Clinicopathological and molecular characteristics of Epstein–Barr virus-associated gastric carcinoma: A meta-analysis
Abstract
There is conflicting data regarding the clinicopathological significance of the risk factors associated with Epstein–Barr virus (EBV)-associated gastric carcinoma (EBVaGC). To address this controversy, we performed a meta-analysis for the clinicopathological and molecular characteristics of EBVaGC. The relevant published studies were reviewed according to the defined selection criteria. The effect sizes of the outcome parameters were estimated by an odds ratio or a weighted mean difference. This meta-analysis included 48 studies that encompassed a total of 9738 patients. The frequency of EBVaGC was 8.8%, and EBVaGC was significantly associated with ethnicity. It was more predominant in men and in younger individuals. Interestingly, EBVaGC was more prevalent in Caucasian and Hispanic patients than in Asian ones. EBVaGC developed most often in the cardia and body, and it generally showed the diffuse histological type. EBV was highly prevalent in the patients with lymphoepithelial carcinoma. EBVaGC was closely associated with remnant cancer and a CpG island methylator-high status, but not with Helicobacter pylori infection, a TP53 expression, and p53 mutation. In addition, EBVaGC was not significantly associated with the depth of invasion, lymph node metastasis, or the clinical stage. The clinicopathological and molecular characteristics of EBVaGC are quite different from those of conventional gastric adenocarcinoma. However, further study is needed to determine the effect of EBV on the survival of EBVaGC patients.
Introduction
Epstein–Barr virus (EBV) is a herpes virus that was originally identified in a human Burkitt lymphoma cell line.1 It is now known that EBV infects more than 90% of the human population.1 While the vast majority of EBV infections remain asymptomatic, a small portion of these EBV-infected individuals develop hematopoietic, epithelial, and mesenchymal tumors. These tumors include Burkitt lymphoma, Hodgkin lymphoma, nasopharyngeal carcinoma, gastric adenocarcinoma, and on rare occasion, leiomyosarcoma in immunosuppressed patients.1
The presence of EBV in a patient with gastric cancer was first reported in a case of lymphoepithelioma type.2 Subsequently, Shibata and Weiss reported cases of EBV infection in typical gastric adenocarcinomas.3 Subsequent reports of the prevalence of EBV-associated gastric carcinoma (EBVaGC) have ranged from 1.3% to as high as 20.1% in different countries.3–50 EBVaGC is a relatively non-endemic disease that is distributed throughout the world, while Burkitt lymphoma and nasopharyngeal carcinoma are endemic to equatorial Africa and southeast China, respectively. Numerous studies have suggested that there are both regional and ethnic differences in the incidence of EBVaGC.3–50
Epstein–Barr virus-associated gastric carcinoma is defined as the presence of EBV in gastric tumor cells, and this is always identified by performing EBV-encoded RNA (EBER) in situ hybridization. Almost all cancer cells contain large amounts of the EBER-1 and EBER-2 (107 copies per infected cell) in their tumor nuclei. EBVaGC has several characteristic clinicopathological features such as a high incidence of EBV in both lymphoepithelial carcinoma (LEC) and remnant gastric cancers, a male predominance, and it most frequently occurs in the cardia and body.3–52 Nonetheless, the relationship between EBVaGC and the patient's age, the histological subtype, the tumor depth, lymph node metastasis, and the clinical stage has been the subject of significant controversy. In an attempt to address this controversy, we performed a meta-analysis to determine the association between EBVaGC and the clinicopathological characteristics. In addition, we examined whether EBVaGC is associated with Helicobacter pylori infection, a TP53 expression, and a CpG island methylator phenotype (CIMP) status.
Methods
Eligibility criteria for the meta-analysis
We performed an extensive search for articles that examined the association between EBV and the clinicopathological characteristics of gastric carcinoma. The following types of articles were included: (i) articles published with the evidence that EBV was detected only in primary gastric adenocarcinoma tissue by EBER in situ hybridization; articles that dealt with different cancer types and cell lines were excluded; (ii) articles published before December 2007 in English; and (iii) the most informative article when multiple articles were published by the same authors or groups. The following articles were excluded: (i) review articles without original data; (ii) articles lacking or containing inappropriately reported clinicopathological data; and (iii) case reports.
Collection of published studies
A literature search was performed by using the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed). The keywords for our search consisted of ‘Epstein-Barr virus + gastric cancer’ and ‘Epstein-Barr virus + gastric adenocarcinoma’, which retrieved 331 and 125 citations, respectively. We selected the relevant studies on the basis of the summary analysis. We took care to avoid obtaining duplicate data by examining the authors' names and affiliations for each publication. Overlapping articles and the articles unrelated to our analysis were excluded.
Data pooling and statistics
Meta-analysis was performed as previously described.53 An effect size for each of the studies we analyzed was calculated by an odds ratio (OR) using the Mantel–Haenszel method or the weighted mean difference (WMD) according to the Cohen method. The decision to use one statistical method over the other depended on whether the measured event was dichotomous or continuous, whereas the choice to use a random or fixed effect model for analysis depended on Q statistics. Statistical analysis was performed by using Comprehensive Meta-analysis Software version 2.0 (Biostat, Englewood, NJ, USA); P values less than 0.05 were considered statistically significant.
Results
A total of 48 articles that were identified with our search terms fulfilled the eligibility criteria.3–50 The main data of the eligible studies is summarized in Table 1. The number of patients in the studies ranged from 19 to 970, for a total of 9738 patients. EBV was detected in 857 (8.8%) of the 9738 gastric adenocarcinoma patients.
| Study | No. of cases | Country (no. of cases, race) | EBVaGC no. (%) | Gender (EBV+/−) | Histological type (EBV+/−) | Tumor depth (EBV+/−) | |||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Intestinal | Diffuse | EGC | AGC | ||||
| Shibata and Weiss3 | 138 | USA (C) | 22 (16.0) | 21/78 | 1/38 | 15/80 | 7/36 | NA | NA |
| Tokunaga et al.4 | 970 | Japan (A) | 67 (6.9) | 58/572† | 11/358† | 35/479† | 34/451† | 22/275† | 47/655† |
| Rowlands et al.5 | 174 | UK(120, C) | 5 (4.2) | 5/105† | 2/62† | 1/80† | 0/44† | NA | NA |
| Japan (54, A) | 4 (7.4) | ||||||||
| Shousha and Luqmani6 | 19 | UK(C) | 1 (5.3) | 0/14 | 1/4 | 0/11† | 0/0† | NA | NA |
| Ott et al.7 | 39 | Germany (C) | 7 (17.9) | NA | NA | 1/16† | 2/16† | NA | NA |
| Yuen et al.8 | 74 | Hong Kong (A) | 7 (9.5) | 7/45 | 0/22 | 7/48 | 0/14 | 0/13 | 7/54 |
| Harn et al.9 | 55 | Taiwan (A) | 6 (10.9) | 4/24 | 2/25 | NA | NA | NA | NA |
| Moritani et al.10 | 132 | Japan (A) | 15 (11.4) | 9/78 | 6/39 | 4/57† | 1/57† | 8/55 | 7/62 |
| Shin et al.11 | 89 | Korea (A) | 12 (13.5) | 9/50 | 3/27 | NA | NA | NA | NA |
| Selves et al.12 | 59 | France (C) | 5 (8.5) | 4/39 | 1/15 | 5/23 | 0/21 | 1/5 | 4/49 |
| Ojima et al.13 | 412 | Japan (A) | 83 (20.1) | 58/192 | 25/137 | 0/0 | 23/324 | 8/103 | 75/226 |
| Gulley et al.14 | 95 | USA (70, H) | 9 (12.9) | 9/50 | 2/33 | 4/47 | 7/37 | NA | NA |
| Mexico (25, non-H) | 2 (8.0) | ||||||||
| Qiu et al.15 | 241 | China (90, A) | 7 (7.8) | 11/146 | 5/79 | 4/111 | 12/114 | NA | NA |
| Japan (151, A) | 9 (6.0) | ||||||||
| Galetsky et al.16 | 206 | Russia (C) | 18 (8.7) | 15/86† | 2/81† | 9/78† | 8/89† | NA | NA |
| Yanai et al.17 | 117 | Japan (A) | 12 (10.2) | 9/74 | 3/31 | 3/68 | 9/44 | 7/66 | 5/46 |
| Chong et al.18 | 98 | Japan (A) | 19 (19.4) | 15/50 | 4/29 | 2/41† | 6/28† | NA | NA |
| Hayashi et al.19 | 590 | Japan (510, A) | 29 (5.7) | NA | NA | NA | NA | NA | NA |
| Brazil (80, H) | 4 (5.0) | ||||||||
| Takano et al.20 | 513 | Japan (A) | 33 (6.4) | 28/339 | 5/141 | NA | NA | 18/222 | 15/258 |
| Kume et al.21 | 344 | Japan (A) | 40 (11.6) | 30/24‡ | 10/16‡ | NA | NA | 11/10‡ | 29/30‡ |
| Tanaka et al.22 | 252 | Japan (A) | 15 (6.0) | NA | NA | NA | NA | NA | NA |
| Ioachim et al.23 | 22 | USA (C) | 1 (4.5) | NA | NA | 0/19 | 0/2 | NA | NA |
| Chapel et al.24 | 56 | France (C) | 7 (12.5) | 7/24 | 0/25 | 4/11† | 3/28† | NA | NA |
| zur Hausen et al.25 | 132 | The Netherlands (C) | 10 (7.6) | NA | NA | NA | NA | NA | NA |
| Wu et al.26 | 139 | Taiwan (A) | 19 (13.7) | 13/76 | 6/44 | 11/68 | 8/52 | 3/25 | 16/95 |
| Chang et al.27 | 306 | Korea (A) | 17 (5.6) | 16/192 | 1/97 | 2/115† | 11/149† | 3/93 | 14/196 |
| Koriyama et al.28 | 300 | Brazil (149, A; 126, C; 25, B) | 7 (4.7); 13 (10.3); 4 (16.0) | 18/183 | 6/93 | 8/119 | 16/155 | NA | NA |
| Corvalan et al.29 | 185 | Chile (C) | 31 (16.8) | 21/98 | 8/56 | 10/104 | 21/50 | NA | NA |
| Lo et al.30 | 51 | Hong Kong (A) | 5 (9.8) | NA | NA | NA | NA | NA | NA |
| Kattoor et al.31 | 215 | India (A) | 10 (4.7) | 10/151 | 0/54 | 2/85† | 8/62† | NA | NA |
| Hao et al.32 | 378 | China (A) | 29 (7.7) | 24/230 | 5/117 | 4/136 | 25/209 | NA | NA |
| Hoshikawa et al.33 | 21 | Japan (A) | 3 (14.3) | NA | NA | NA | NA | NA | NA |
| Kang et al.34 | 233 | Korea (A) | 21 (9.0) | 17/41‡ | 4/15‡ | NA | NA | NA | NA |
| Carrascal et al.35 | 178 | Columbia (H) | 23 (12.9) | 19/89 | 4/65 | 11/80 | 12/74 | NA | NA |
| Czopek et al.36 | 40 | Poland (C) | 5 (12.5) | 4/26 | 1/9 | 0/13† | 4/15† | NA | NA |
| Karim and Pallesen37 | 50 | Malaysia (A) | 5 (10.0) | 4/28 | 1/17 | 4/33 | 1/12 | NA | NA |
| Oda et al.38 | 97 | Japan (A) | 5 (5.2) | NA | NA | NA | NA | NA | NA |
| van Beek et al.39 | 566 | The Netherlands (C) | 41 (7.2) | 38/286 | 3/239 | 26/261† | 2/112† | NA | NA |
| Lopes et al.40 | 53 | Brazil (H) | 6 (11.3) | 5/30† | 1/16† | 1/26† | 0/18† | NA | NA |
| Morewaya et al.41 | 150 | Papua New Guinea (A) | 2 (1.3) | 2/88 | 0/60 | 0/72 | 2/76 | NA | NA |
| Alipov et al.42 | 139 | Kazakhstan (83, C; 56, A) | 9 (10.8); 5 (8.9) | 12/74 | 2/51 | 1/47 | 13/78 | NA | NA |
| Yoshiwara et al.43 | 254 | Peru (H) | 10 (3.9) | 5/115 | 5/123 | 4/127 | 6/117 | NA | NA |
| Sakuma et al.44 | 140 | Japan (A) | 24 (17.1) | 20/78 | 4/38 | 7/63 | 17/53 | 6/39 | 18/77 |
| Anwar et al.45 | 52 | Pakistan (A) | 1 (1.9) | 1/36 | 0/15 | 1/19 | 0/32 | NA | NA |
| Herrera-Goepfert et al.46 | 330 | Mexico (H) | 24 (7.3) | 13/160 | 11/146 | 4/137 | 20/169 | 2/12† | 22/294† |
| Luo et al.47 | 185 | China (A) | 13 (7.0) | 13/121 | 0/51 | NA | NA | NA | NA |
| Begnami et al.48 | 208 | Brazil (H) | 25 (12.0) | 20/68‡ | 5/35‡ | 7/62‡ | 18/41‡ | NA | NA |
| Campos et al.49 | 368 | Columbia (H) | 42 (11.4) | 34/196 | 8/130 | 15/189 | 27/137 | 4/36† | 20/155† |
| Abdirad et al.50 | 273 | Iran (A) | 9 (3.3) | 8/188 | 1/76 | 1/151† | 8/113† | 0/2 | 9/262 |
| Total | 9738 | NA | 857 (8.8) | 618/4535 | 159/2708 | 213/3076 | 331/3029 | 93/956 | 288/2459 |
- † The cases have insufficient clinical information.
- ‡ Case–control study.
- AGC, advanced gastric cancer; A, Asian; B, Black; C, Caucasian; EBV, Epstein–Barr virus; EBVaGC, EBV-associated gastric adenocarcinoma; EGC, early gastric cancer; H, Hispanic; NA, not available.
Ethnicity
To evaluate racial differences in EBVaGC, we divided the population into Caucasian, Hispanic, and Asian, which produced groups of 1809, 1541, and 6358 patients, respectively.3–50 EBVaGC was detected in 176 (9.7%) of the 1809 Caucasian patients, in 143 (9.3%) of the 1541 Hispanic patients, and in 533 (8.4%) of the 6358 Asian patients. The overall ORs for EBVaGC in the Caucasian and Hispanic patients were 2.127 (95% CI: 1.383–3.272; P = 0.001) (Fig. 1) and 3.536 (95% CI: 2.143–5.836; P < 0.001) (Fig. 2), respectively. No significant association was found between EBVaGC and Asian patients (OR = 0.698; 95% CI: 0.465–1.046; P = 0.082).
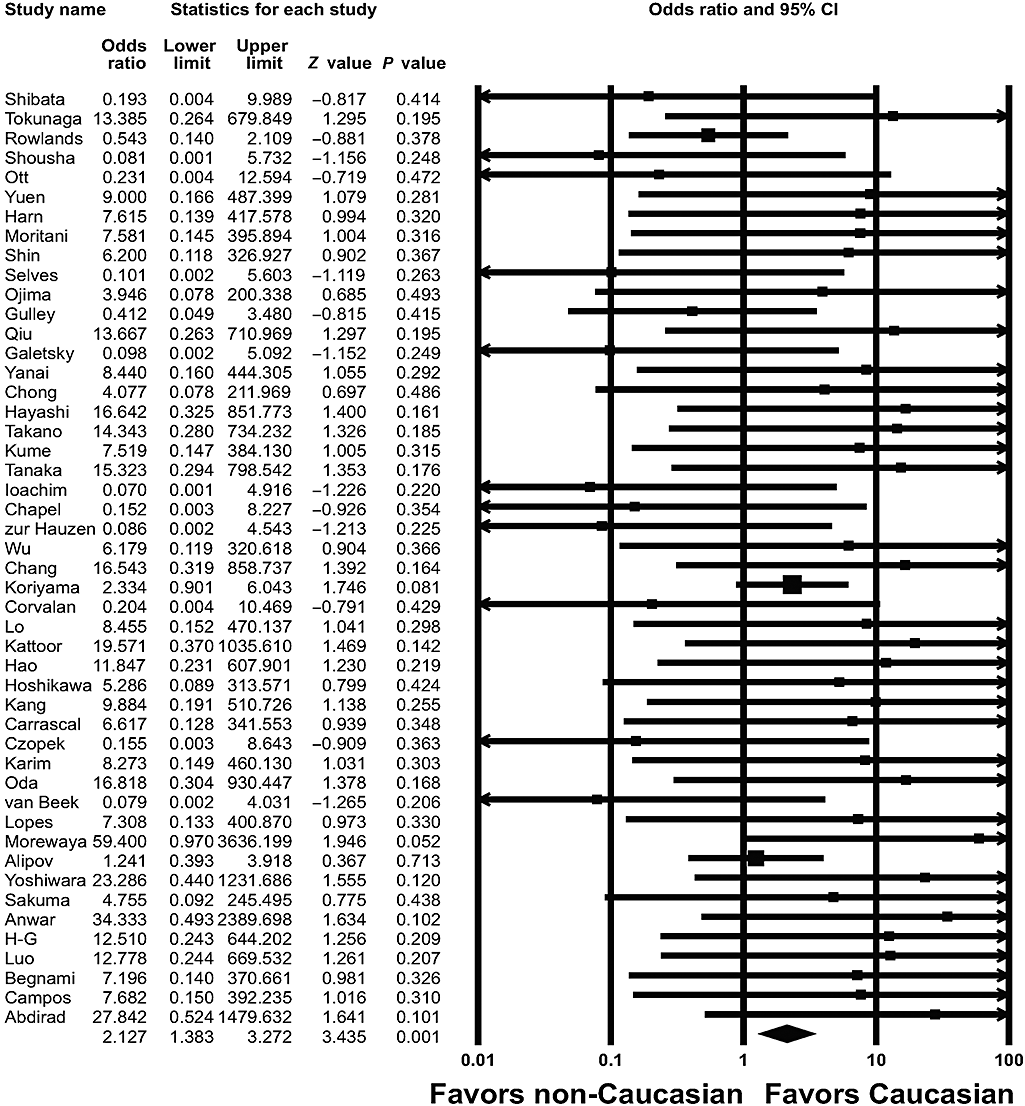
Odd ratios with corresponding 95% CIs of the individual studies and the pooled data for the association of Epstein–Barr virus-associated gastric carcinoma with Caucasian ethnicity. The graph demonstrates the effect sizes and 95% CIs for each study and the overall data.
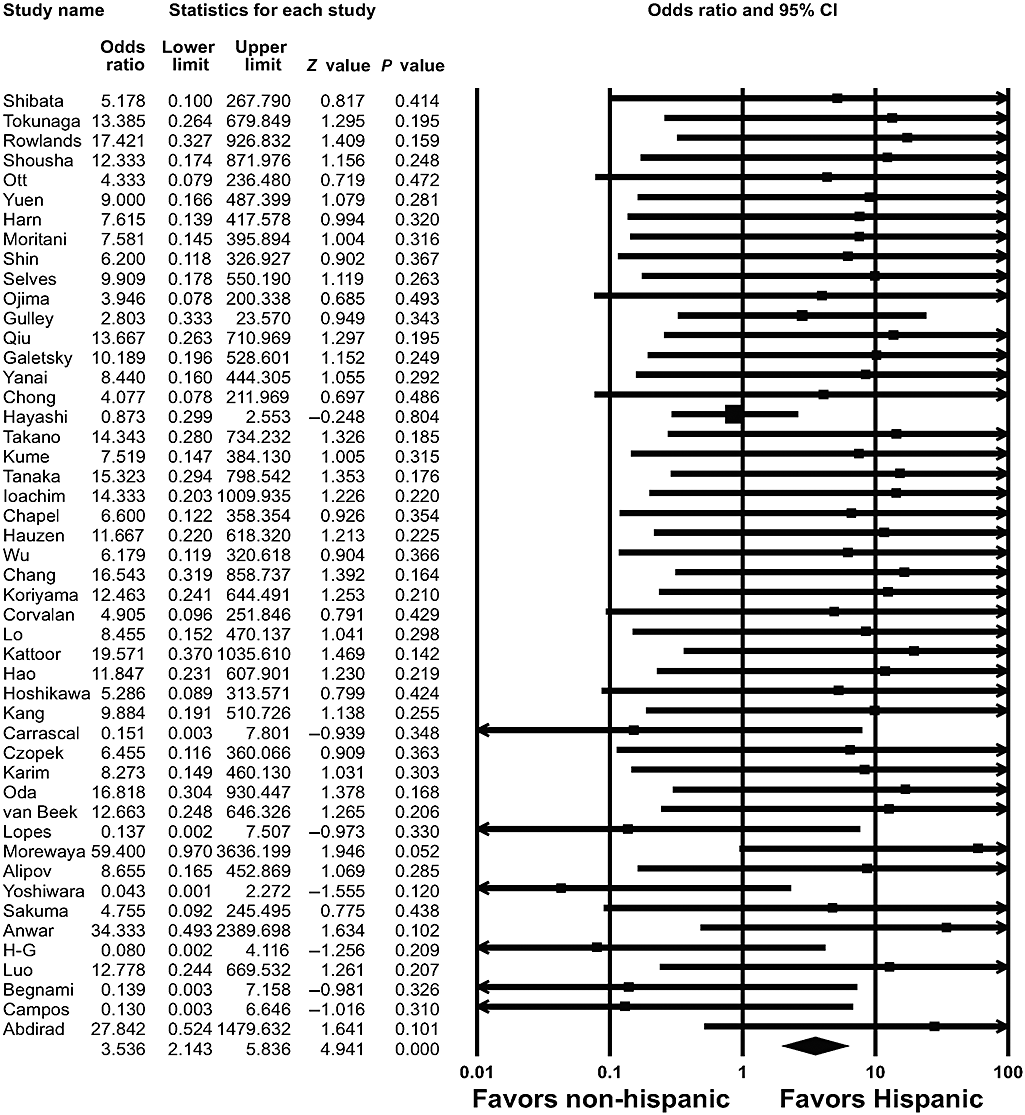
Pooled estimates of the association between Epstein–Barr virus-associated gastric carcinoma and Hispanic ethnicity.
Gender
Gender information was available for 40 of the 48 studies for a total of 5153 male and 2867 female patients.3–6,8–18,20,21,24,26–29,31,32,34–37,39–50 EBVaGC was detected in 618 (12.0%) of the 5153 male and in 159 (5.5%) of the 2867 female patients. Based on these findings, there was a high association of EBVaGC with the male gender, but not with the female gender (OR = 2.108; 95% CI: 1.742–2.550; P < 0.001).
Age
Four studies presented clinical data for the mean age with accurate P values.18,24,34,39 The mean ages of the patients with EBVaGC ranged from 50 to 68 years, whereas the mean ages of patients with EBV-negative carcinoma ranged from 56 to 72 years. Thus, a strong association between mean age and EBVaGC was identified (WMD = −0.372; 95% CI: −0.600-−0.145; P = 0.001).
Tumor location
To evaluate the prevalence of EBVaGC according to the anatomic sites of the stomach, we analyzed the tumor location of EBVaGC. The stomach was divided into the cardia (upper 1/3), body (mid 1/3), and antrum (lower 1/3) regions. The 33 studies available for this meta-analysis consisted of 1086 cases in the cardia, 1826 in the body, and 3118 in the antrum.4–6,8,10–13,15–18,20,24,26,28,29,31,32,34–36,39–44,46–50 EBVaGC was detected in 128 (11.8%) of the 1086 cases in the cardia, in 238 (13.0%) of the 1826 cases in the body, and in 183 (5.9%) of the 3118 cases in the antrum, indicating that there was a significant association between the tumor location in the stomach and EBVaGC. The overall OR for EBVaGC in the cardia and body was 1.687 (95% CI: 1.330–2.139; P < 0.001) (Fig. 3) and 2.144 (95% CI: 1.614–2.848; P < 0.001), respectively; statistical heterogeneity was found among these studies (Q = 46.388, d.f. = 32, P = 0.048). In contrast, the overall OR for EBVaGC in the antrum was 0.415 (95% CI: 0.340–0.505; P < 0.001), and there was no statistical heterogeneity among these studies (Q = 43.497, d.f. = 32, P = 0.085).
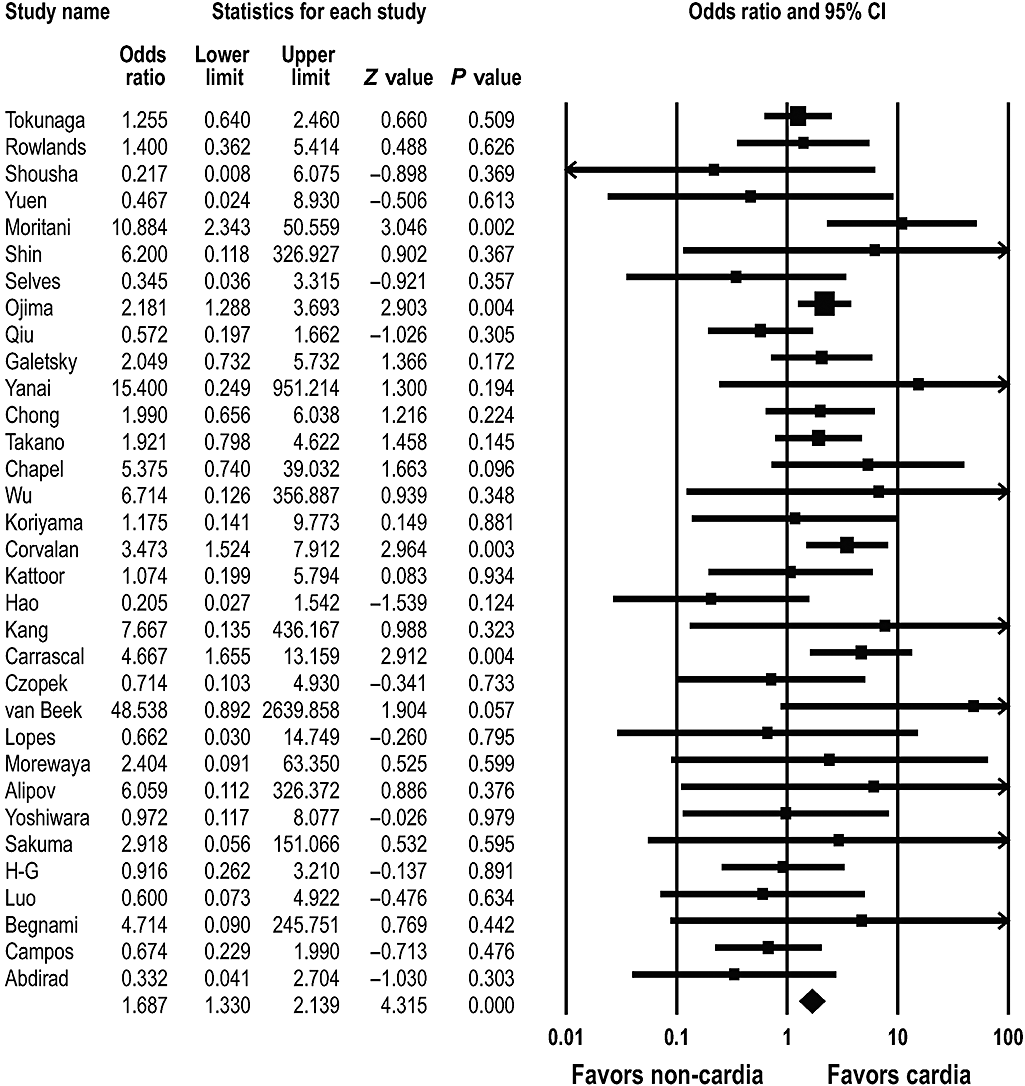
Pooled estimates of the association between Epstein–Barr virus-associated gastric carcinoma and tumor occurrence in the cardia.
Histological subtype (Lauren's classification)
We identified 34 studies that addressed the frequency of EBVaGC according to the histological subtype.3–5,7,8,10,12–18,24,26–29,31,32,35–37,39–46,48–50 EBVaGC was present in 331 (9.9%) of 3360 diffuse type cancers and in 213 (6.5%) of 3289 intestinal type cancers; significant statistical heterogeneity was found in the above-mentioned studies (Q = 66.391, d.f. = 33, P = 0.001). The overall OR for EBVaGC in the diffuse type cancer was 1.745 (95% CI: 1.255–2.427; P = 0.001) (Fig. 4).
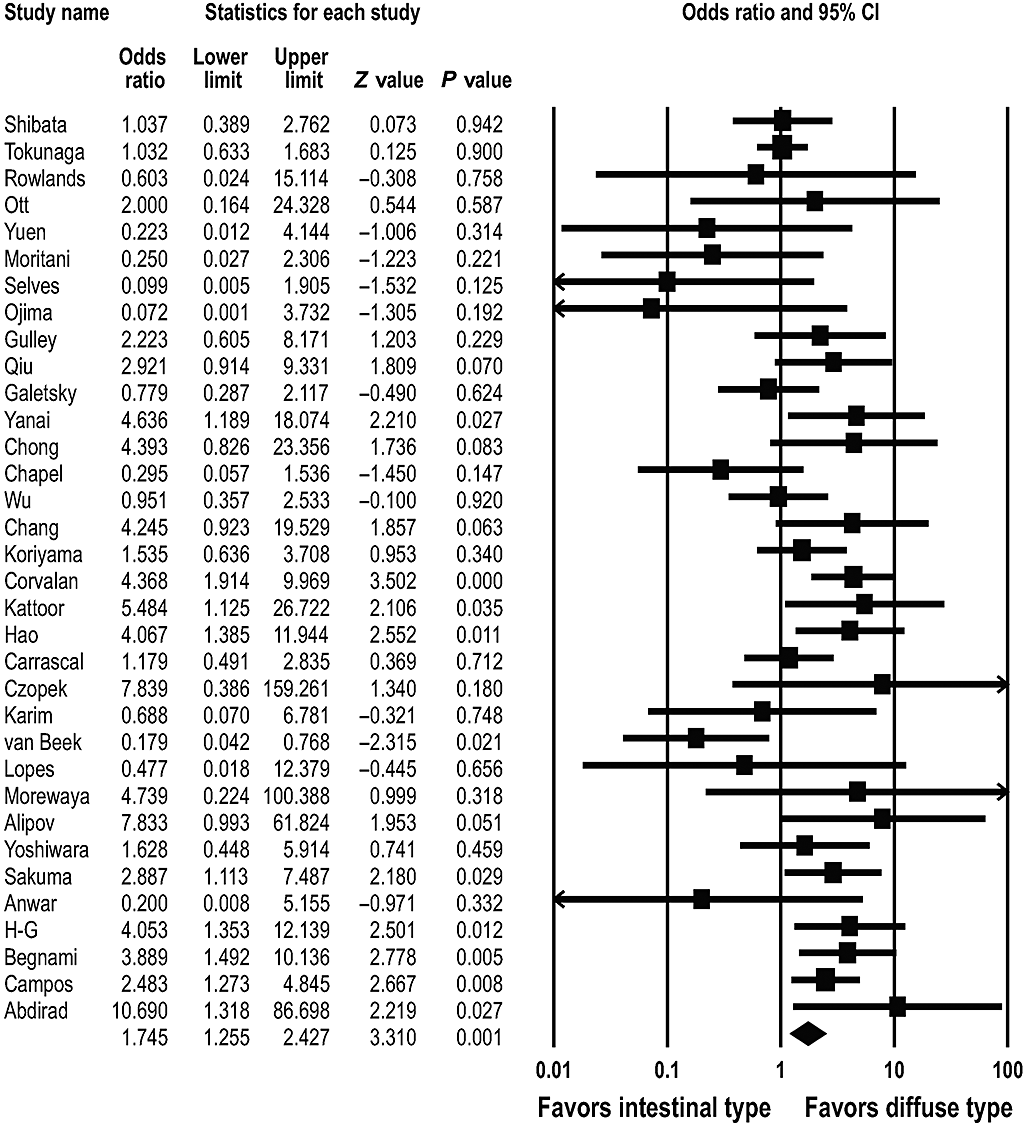
Pooled estimates of the association between Epstein–Barr virus-associated gastric carcinoma and the histological subtype.
Tumor depth
Fourteen studies consisted of 2747 advanced gastric cancers and 1049 early gastric cancers.4,8,10,12,13,17,20,21,26,27,44,46,49,50 EBVaGC was present in 288 (10.5%) of the 2747 advanced gastric cancers and in 93 (8.9%) of the 1049 early gastric cancers. There was no significant association between EBVaGC and the tumor invasion of the muscularis propria (OR = 1.141; 95% CI: 0.871–1.496; P = 0.339). With respect to the tumor depth, seven studies described 968 cases with T1 or T2 stage and 771 cases with T3 or T4 stage.8,13,24,27,36,39,50 EBVaGC was found in 97 (10.0%) of the T1 and T2 cases and 72 (9.3%) of the T3 and in T4 cases. There was no significant association with respect to the tumor depth (OR = 0.931; 95% CI: 0.644–1.346; P = 0.705).
Lymph node metastasis
Ten studies were identified that evaluated the association of EBVaGC and lymph node metastasis, and these consisted of 1022 cases with and 719 cases without lymph node metastasis.18,21,24,26,27,36,39,44,47,48 EBVaGC was observed in 102 (10.0%) of the 1022 cases with lymph node metastasis and in 107 (14.9%) of the 719 cases without lymph node metastasis. There was no significant association between lymph node metastasis and EBVaGC (OR = 0.852; 95% CI: 0.614–1.181; P = 0.336).
Clinical stage
Four studies presented data on EBVaGC according to the clinical stage.11,36,39,47 These studies were composed of 550 cases with stage I and II and 330 cases with stages III and IV. EBVaGC was detected in 47 (8.5%) of the lower stage cancers and in 24 (7.3%) of the higher stage cancers. There was no significant association between the clinical stage and the presence of EBVaGC (OR = 0.747; 95% CI: 0.422–1.322; P = 0.316).
Lymphoepithelial carcinoma
To compare the prevalence of EBV infection between LEC and non-LEC adenocarcinoma, the cases with LEC and gastric carcinoma with lymphoid stroma were extracted from the publications. Twenty studies were appropriate for determining the frequency of EBVaGC in LEC and/or gastric carcinoma with lymphoid stroma.4–10,12,13,19,23,24,26,27,29,34,36,38,40,46 EBV was detected in 152 (86.4%) of the 176 LEC cases and in 224 (6.1%) of the 3694 non-LEC cases. The prevalence of EBV involvement in LEC was significantly higher than that for the non-LEC cases (OR = 80.881; 95% CI: 49.600–131.888; P < 0.001) (Fig. 5).
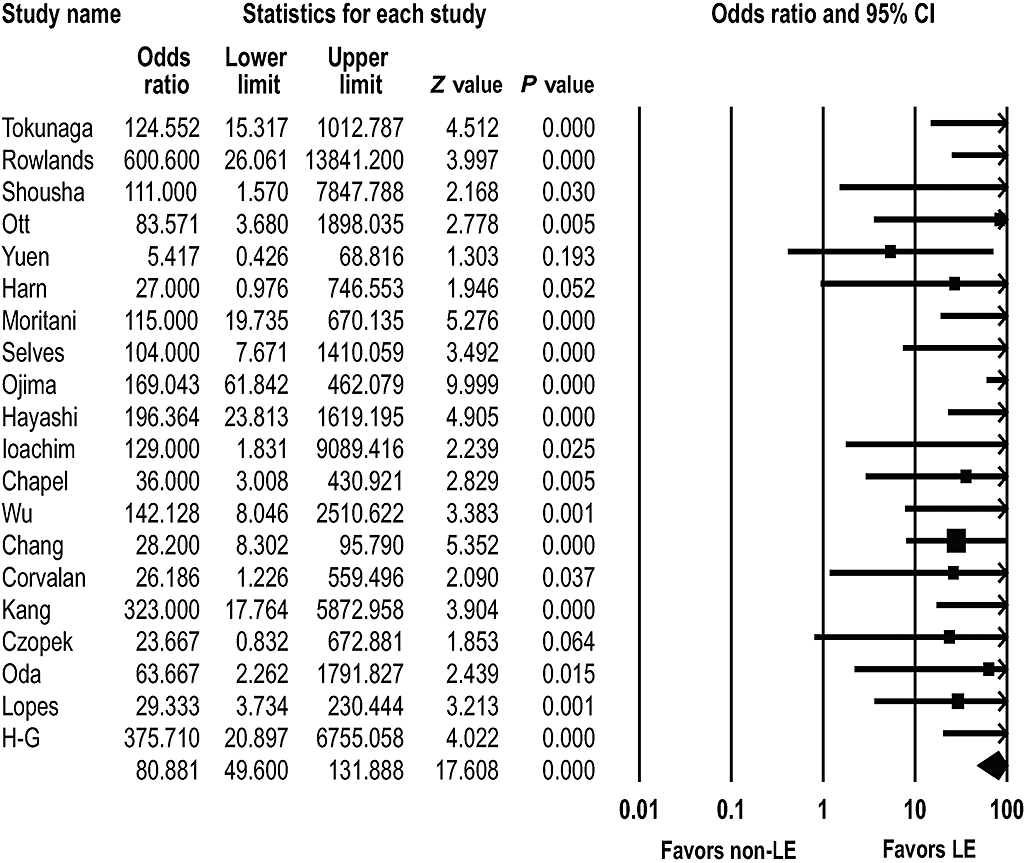
Pooled estimates of the association between Epstein–Barr virus infection and lymphoepithelial (LE) carcinoma.
Gastric remnant carcinoma
Six studies presented data on the prevalence of EBVaGC in remnant and non-remnant gastric cancers.12,24,29,47,51,52 EBVaGC was detected in 22 (25.6%) of the 86 remnant cancer patients and in 185 (7.1%) of the 2603 non-remnant cancer patients. The prevalence of EBV involvement in gastric remnant cancer was much higher than the non-remnant cancer (OR = 5.067; 95% CI: 3.011–8.527; P < 0.001) (Fig. 6).
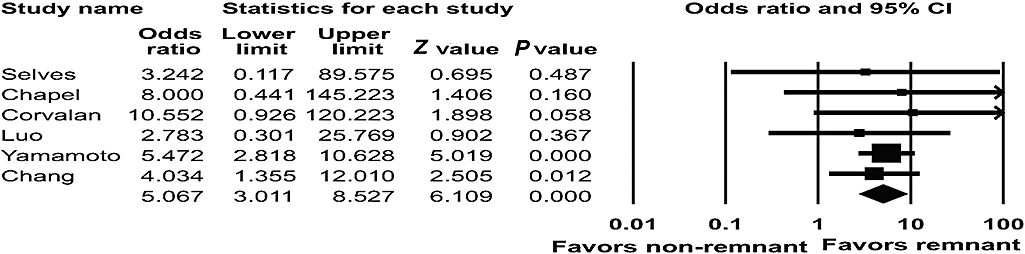
Pooled estimates of the association between Epstein–Barr virus infection and gastric remnant cancer.
Three studies described EBV-associated gastric remnant cancer according to the type of surgical procedure that had been performed.51,54,55 EBV-associated gastric remnant cancer was observed in seven (14.0%) of 50 Billroth I anastomosis patients and in 30 (33.0%) of 91 Billroth II anastomosis patients. There was a significant association between the type of operation and EBV involvement (OR = 2.531; 95% CI: 1.023–6.262; P = 0.045).
Several studies reported on EBV-associated gastric remnant cancer according to gender,52,54–56 tumor location, the histological subtype,51,52,54,55 or the depth of invasion;52,55 our analysis indicated that there was no statistically significant association between EBV-associated gastric remnant cancer and these parameters.
Helicobacter pylori infection
Six studies addressed the significance of H. pylori infection in EBVaGC.12,26,47,57–59H. pylori infection was found in 42 (53.2%) of 79 EBVaGC cases and in 247 (56.3%) of 439 EBV-negative cases. Thus, there was no significant association between H. pylori infection and EBVaGC (OR = 0.799; 95% CI: 0.473–1.351; P = 0.402).
TP53 expression and p53 mutation
Seventeen studies determined the presence of a TP53 expression in EBVaGC by performing immunohistochemistry.14,21,24,26,27,31,32,48,54,60–67 A TP53 expression was observed in 149 (36.2%) of 412 EBVaGC and in 717 (47.9%) of 1496 EBV-negative carcinomas. While statistical heterogeneity was found among the studies (Q = 36.368, d.f. = 16, P = 0.003), there was no significant association between a TP53 expression and EBVaGC (OR = 0.661; 95% CI: 0.431–1.015; P = 0.058). Although the immunohistochemical expression of TP53 is usually associated with p53 mutation, we also examined the association between p53 mutation and EBVaGC. Two studies determined p53 mutation in EBVaGC by single-strand conformational polymorphism analysis and/or direct DNA sequencing.68,69 However, no statistical significance was found between p53 mutation and EBVaGC (OR = 0.434; 95% CI: 0.095–1.981; P = 0.281).
CpG island methylator phenotype
Three studies were concerned the CIMP status in EBVaGC.69–71 Thirty-two (94.1%) of 34 EBVaGC cases and 47 (21.8%) of 216 EBV-negative gastric cancers were in the CIMP-high group. There was a close relationship between a CIMP-high status and EBVaGC (OR = 42.844; 95% CI: 11.274–162.820; P < 0.001) (Fig. 7).
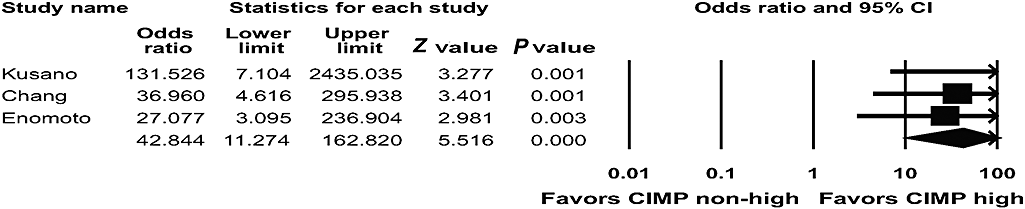
Pooled estimates of the association between Epstein–Barr virus-associated gastric carcinoma and the CpG island methylator phenotype (CIMP) status.
Discussion
This meta-analysis revealed that EBVaGC is highly correlated with ethnicity, it is more predominant in men and in younger individuals, and it most frequently occurs in the body and cardia of the stomach. EBVaGC is also highly associated with the diffuse histological type, LEC, postoperative remnant cancer, and a CIMP-high status, but it is not associated with tumor depth, lymph node metastasis, the clinical stage, and H. pylori infection.
The association between ethnicity and EBVaGC, except for LEC, is noteworthy. It is interesting that the occurrence of EBVaGC was closely associated with Caucasian and Hispanic populations, with twofold and threefold ORs, respectively. Paradoxically, our meta-analysis showed that the development of EBVaGC is lowest within the geographic regions that are endemic for gastric adenocarcinoma. The discrepancy between the incidence of EBVaGC and conventional gastric cancer in endemic areas suggests that the risk factors for EBVaGC may be rather different from those of conventional gastric cancer.
Epstein–Barr virus-associated gastric carcinoma was found to be highly associated with the male gender. Numerous studies have reported that EBVaGC occurs at a significantly higher frequency in male patients,3,4,9,16,27,28,31,32,35,39,42,47,49 while far fewer studies have reported that EBVaGC occurs at a similar frequency in male and female genders.10,43,46 Our meta-analysis suggests that the apparent gender preponderance of EBVaGC can be attributed to both the male genetic status and male lifestyle.
Epstein–Barr virus-associated gastric carcinoma was significantly associated with younger individuals, based on four studies that presented the mean age and accurate P values. Kang et al.34 and van Beek et al.39 reported that EBVaGC is highly associated with a younger age, while Chong et al.18 and Chapel et al.24 reported that EBVaGC is not related to age. In addition, several additional studies beyond those above-mentioned have also reported that EBVaGC is significantly associated with a younger age.14,31,35 Therefore, we speculated that EBVaGC may develop via the reactivation of EBV in adults with higher risk factors, rather than developing in aged persons with suppressed immune systems.
This meta-analysis confirmed that EBVaGC arises more frequently in the body and cardia. The overall ORs for EBVaGC in the body, cardia, and antrum were 2.1, 1.7, and 0.4, respectively, suggesting that the body and cardia of the stomach may be a more suitable environment for EBV infection than the antrum.
Our meta-analysis indicated that the diffuse type EBVaGC was more common than the intestinal type EBVaGC. Many studies have reported that EBVaGC is significantly associated with the diffuse type,17,27,29,32,42,44,48–50 whereas only one study by van Beek et al.39 reported that EBVaGC is significantly associated with the intestinal type. Additionally, Arikawa et al. reported that EBVaGC is associated with a lace-like growth pattern and lymphocytic infiltration in and around tumor nests.57
Epstein–Barr virus-associated gastric carcinoma was not associated with the depth of tumor invasion, lymph node metastasis, or the clinical stage. Ojima et al.13 reported that EBVaGC exhibits an advanced tumor depth, while Takano et al.20 reported that EBVaGC is limited to the submucosa. The study by van Beek et al.39 reported that EBVaGC is closely related to a lower rate of lymph node metastasis and lower clinical stages, yet other studies11,18,24,26,27,36,44,47,48 failed to conclusively demonstrate any relationship between EBVaGC and lymph node metastasis or the clinical stage. This meta-analysis suggested that the presence of EBV in gastric carcinoma may not be a significant prognostic factor.
There has been considerable controversy with respect to the effects of EBVaGC on patient survival. Several studies have described the differences in patient survival between EBVaGC and EBV-negative gastric cancers;14,21,26,27,36,39,72 however, we could not analyze the results of these studies because of insufficient data. Nonetheless, van Beek et al. reported that EBVaGC is significantly associated with better cancer-specific survival and a longer disease-free period.39 In addition, Wu et al.26 reported that the survival rates of gastric LEC are much better than those of EBV-positive non-LEC and EBV-negative gastric cancers, although the survival rates were not different between EBV-positive non-LEC and EBV-negative gastric cancer. Other studies14,21,27,36,72 failed to show a significant relationship between the patient survival and EBVaGC, as compared with EBV-negative gastric cancer.
Our meta-analysis confirmed that EBVaGC is significantly associated with gastric LEC. Consistent with previous studies, the overall OR of EBV involvement in gastric LEC was 80.9.
Epstein–Barr virus-associated gastric carcinoma was found to be associated with gastric remnant cancer. The overall OR of EBV involvement in gastric remnant cancer was 5. Specifically, EBV-associated remnant cancer developed more frequently in the patients with Billroth II anastomosis, suggesting that mucosal damage after undergoing Billroth II gastrectomy is linked to EBV-associated gastric remnant cancer. One possibility to explain this observation is that the gastric mucosa might be injured by duodenogastric reflux, which contains pancreatic and bile juice.
Epstein–Barr virus-associated gastric carcinoma was significantly associated with a CIMP-high status. CIMP is characterized by simultaneous methylation of CpG islands in multiple genes, and this is now recognized as an important mechanism in gastrointestinal carcinogenesis. The association found here suggests that presence of EBV results in methylation of many of the tumor suppressor genes that are related to gastric carcinogenesis.
There are several limitations of our study. First, we could not exclude the possibility of immigrants of Asian origin living in the USA or other geographic regions, in the meta-analysis of the association of EBVaGC with ethnicity. Second, as was mentioned earlier, we could not analyze the patient survival of EBVaGC compared with that of EBV-negative gastric cancer because the number of studies and their data presentation were inappropriate for this meta-analysis. Third, although p73 and Hoxa10 are known to be specifically methylated in EBVaGC,73 we could analyze only the CIMP status of EBVaGC because the epigenetic studies used different sets of indicator genes. Finally, we analyzed only the articles that were published before December 2007. After that, several papers have reported about the signaling pathway of LMP2A,74 DNA methylation-mediated repression of tumor suppressor genes,73 and bortezomib-induced EBV thymidine kinase-targeted radiotherapy,75 but more of this type of data need to be accumulated before performing meta-analysis on it.
In summary, our study indicates that EBVaGC has unique clinicopathological and molecular characteristics. The relative frequency of EBVaGC was significantly higher among the Caucasian and Hispanic populations. EBVaGC can be characterized as being predominant in men and also in younger individuals. It generally exhibits a diffuse histological type, and it occurs most frequently in the body and cardia. EBVaGC was also found to be highly associated with LEC, remnant cancer, and a CIMP-high status, whereas it was not related to the depth of invasion, lymph node metastasis, the clinical stage, a TP53 expression, or H. pylori infection.




