tested. However, modeling results estimate that approximately 85% of cases have been notified in Australia.1 In addition, testing amongst the IDU population, who contribute most of the newly acquired cases, is thought to be high (60% of surveyed clients using needle and syringe programs reported testing in the last year and only 7% reported they had never been tested in 2007).12 Hence, even though notifications are an underestimate of the true prevalence of infection, the data appear to be relatively complete. Further to this, it is likely that screening for HCV infection in at-risk populations has increased over time (as evidenced by the increased proportion of newly acquired cases detected through screening). Therefore the downward trend in total notifications reported here is unlikely to be due to a reduction in testing.
Trends in notifications specifically classified as newly acquired should be interpreted with more caution. Cases need to be further investigated by the local or state health authority to confirm whether the infection was recently acquired, which is resource intensive. Therefore case follow-up procedures have not been implemented in a consistent manner over time or across Australia.5,10,25,26 This not only means there is significant under-ascertainment of newly acquired cases but also that the number reported depends on the intensity and mechanisms of surveillance. Increased screening may also bias detection towards cases from certain at-risk populations, such as IDU, who are more likely to be tested. However, we found that the proportion reporting IDU as the most likely exposure did not change over time, despite an increased proportion of cases detected through screening programs. In addition, even amongst cases with a symptomatic illness, IDU was still the main (87%) risk factor reported.
In terms of the other data sources reviewed, although the ANZLTR collects data on all liver transplants in Australia the numbers of transplants are limited by the availability of donors. To address this issue, the ANZLTR are now also collecting information about patients on transplant waiting lists and this will provide a more accurate representation of the burden of end-stage liver disease for future studies. Finally, only numbers of public patients prescribed HCV treatments were available. However, the vast majority of patients receive their treatment through the public system.
Recommendations for HCV surveillance
Mechanisms for surveillance of newly acquired cases require consistency over time and across states and territories so that trends can be interpreted with confidence. Given that active follow up is resource intensive, population-based surveillance could be restricted to young adults and/or particular geographic regions with the caveat that the data may not be representative of all cases but will provide robust data to describe trends over time.10,27
In terms of enhanced data collected about newly acquired cases, we found that 70% of cases reporting the risk factors of either imprisonment, sexual contact or a skin penetration procedure also reported IDU. This high correlation with IDU underscores the need for a standardized data collection instrument and to report the prevalence of each risk factor separately (rather than as a hierarchy where cases with IDU are excluded from other risk estimates)28 in order to accurately determine the contribution of each of these potential risk factors.
Population-based surveillance for newly acquired cases could be complemented by expanding sentinel surveillance in clinics that service at-risk populations, especially new IDUs.9,29 However, detection of newly acquired cases in this setting is dependent on the frequency of screening each individual. Surveillance for chronic HCV infections also needs to be expanded. There are a significant number of people that are in the early stages of chronic HCV infection and the burden of morbidity in this group is unknown. With this in mind, a data linkage project is underway to look at hospital admissions in a cohort of 128 000 notified HCV cases in the state of New South Wales. In addition, a clinic-based cohort study of 1500 patients with chronic HCV has been established in four Australian states to examine factors associated with treatment uptake and outcomes including liver disease progression.
Conclusion
In conclusion, the evidence presented here suggests a decline in HCV incidence in Australia. However, the burden of advanced HCV disease continues to increase as indicated by the rising number and proportion of liver transplants due to HCV. To reduce this burden, treatment uptake needs to further improve on recent encouraging trends. In terms of surveillance methodology, consistent surveillance mechanisms over time and across Australia are required together with an expansion of surveillance for cases of newly acquired and chronic HCV infections.
Acknowledgments
The authors would like to acknowledge the following individuals and organizations for their expert advice and provision of data: state and territory surveillance coordinators: Kate Ward, Mark Bartlett, Nasra Higgins, David Coleman, Kelly Shaw, Tess Davey, Peter Markey, Riemke Kampen, Katherine Davis, and Caroline Giele; Glenda Balderston, the Australia and New Zealand Liver Transplant Registry; Jess Dalla, Naomi Cole & Emma Gibbs, Highly Specialised Drugs Special Access Programs, Department of Health and Ageing; and the Australian Government Department of Health and Ageing. Margaret Hellard and Lisa Maher are supported by NHRMC Research Fellowships. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing and is affiliated with the Faculty of Medicine, The University of New South Wales.




