The Effects of Increased Testicular Temperature on Testis-Specific Isoform of Na+/K+-ATPase in Sperm and its Role in Spermatogenesis and Sperm Function
Contents
Impaired testicular thermoregulation is commonly implicated in abnormal spermatogenesis and impaired sperm function in animals and humans, with outcomes ranging from subclinical infertility to sterility. Bovine testes must be maintained 4–5°C below body-core temperature for normal spermatogenesis. The effects of elevated testicular temperature have been extensively studied in cattle using a scrotal insulation model, which results in abnormal spermatogenesis and impaired sperm morphology and function. Using this model and proteomic approaches, we compared normal and abnormal sperm (from the same bulls) to elucidate the molecular basis of impaired function. We identified a cohort of sperm functional proteins differentially expressed between normal vs abnormal sperm, including a testis-specific isoform of Na+/K+-ATPase. In addition to its role as a sodium pump regulating sperm motility, Na+/K+-ATPase is also involved as a signalling molecule during sperm capacitation. In conclusion, because of its involvement in regulation of sperm function, this protein has potential as a fertility marker. Furthermore, comparing normal vs abnormal sperm (induced by scrotal insulation) is a useful model for identifying proteins regulating sperm function.
Regulation of Testicular Temperature
In mammals with a scrotum, testes are maintained 2–6°C (Waites 1970; Setchell 1978) below core body temperature to ensure normal spermatogenesis; this is achieved by the combined action of the scrotum, testes and testicular vasculature (Cook et al. 1994). The highly coiled testicular artery and the associated venous network (pampiniform plexus), which are collectively termed the testicular vascular cone, enable counter-current heat exchange between arterial and venous blood vessels, thereby cooling arterial blood before it enters the testis (Cook et al. 1994). In addition, the scrotal musculature (Setchell and Carrick 1973) and tunica dartos keep the testis away from the body, scrotal sweat glands provide evaporative cooling (Blazquez et al. 1988), and radiation of heat from the scrotal surface (Coulter et al. 1988) provides additional cooling.
Increased ambient temperature and seasonal climatic changes (Chacon et al. 2002) decrease semen quality and sperm production in cattle (Meyerhoeffer et al. 1985; Coulter et al. 1997) and swine (Wettemann et al. 1976; Suriyasomboon et al. 2005). In humans, several environmental, lifestyle-related risk factors and pathological conditions of the testis (e.g. varicocele) have been implicated in the elevation of scrotal/testicular temperature and subsequent deterioration of semen quality (Chen and Chen 2011, Sheykin et al. 2005, Hjollund et al. 2000).
The current dogma is that the testis operates on the brink of hypoxia under physiological conditions (Waites and Setchell 1964) and in situations of increased scrotal/testicular temperatures, metabolism and oxygen utilization increase, but blood flow to the testis remains constant, resulting in frank hypoxia (Setchell 1998). We tested this hypothesis by exposing mice to environmental temperatures of 20 and 36°C and concurrently exposing them to hyperoxic conditions to prevent hypoxia induced by elevated testicular temperature. Hyperoxia (known to increase testicular oxygen saturation) did not protect against hyperthermia-induced deterioration in sperm quality. Furthermore, because sperm characteristics were not significantly different between mice exposed to hypoxic vs normoxic or hyperoxic conditions at 20°C, we concluded that the effects of increased testicular temperature on semen quality were because of hyperthermia per se and not hypoxia (Kastelic et al. 2008). It was noteworthy that similar results were obtained with a scrotal insulation model in rams (Kastelic J, Wilde R, Bielli A, Genovese P, Bilodeau-Goeseels S, Thundathil J, unpublished).
Abnormal Spermatogenesis Induced by Scrotal Insulation as an Experimental Model for Investigation of Spermatogenesis and Sperm Function in Bulls
Effect of scrotal temperature on spermatogenesis and sperm function
Increase in testicular temperature, because of elevated ambient temperature or scrotal insulation, impairs spermatogenesis and reduces semen quality and sperm production. Pachytene spermatocytes, spermatids and epididymal sperm are the germ cells most susceptible to heat (Rockett et al. 2001; Zhu and Setchell 2004); this sensitivity has been well documented in mice (Meistrich et al. 1973), bulls (Vogler et al. 1991), humans (Levine et al. 1990) and rams (Mieusset et al. 1991).
Elevated testicular/epididymal temperature (because of scrotal insulation) has deleterious effects on sperm morphology (Vogler et al. 1993; Barth and Bowman 1994), motility (Brito et al. 2004; Fernandes et al. 2008; Newton et al. 2009), viability (Vogler et al. 1991; DeJarnette et al. 2000), sperm fertilizing capacity and developmental competence of resulting embryos (Thundathil et al. 2000, 2001a,b; Walters et al. 2005b, 2006; Fernandes et al. 2008). Specific sperm abnormalities emerge at consistent intervals after scrotal insulation (Barth and Bowman 1994; Acevedo 2001; Walters et al. 2005a; Fernandes et al. 2008; Newton et al. 2009); the type of abnormality corresponds with where germ cells are in the spermatogenic process when testicular temperature was increased (Hales et al. 2005). Furthermore, the appearance of morphologically abnormal sperm was consistently accompanied by reduced sperm motility. Because sperm use ATP from mitochondrial oxidative phosphorylation to support motility (Ruiz-Pesini et al. 1998), perhaps heat stress has a direct effect on germ cell mitochondria.
Abnormal sperm fail during various steps of gamete interaction, depending on the nature of the abnormality (Thundathil et al. 1998, 1999, 2000, 2001a,b). Furthermore, apparently normal sperm coexisting in a semen sample with a high percentage of abnormal sperm have impaired sperm function. Some forms of abnormal sperm can penetrate the oocyte and initiate fertilization, but the resulting zygotes fail at various stages of preimplantation development (Thundathil et al. 2000, 2001a, b; Walters et al. 2005a). Some of these impaired sperm functions may be due to the effects of elevated testicular temperatures on sperm DNA (Steinberger 1991; Sailer et al. 1997; Banks et al. 2005), sperm proteins or both.
Effect of increased testicular temperature on expression pattern of sperm proteins
Scrotal insulation induces abnormal spermatogenesis and impairs sperm function. Because sperm DNA is transcriptionally inactive, sperm functions are regulated by proteins already present in sperm without additional protein synthesis. Therefore, we hypothesized that elevated testicular temperature alters the content of proteins in sperm, impairing their function. To test this hypothesis, we used scrotal insulation to induced abnormal spermatogenesis in Holstein bulls, with pre- and post-insulation sperm compared for expression of sperm proteins (Newton et al. 2009). Whereas expression of Hexokinase-1, the α-4 subunit of Na+/K+-ATPase and the testicular isoform of angiotensin converting enzyme (tACE) were decreased, expression of tissue inhibitor metalloprotease-2 (TIMP-2) was higher in morphologically abnormal sperm. Tissue inhibitor metalloprotease-2 is abundant in epididymal fluids (Belleannéea et al. 2011) and the equilibrium between the production of proteinases and proteases and their inhibitors (e.g. TIMP-2) is critical for sperm maturation in the epididymis (Me′tayer et al. 2002). Perhaps proteinases and proteases arising from the damaged membrane of abnormal sperm increase TIMP-2 production in an attempt to preserve the membrane integrity, which may account for the increase in TIMP-2 content seen in insulated bulls compared with their controls. Similarly, there was a significant decrease in PH-20 (a sperm adhesion molecule (SPAM1) implicated in zona-pellucida binding; Fleming et al. 2004) in ram sperm following scrotal insulation. Therefore, scrotal insulation is a suitable research model to identify specific proteins involved in regulation of sperm function. Because our recent research focus is on Na+/K+-ATPase (a protein differentially expressed between pre- vs post-insulation sperm samples), the remainder of this review is focused on current knowledge regarding the role of this protein in regulation of sperm function.
Structure of Na+/K+-ATPase
Na+/K+-ATPase is a ubiquitous integral membrane protein responsible for maintaining Na+ and K+ gradients across the plasma membrane of most mammalian cells. It utilizes energy from hydrolysis of one molecule of ATP to exchange three cytoplasmic Na+ ions for two extracellular K+ ions (Blanco and Mercer 1998; Kaplan 2002). This enzyme has a pivotal role in several cellular functions that depend (directly or indirectly) on transmembrane Na+ and K+ concentrations. In that regard, this enzyme contributes to the maintenance of cell volume and pH, resting membrane potential, osmotic balance and generation of the Na+ gradient for coupled transmembrane ion transport (Sweadner 1989; Skou and Esmann 1992). The functional Na+/K+-ATPase consists of two subunits, the α subunit (112 kDa) and the β subunit (35–60 kDa, depending on glycosylation; Blanco and Mercer 1998). The α polypeptide is a multispanning membrane protein, which functions as a catalytic unit, with roles in ion translocation and ATP hydrolysis (Jorgensen et al. 2003). Furthermore, it has a binding site for digoxin and ouabain, potent inhibitors of Na+/K+-ATPase (Jorgensen et al. 2003). The β subunit is a single membrane-spanning polypeptide, essential for the enzyme’s activity, as well as folding and localization within the membrane (Geering 2002).
Expression of Na+/K+-ATPase in the Testis and Sperm
There are four α isoforms (α1, α2, α3, and α4) and three β isoforms (β1, β2, and β3) in mammalian tissues (Blanco and Mercer 1998; Mobasheri et al. 2000). The α1 and β1 isoforms are expressed in almost every cell (function as ‘housekeeping’ Na+/K+-ATPase), whereas other α polypeptides have a more restricted expression and may have specific roles (Mobasheri et al. 2000). Testes (Shamraj and Lingrel 1994; Underhill et al. 1999; Blanco et al. 2000) and Sertoli cells (Konrad et al. 2011) express the α4 isoform along with the highly ubiquitous α1 isoform (Underhill et al. 1999; Blanco et al. 2000). Moreover, two-thirds of total Na+/K+-ATPase activity of sperm are attributed to the α4 isoform (Wagoner et al. 2005). The α1 and α4 subunits are co-expressed in sperm with the β1 and β3 isoforms; the α4 isoform associates with both the β subunits equally, with similar kinetic properties (Arystarkhova and Sweadner 1997). In addition to α1, α4, β1 and β3 isoforms, the α3 and β2 subunits are also present in bovine sperm (Hickey and Buhr 2012). The α4 isoform has high affinity for Na+ but low affinity for K+, and a very high sensitivity to ouabain, in contrast with other isoforms (Woo et al. 1999).
Because of the tissue-specific expression of α4 isoform of Na+/K+-ATPase in the testis, this protein has received considerable attention regarding its role in regulation of spermatogenesis. In rats (Woo et al. 2000; Wagoner et al. 2005) and humans (Hlivko et al. 2006), expression of α4 isoform peaked in mature testis, whereas the α1 isoform was held constant throughout spermatogenesis. Although the α4 isoform was mostly present in the mid-piece and principal piece region of the rat and human sperm flagellum, respectively (Woo et al. 2000; Hlivko et al. 2006), α1 was present throughout the rat sperm flagellum (Wagoner et al. 2005). In our studies with bovine sperm, α4 was restricted to the head (Newton et al. 2010). Perhaps there are differences among species in the role of the α4 isoform in regulation of sperm function.
Na+/K+-ATPase and Regulation of Sperm Motility
Na+/K+-ATPase activity appears to influence sperm motility, because of its indirect role in the regulation of pH and resting membrane potential. The α4 isoform functionally couples to the Na+/H+ exchanger (NHE) to regulate intracellular pH in sperm (Woo et al. 2002). The NHE is a transmembrane protein (present in sperm flagella) that use the Na+ gradient (established by the Na+/K+-ATPase) to remove H+ from the cell in exchange for Na+ (Counillon and Pouyssegur 2000; Shull et al. 2000). Therefore, inhibition of Na+/K+-ATPase eliminates the Na+ gradient used by the NHE to move H+ out of the cell. Loss of NHE activity may lead to acidification of the intracellular compartment, which suppresses movement of dynein and reduces flagellar movement (Woo et al. 2002).
Na+/K+-ATPase affects sperm motility in various species. Inhibition of α4 isoform with ouabain inhibits several motion characteristics in rat sperm, including total and progressive motility and several sperm kinematic parameters (Jimenez et al. 2010). In a subsequent study, these researchers overexpressed the α4 isoform of rat Na+K+-ATPase in mouse sperm; the resulting transgenic sperm had hyperactivated motility under non-capacitating conditions, with additional augmentation under capacitating conditions (Jimenez et al. 2011b). These authors speculated that the α4 isoform may contribute to sperm capacitation by enhancing hyperactivated motility. Sanchez et al. (2006) observed that incubation of human sperm at room temperature with ouabain (at concentrations inhibitory to either α4 or both α4 and α1) inhibited total motility of sperm. However, these treatments did not modulate other sperm motion parameters. Mice deficient in the α4 isoform (knockout) were sterile and had significantly reduced sperm motion parameters, further indicating the role of α4 isoform of Na+/K+-ATPase in sperm motility (Jimenez et al. 2011a). Yet, it is important to note that sperm from these knockout mice were morphologically abnormal (a characteristic bend in the flagellum). This sperm defect may also have contributed to reduced motility.
Overall, the above-mentioned studies implicated Na+/K+-ATPase in modulating the motility characteristics of sperm from several species. However, in our studies with bovine sperm, the α4 isoform of Na+/K+-ATPase was exclusively localized to the sperm head (Newton et al. 2010), and incubation of ejaculated fresh sperm with ouabain did not significantly decrease total sperm motility (Thundathil et al. 2006), suggesting species differences in the role of Na+/K+-ATPase in regulation of sperm function. Because ouabain is secreted from the bovine adrenal gland (Laredo et al. 1994) and is present in bovine vaginal fluid (Daniel et al. 2010), we inferred that the interaction of ouabain with Na+/K+-ATPase may be involved in the regulation of sperm functions other than motility in this species.
Na+/K+-ATPase and Regulation of Sperm Capacitation
Role of Na+/K+-ATPase as an ion exchanger during capacitation
During capacitation, sperm undergo a series of structural and functional modifications in the female reproductive tract (Yanagimachi 1994). These biochemical changes include hyperpolarization (Arnoult et al. 1999; Munoz-Garay et al. 2001), increased pH (Visconti and Kopf 1998), protein tyrosine phosphorylation (Visconti et al. 1995; Leclerc et al. 1998; Visconti and Kopf 1998) and increased intracellular calcium (Breitbart 2002). The influence of the external ionic composition on capacitation strongly suggests the involvement of ion channels in these cellular processes (Darszon et al. 1999).
Previous studies demonstrated a role for Na+/K+-ATPase in capacitation; for example, there was a negative correlation between Na+/K+-ATPase activity and capacitation in guinea pig sperm (Hai-Ying et al. 1990). Furthermore, ouabain promoted capacitation of mouse (Fraser et al. 1993) and bovine (Thundathil et al. 2006) sperm. Similarly, we have indications that ouabain induces tyrosine phosphorylation and capacitation of mouse and human sperm (Thundathil JC, unpublished data). It is very likely that inhibition of the Na+/K+-ATPase activity can lead to hyperpolarization and increase in intracellular concentrations of calcium and sodium, via its interaction with a Na+/Ca2+ exchanger, leading to capacitation. In addition, extensive studies in somatic cells suggest a signalling role for this protein in capacitation.
Na+/K+-ATPase as a signalling molecule in somatic cells
Na+/K+-ATPase functions as a classical receptor, capable of converting ouabain binding into activation of signalling pathways involved in regulation of various physiological processes in somatic cells (Xie 2003). Although Na+/K+-ATPase have no intrinsic kinase activity, interaction of this protein with ouabain leads to its coupling with Src kinase (Schmidt-Ruppin A-2 protein kinase) and its conversion to a functional receptor tyrosine kinase (Pierre and Xie 2006). Subsequently, this Na+/K+-ATPase/ouabain/Src functional complex can transactivate the epidermal growth factor receptor (EGFR). Transactivation of EGFR plays a central role in relaying ouabain-Na+/K+-ATPase signalling to downstream lipid and Serine/Threonine protein kinase pathways, initiating generation of second messengers (e.g. Ca2+) from intracellular stores and generation of reactive oxygen species (ROS) from mitochondria (Ullrich and Schlessinger 1990; Haas et al. 2000; Liu et al. 2000), as well as activation of the mitogen-activated protein kinase (MAPK) cascade (Haas et al. 2002). The upstream signalling mechanisms leading to MAPK pathway activation include recruitment of Shc (Src homology 2 domain containing protein) by transactivated EGFR, Grb2 (Growth factor receptor-bound protein 2), Sos (Son of sevenless) and Ras (Rat sarcoma protein) to the plasma membrane (Haas et al. 2002), and their subsequent activation. The MAPK pathway assembly consists of three kinases: Raf (Rapidly accelerated fibrosarcoma protein; MAPK kinase kinase), MEK (MAPK kinase) and ERK1/2 (Extracellular regulated kinase 1/2). Upon activation, these molecules can subsequently activate downstream signalling molecules (Kolch 2000).
Interaction of ouabain with the α1 isoform of Na+/K+-ATPase leads to formation of a signalplex involving Na+/K+-ATPase, Src, phospholipase C-γ1 (PLC-γ1) and inositol 1, 4, 5-triphosphate (IP3) receptor isoform 2 (IP3R2) in pig LLC-PK1 cells derived from renal proximal tubule (Yuan et al. 2005). This signalplex activates PLC-γ1 and increases hydrolysis of phosphatidylinositol-4, 5-bisphosphate (PIP2), generating inositol 1, 4, 5-triphosphate (IP3). Because Na+/K+-ATPase was able to tether PLC-γ1 and IP3R2, IP3 can activate IP3R2, which in turn releases intracellular Ca2+ (Yuan et al. 2005). In addition, activation of PLC-γ1 generates diacyl glycerol (DAG), which ultimately activates protein kinase C (PKC) and Raf, an essential step for activation of ERK1/2 (Mohammadi et al. 2001).
Na+/K+-ATPase as a signalling molecule during sperm capacitation
Based on known signalling roles of Na+/K+-ATPase in somatic cells, it is clear that ouabain signalling induces ERK1/2 activation via EGFR transactivation and activation of PLC and PKC. In addition, interaction of ouabain with Na+/K+-ATPase generates ROS from the mitochondria through the Src/EGFR/Ras pathway (Xie et al. 1999; Liu et al. 2000). Therefore, the sequalae of Na+/K+-ATPase signalling events in somatic cells resemble some events associated with sperm capacitation, namely increase in intracellular Na+ and Ca2+ concentrations, generation of ROS and activation of ERK1/2. Increase in Ca2+ concentrations induces tyrosine phosphorylation (a hallmark of capacitation) in sperm via protein kinase A (PKA)-mediated activation of protein tyrosine kinase (PTK) (de Lamirande and O’Flaherty 2008). Activation of ERK1/2 induces tyrosine phosphorylation in sperm via PTK, and there is cross-talk between ROS and elements of ERK1/2, PKA and PTK (O’Flaherty et al. 2006). Therefore, we hypothesized that Na+/K+-ATPase is involved as a signalling molecule during sperm capacitation. Under capacitating conditions, ouabain induced tyrosine phosphorylation and acrosome reaction in a dose-dependent manner in fresh bovine sperm (Thundathil et al. 2006; Newton et al. 2010).
Previous studies suggested that signalling molecules implicated in mediating Na+/K+-ATPase-ouabain signalling in somatic cells is involved in sperm capacitation. Phosphorylated Src (p-Src) has been localized in various regions of capacitated sperm (human: Mitchell et al. 2008; Varano et al. 2008; mouse: Baker et al. 2006; bull: Etkovitz et al. 2009). Furthermore, PKA, Src and EGFR are present in bovine sperm (Etkovitz et al. 2009). The MAPK pathway is involved in the regulation of sperm capacitation (pig: Awda and Buhr 2010; mouse: Nixon et al. 2010; human: de Lamirande and Gagnon 2002; Thundathil et al. 2003). Therefore, we tested involvement of these signalling molecules during capacitation induced by Na+/K+-ATPase-ouabain signalling in bovine sperm and demonstrated that PKA, RTK and Src kinases are involved in this process (Newton et al. 2010). In addition, a recent study implicated the ERK pathway in this process (Anpalakan 2010).
Our previous study (Newton et al. 2010) demonstrated that preincubation of bovine sperm with a PKA inhibitor (H89) inhibits ouabain-induced tyrosine phosphorylation. Perhaps generation of ROS and increase in intracellular calcium during ouabain signalling activate PKA, leading to tyrosine phosphorylation. In addition, activated PKA interacts with PKC and activates phospholipase D (PLD), which subsequently hydrolyses phosphatidyl choline (PC) to phosphatidic acid (PA), mediating polymerization of globular (G)-actin to filamentous (F)-actin. Actin polymerisztion is involved in capacitation and the acrosome reaction in bovine sperm (Yagi and Paranko 1995; Cohen et al. 2004).
Based on our knowledge of Na+/K+-ATPase-ouabain signalling in somatic cells and experimental evidence regarding the involvement of this process in sperm capacitation, we proposed a novel model for bovine sperm capacitation (Fig. 1). Further studies are required to confirm the interaction of several of these signalling molecules and cross-talk between pathways. Existence of ubiquitous and testis-specific isoforms of Na+/K+-ATPase in sperm, sensitivity of both isoforms to ouabain, and distinct differences among species in localization of α4 isoform of this protein in sperm make it difficult to elucidate contributions of specific isoforms of Na+/K+-ATPase to capacitation.
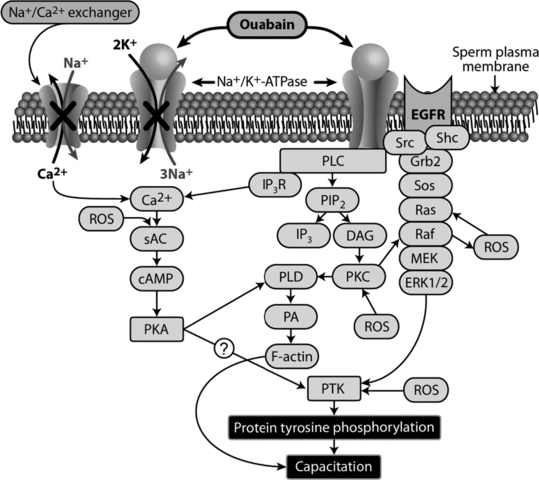
A proposed model for bovine sperm capacitation induced by the interaction of Na+/K+-ATPase and ouabain. This model is based on our current knowledge regarding the role of Na+/K+-ATPase as an ion pump and signalling molecule in somatic cells and available data on the involvement of this protein in bovine sperm capacitation. Ouabain signalling activates ERK1/2, PLC, and protein kinase C (PKC), leading to protein tyrosine kinase (PTK)-mediated tyrosine phosphorylation of proteins and capacitation. The reactive oxygen species generated during this process also contributes to tyrosine phosphorylation of sperm proteins by cross-talking with elements of ERK1/2, protein kinase A (PKA), PKC and PTK. In addition to this signalling process, the interaction of Na+/K+-ATPase with ouabain inhibits ion transport activities of Na+/K+-ATPase and the Na+/Ca2+ exchanger (indicated by ‘X’), leading to an increase in intracellular Ca2+, PKA activation and PTK-mediated tyrosine phosphorylation of proteins, and contributing to sperm capacitation
Na+/K+-ATPase as a Fertility Marker
Because Na+/K+-ATPase regulate various sperm functions, including motility and capacitation, this protein has potential for predicting sperm function and fertility. Premature capacitation occurs in frozen-thawed bovine semen, reducing the fertile lifespan of sperm in the female reproductive tract (Cormier and Bailey 2003). One of the primary sites of cryopreservation-induced damage is the sperm plasma membrane; the membrane covering the sperm head is inevitably damaged (Bailey et al. 2000). Cryopreservation procedures inhibited Na+/K+-ATPase activity in sperm head plasma membranes extracted from frozen-thawed bovine sperm (Zhao and Buhr 1996). In our preliminary studies (Rajamanickam GD, Dance A, Thundathil JC, unpublished data) on membrane protein extracts from whole bovine sperm, the α4 isoform was the predominant form of Na+/K+-ATPase; furthermore, following cryopreservation, the content of this enzyme remained unchanged, although enzyme activity decreased. Moreover, we demonstrated that Na+/K+-ATPase activity of frozen-thawed sperm differs among beef bulls (Rajamanickam et al. 2011). However, the association between content and activity of Na+/K+-ATPase and its relationship to morphology, sperm function and fertility remain to be elucidated.
Conclusion and Future Directions
Inducing abnormal spermatogenesis with scrotal insulation and comparing the abnormal vs normal sperm is an effective strategy to identify important sperm functional proteins. Using this approach, we demonstrated decreased expression of Na+/K+-ATPase in abnormal sperm, the relevance of this protein in regulation of sperm function, and its potential as a fertility marker.
It is very likely that Na+/K+-ATPase has effects beyond regulation of sperm motility and capacitation. For example, Konrad et al. (2011) demonstrated that Na+/K+-ATPase isoforms could be detected in Sertoli cells and that expression of the α4 isoform is directly linked to ouabain-mediated signalling, leading to phosphorylation of CREB (cAMP response element binding) transcription factor, which has an important role in spermatogenesis. Larre and Cereijido (2010) demonstrated that non-toxic concentrations of ouabain modulated the sealing of tight junctions in epithelial cells by altering the expression and localization of various claudins, an integral membrane protein component of tight junctions, through signalling pathways involving c-Src and ERK1/2. It is very likely that Sertoli cell tight junction proteins (e.g. claudins), which constitute the blood-testis barrier, could be regulated through the same mechanism, suggesting a new role for Na+/K+-ATPase. In ejaculated bovine sperm, the α4 isoform of Na+/K+-ATPase is distributed in the entire sperm head. However, this protein redistributed to post-acrosomal region during capacitation, suggesting potential roles in gamete interaction and perhaps fertilization (Newton et al. 2010). Certainly, these novel areas are the future directions for research, which will advance our current knowledge in the role of this protein in spermatogenesis, sperm function and fertilization.
Acknowledgements
This study received funding support from the Natural Sciences and Engineering Research Council of Canada, Alberta Livestock and Meat Agency, and Alberta Innovates and Bio Solutions.
Conflicts of interest
Authors report no conflicts of interest.




