Effects of Maternal Environment During Gestation on Ovarian Folliculogenesis and Consequences for Fertility in Bovine Offspring
Contents
Mammals such as cattle, swine, sheep and humans are born with a highly variable number of ovarian follicles and oocytes in the ovaries that dwindle during ageing and are never replenished. This variation in the ovarian reserve is reflected in the numbers of antral follicles in the ovaries at all ages after birth. As numbers of follicles in ovaries are determined during gestation, the role of maternal nutrition and health during gestation (at time of ovarian development in their foetuses) has been investigated as factors that may impact oogonia proliferation and thus follicle numbers post-natally. These studies have found that both nutrition and health impact numbers of follicles in their offspring. The idea that numbers of follicles and oocytes in ovaries impact fertility is a long-held belief in reproductive biology. This has recently been tested in cattle, and it has been shown that cows with a relatively high number of antral follicles in ovaries have higher pregnancy rates, shorter calving to conception intervals and fewer artificial inseminations during the breeding season compared with cows with a lower number of follicles, and similarly, heifers with many follicles had higher pregnancy rates than those with fewer follicles. Studies summarized in this review highlight the importance of the maternal environment during gestation in determining the size of the ovarian reserve in their offspring and also the contribution of the ovarian reserve to subsequent fertility in cattle.
Introduction
Successful reproduction and fertility is the cornerstone of most animal-based agricultural enterprises; with none being more important than cattle-based industries for the production of milk and meat. The development of ovarian follicles has been investigated in many studies in the literature, but only recently have the numbers of ovarian follicles in ovaries and the factors that affect the size of the ovarian follicle reserve in cattle received attention (Evans et al. 2010; Ireland et al. 2011).
Ovarian follicles and the variation in the ovarian follicle reserve
Ovarian follicles are endocrine structures located within ovaries of all mammals that are essential for female reproductive function. Each follicle nurtures oocyte (egg) development and each individual oocyte is usually surrounded by a single follicle. The follicle and oocyte develop in tandem over a long period of time culminating in a species-specific number of follicles releasing their oocytes (ovulating) into the reproductive tract for fertilization. The events of follicle development are similar in a wide range of species (Evans 2003; Mihm and Evans 2008). Ovarian follicles are formed in ovaries during the foetal period in most species (also including the early prenatal period in rodents), resulting in a large pool of resting primordial follicles that develop during the lifetime of animals. Mammals such as cattle (Erickson 1966a,b), swine (Black and Erickson 1968), sheep (McNatty et al. 1995; Steckler et al. 2005) and human beings (Block 1952) are born with a highly variable number of morphologically healthy follicles and oocytes in the ovaries that dwindle rapidly during ageing and are never replenished. Development of primordial into larger pre-antral then into ovulatory follicles takes approximately 4–6 months in cattle. Once pre-antral develop into antral follicles, their antral cavity fills with a serum-like fluid rich in hormones and growth factors, and the diameter continues to increase until ovulation or atresia. These antral follicles develop in cohorts (groups) in a pattern that has been described as a follicle wave (Ireland et al. 2000; Evans 2003). The wave-like pattern of follicle growth was first proposed by Rajakoski (1960) after a large study examining the number of follicles >1 mm in diameter in the ovaries of slaughtered cattle on different days of the cycle (Ireland et al. 2000). The development of ultrasonography as a tool to non-invasively monitor the growth and regression of individual ovarian follicles repeatedly in the same animal (Pierson and Ginther 1984) firmly established that antral follicles grow in cohorts, in two or three wave-like patterns during oestrous cycles in cattle (Evans 2003), including humans (Baerwald et al. 2003).
The observations that the number of primordial follicles is highly variable at birth in cattle (Erickson 1966b), that the number of different follicle types vary greatly among adult cattle, and that they reliably ovulate one, or occasionally two, follicle(s) during each oestrous cycle prompted further investigations into the physiological significance of the high variation in ovarian follicle numbers in an agriculturally important single-ovulating species like cattle.
Variation in the numbers of ovarian follicles
A number of studies have systematically catalogued numbers of antral follicles on different days of the oestrous cycle in cattle and have established that numbers of follicles in ovarian follicular waves of the oestrous cycle are highly variable among animals but very highly repeatable (0.85–0.95) within individuals (Burns et al. 2005; Ireland et al. 2007, 2008; Jimenez-Krassel et al. 2009; Mossa et al. 2010). This observation holds true when considering the peak or nadir numbers associated with waves or the mean numbers across all days of the cycle. However, the follicle count must include all follicles ≥3 mm in diameter in both ovaries (the antral follicle count or AFC), which explains why many studies in the last 20 years that focused on counting follicles ≥5 mm in diameter did not observe the high variability of follicle numbers growing during follicular waves among animals, nor the remarkably high repeatability of follicle numbers during waves in individuals. For example, we have noted that AFC during different follicular waves of an oestrous cycle may be consistently lower than five during follicular waves in some animals and >50 in others (Burns et al. 2005; Ireland et al. 2007; Mossa et al. 2010) (Fig. 1). Moreover, this high repeatability of follicle numbers during waves persists for at least 1 year (Burns et al. 2005). Taken together, these results firmly establish that cattle can be phenotyped reliably based on AFC.
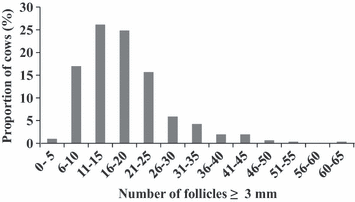
Frequency distribution of the total number of ovarian follicles ≥3 mm in diameter (antral follicle count) in post-partum Holstein–Friesian dairy cows (n = 383) measured using ultrasonography (Mossa et al. 2012)
Whether the high variation in number of antral follicles or AFC, which accounts for much <1% of the total number of follicles in ovaries, was associated with the inherently high variation in numbers of morphologically healthy follicles in ovaries (ovarian reserve) is unknown. To address this question, ovaries were surgically removed from age-matched heifers (adult cycling animal 12–18 months of age) that had high (≥25 follicles ≥3 mm in diameter) or low (≤15) AFC, and numbers of all follicles in ovaries were counted in histological sections (Ireland et al. 2008). This study showed that AFC was highly correlated with total number of morphologically healthy follicles in ovaries, and for example, cattle with a low AFC also had 80% fewer total number of healthy primordial, pre-antral and antral follicles in ovaries compared with cattle with a high AFC (Fig. 2).
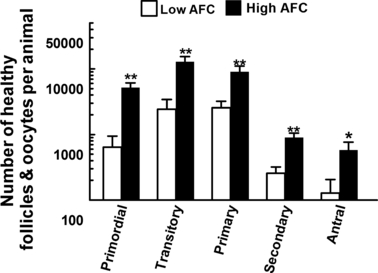
Number of morphologically healthy primordial, transitory, primary, secondary and antral follicles in ovaries of cattle with a low (≤15 follicles, n = 5) vs a high (≥25 follicles, n = 5) number of follicles ≥3 mm in diameter (antral follicle count, AFC) during ovarian follicular waves. The ovary contralateral to the recent ovulation was removed surgically 1–2 days after ovulation and histologically examined. Asterisks (*p < 0.05, **p < 0.01) indicate difference between means for the low vs high group. From Ireland JJ Ireland, DM Scheetz, F Jimenez-Krassel, JK Folger, GW Smith, (2008)
Causes of the variation in AFC
Follicle formation during foetal development
In ruminants, follicle formation occurs during foetal life, starting with migration of primordial germ cells to the genital ridge followed by differentiation into oogonia (Pepling 2006), which is completed by the first or second month of foetal life (Smitz and Cortvrindt 2002; Binelli and Murphy 2010). After arrival of the primordial germ cells into the gonadal ridge, the germ cells continue to proliferate and differentiate into primordial follicles, comprised of an oocyte surrounded by squamous granulosa cells (primordial follicles are arrested in meiosis) (Webb and Campbell 2007). In cattle, differentiation of primordial follicles starts between 90 and 140 days of foetal life (Erickson 1966b; Russe 1983; Tanaka et al. 2001; Yang and Fortune 2008). In addition, it is well established that the population of oogonia in the developing bovine ovary reaches an estimated maximum number of 2.1 million (Smitz and Cortvrindt 2002), which rapidly declines during mid- to late-foetal life owing to apoptosis until the population of follicles is reduced at birth to approximately 130 000 with a high variation among individuals (Erickson 1966b; Aerts and Bols 2010).
David Barker [hence the Barker hypothesis (Barker 1992) or more recently the thrifty phenotype hypothesis (Hales and Barker 2001)] suggests that environmental influences early in human foetal life are reflected in impaired growth, development and metabolism, leading to increased risk for diseases in adulthood. The hypothesis proposes that some diseases originate through foetal adaptations to malnutrition that permanently alter body function. This hypothesis is supported by animal models (McMillen and Robinson 2005). Most studies have examined the link between malnutrition with cardiovascular disease, obesity and diabetes (McMillen and Robinson 2005; Boo and Harding 2006), but only a few studies have examined the impact of maternal environment on reproduction. Hence, it is reasonable to speculate that the maternal environment during gestation, at the time of ovarian development in their foetuses, may impact oogonia proliferation and thus follicle numbers post-natally.
Evidence that maternal disease during gestation negatively impacts AFC in their offspring
We have recently examined the effect of disease during pregnancy in cattle on ovarian development and function of their offspring. Somatic cells normally present in milk of dairy cows are predominantly white blood cells. When somatic cells are counted, results are referred to as the somatic cell count (SCC). The major factor affecting SCC in milk is a mammary gland infection. Somatic cell count in milk samples collected from individual dairy cows at various intervals (e.g. monthly) during lactation is commercially determined and recorded in dairy herds. Consequently, high SCC in milk (e.g., >200 000) is useful as an index for potential previous or current udder infections/inflammation that may have been caused by mastitis or injury (Caraviello et al. 2005). In a preliminary study, we have recently shown that dairy cows with a high number of SCC measurements >200 000 during pregnancy produce daughters with relatively low circulating concentrations of anti-Müllerian hormone (AMH) as adults. Anti-Müllerian hormone is a glycoprotein produced exclusively by granulosa cells of healthy growing follicles (La Marca and Volpe 2006), and circulating AMH concentrations are not only static during reproductive cycles, but also highly correlated with AFC and size of the ovarian reserve in cattle and other species (see review Ireland et al. 2010). In a recent follow-up study with larger animal numbers, we subjected two groups of 12-month-old Holstein heifers to two injections of prostaglandin F2α (PG) spaced 11 days apart to synchronize oestrous cycles. A single blood sample was removed from the tail vein of each animal 96 h after the last PG injection, and serum AMH concentration was determined using our previously validated AMH assay (intra- and interassay cv ≤ 10%; Ireland et al. 2008). One group of heifers were from dams (n = 25 animals) that had 4 or 5 SCC measurements >200 000 during pregnancy, while the other group of heifers were from dams (n = 20) with 0 or 1 SCC measurement >200 000 during pregnancy. Like our previous preliminary study (Ireland et al. 2010), results showed that the heifers from dams with chronically high SCC had AMH concentrations nearly 50% lower (0.13 ± 0.03 vs 0.075 ± 0.01 ng/ml, p < 0.05) compared with heifers from dams with zero or a low SCC (Fig. 3). Taken together, these results imply that chronic infections during pregnancy of dairy cows diminish the size of the ovarian reserve (and lower AMH) and potentially future reproductive performance in their offspring.
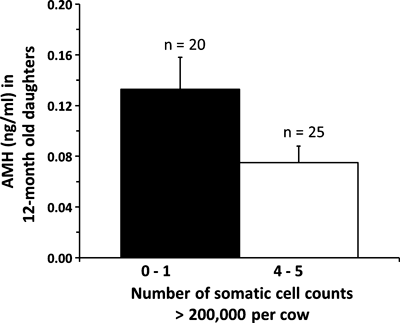
Concentrations of AMH in 12-month-old heifers (96 h after a second injection of prostaglandin) born to dams that had 4 or 5 milk somatic cell count (SCC) measurements >200 000 during pregnancy (n = 25 animals) or born to dams with 0 or 1 SCC measurement >200 000 during pregnancy (n = 20). As AMH concentrations are reflective of the size of the ovarian reserve (see text), these results imply that chronic infections during pregnancy of dairy cows diminish the size of the ovarian reserve in their offspring (Ireland et al. unpublished)
Evidence that maternal nutrition during gestation negatively impacts AFC in their offspring
Most studies examining the thrifty phenotype hypothesis investigate the consequences of nutritional challenges imposed on the dam on health of their offspring as young adults. However, we tested the hypothesis that restriction of maternal nutrition during gestation, at the time of ovarian development in their foetuses, negatively impacts proliferation of primordial germ cells (to be consistent with Introduction) and thus follicle numbers post-natally. We tested this hypothesis by restricting nutrition of cows to 0.6 of their maintenance energy requirements shortly before conception to the end of the first trimester of pregnancy. The first trimester of pregnancy coincides with the peak in number of follicles and oocytes in foetal ovaries (Erickson 1966a). Results show that, although weight of offspring was unaltered at birth, AFC and circulating AMH concentrations in calves born to nutritionally restricted mothers were 60% lower compared with calves born to control mothers (Mossa et al. 2009) (Table 1). Because AFC and AMH concentrations are positively correlated with size of the ovarian reserve (Ireland et al. 2008), these findings imply that maternal nutrition as well as chronic infections during pregnancy may have a previously unrecognized yet important role in regulation of size of the ovarian reserve and perhaps fertility of cattle.
| Weeks of age | Control (n = 13) | Nutrient Restricted (n = 10) |
|---|---|---|
| 7 | 23.8 ± 2.1 | 14.1 ± 0.9** |
| 18 | 26.0 ± 2.8 | 16.2 ± 1.1** |
| 35 | 23.9 ± 2.2 | 16.6 ± 1.2** |
| 56 | 21.9 ± 2.0 | 17.5 ± 1.9 |
| 86 | 23.6 ± 1.9 | 15.8 ± 1.8* |
- *p < 0.05 and **p < 0.01 compared with the control animals at the same age.
Effects of genotype on AFC
Another intriguing observation of the numbers of follicles in cattle is a comparison of Bos indicis vs Bos taurus breeds of cattle. While there are small differences in the characteristics of oestrous cycles and follicle waves between these types of cattle (Sartori et al. 2010), it is clear that there are different number of follicles in follicle waves. At the onset of each follicular wave, there are on average 18 (Ireland et al. 2007) to 24 (Ginther et al. 1996) small antral follicles detected in the ovaries of B. taurus cattle, whereas there appears to be at least double this in B. indicus cattle (Gimenes et al. 2009), with reports of average counts ranging from 40 (Alvarez et al. 2000) to 50 (Buratini et al. 2000; Sartori et al. 2010) follicles.
While these differences in antral follicle numbers between breeds have been suggested to be due to differences in circulating concentrations of insulin and IGF-I (Sartori et al. 2010; Sartori and Barros 2011), it is also possible that they arise owing to differences in the maternal environment during gestation affecting numbers of follicles in the ovarian reserve. In addition, it is interesting to note that while B. indicus cattle have more follicles than B. Taurus breeds, there is some evidence to support the notion that prenatal maternal nutrition also negatively impacts the ovarian reserve in offspring of B. indicus breeds of cattle (Sullivan et al. 2009), as shown in our studies (Mossa et al. 2009).
Consequences of the variation in AFC for fertility
It has long been known that there is very high variation in the size of ovaries and the ovarian reserve (Erickson 1966a,b). Moreover, the idea that quantity of follicles and oocytes in ovaries impacts fertility is a long-held tenet in reproductive biology (Hunter 1787; Erickson 1966b; te Velde and Pearson 2002). However, the association of AFC with fertility has only recently received interest in human beings (Rosen 2011; Styer and Toth 2011).
It is clear that the quantity of follicles and oocytes is highly variable throughout reproductive life spans of adults (see above) and also that oocyte quantity rapidly declines during ageing (Fayad et al. 2004; Kuhn et al. 2006). Furthermore, in women, the decline in fertility approximately 20 years before menopause (cessation of reproductive cycles and depletion of oocytes from ovaries) and high variability of age at menopause (range = 40–60 years old) are both hypothesized to be caused by the high variation in number of follicles and oocytes in women at birth (Faddy and Gosden 1996; te Velde and Pearson 2002). Nevertheless, the extent and mechanisms whereby the inherently high variation in quantity of follicles and oocytes in ovaries of individuals (Block 1952; Erickson 1966a,b; Burns et al. 2005; Ireland et al. 2007, 2008, 2009; Jimenez-Krassel et al. 2009) may cause or contribute to the relatively large differences in fertility among young adults are unknown and have not been previously directly examined for any species.
To test the hypothesis that AFC is positively associated with fertility in cattle, we recently completed a study showing that cows with a high AFC had higher pregnancy rates (Fig. 4), had shorter calving to conception intervals and received fewer services during the breeding season compared with cows with a low AFC (Mossa et al. 2012). This finding is supported by a study in beef heifers showing higher pregnancy rates in heifers with a high vs a lower AFC (Cushman et al. 2009). A similar association of low numbers of follicles with reduced fertility is reported for humans (Baerwald et al. 2003).
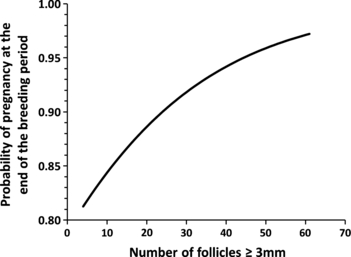
Probability of pregnancy at the end of the breeding period in 306 lactating dairy cows plotted against the numbers of ovarian follicles ≥3 mm in diameter (AFC). Logistic regression, utilizing a logit link function, was used to quantify the association between AFC and pregnancy at the end of the breeding period (p = 0.06) (Mossa et al. 2012)
Evidence to explain the association of low AFC with poor fertility
The mechanisms whereby the high variation in quantity of follicles and oocytes per se may alter ovarian function and fertility are unknown. Nevertheless, we have generated intriguing results (Ireland et al. 2007, 2008, 2009; Jimenez-Krassel et al. 2009) showing that young adult cattle with a low vs a high AFC also exhibit a variety of additional phenotypic characteristics, usually associated with impaired ovarian function in older animals, which are reflective of ovarian ageing and suboptimal fertility.
AFC and superovulation
Responsiveness to superovulation and reduced success of IVF and embryo transfer are well established to be negatively associated with ageing and to correspond to a reduction in number of follicles and oocytes in ovaries in single-ovulating species such as cattle (Kawamata 1994; Cushman et al. 1999; Taneja et al. 2000; Singh et al. 2004) and humans (Beckers et al. 2002; Styer and Toth 2011). Our results show that young adult cattle with low vs high AFC respond poorly to superovulation and have a reduced number of transferable embryos (Ireland et al. 2007).
AFC, progesterone concentrations and endometrial growth
Progesterone is a key physiological marker of corpus luteum function and thus ovarian function, which also has a well-established positive role in uterine function, embryo development and fertility in single-ovulating species such as cattle (Diskin and Morris 2008). In support of the crucial role for progesterone in successful reproduction, low circulating progesterone concentrations are associated with high rates of embryo mortality in cattle (Diskin and Morris 2008) and women (Daily et al. 1994; Elson et al. 2003) and luteal phase defects and infertility in women (Pritts and Atwood 2002). Therefore, we asked whether the high variation in AFC was associated with CL function and circulating progesterone concentrations during oestrous cycles. The combined results for 63 different oestrous cycles for 47 animals illustrated that young adult beef cattle and late lactation dairy cows with a low AFC had progesterone concentrations consistently 30–50% lower than cattle with a high AFC and also that this was reproducible from one oestrous cycle to the next (Jimenez-Krassel et al. 2009). Whether the difference in circulating progesterone concentrations observed for heifers with a low vs a high AFC also impacted uterine development was then examined, and it was found that young adult cattle with a low AFC had chronically much lower circulating progesterone concentrations throughout the early to mid-luteal phase and poor endometrial development during the early luteal phase of oestrous cycles compared with cattle with a high AFC (Jimenez-Krassel et al. 2009). These observations, coupled with many reports of the association between low serum progesterone concentrations with embryo mortality in cattle (Diskin and Morris 2008) and between reduced endometrial thickness and infertility in women (Raga et al. 1999; Basir et al. 2002), imply that rates of embryo mortality may be higher in young adult cattle with a low vs a high AFC and correspondingly reduced quantity of follicles and oocytes in ovaries.
AFC and intrafollicular markers of oocyte quality
We have also investigated markers of follicle, cumulus and oocyte cell function from animals with high vs low AFC and conclude that the high variation in follicle and oocyte numbers is also associated with physiologically significant alterations in follicular differentiation and in turn oocyte quality (Ireland et al. 2009). In particular, intrafollicular estradiol concentrations are approximately twofold higher in animals with a low vs a high AFC (Ireland et al. 2009), and this may have detrimental effects on oocyte maturation and developmental competence in cattle with low follicle numbers because of high physiological concentrations of estradiol block maturation of bovine oocytes in vitro (Beker-van Woudenberg et al. 2004). In addition, we have observed that young adult cattle with a low vs a high AFC have much higher expression of cathepsin mRNA in cumulus cells (Ireland et al. 2009), which is a marker for poor oocyte quality in cattle (Bettegowda et al. 2008).
Conclusions
In cattle, the number of antral follicles in the ovaries is reflective of the size of the ovarian reserve (Ireland et al. 2008), and there is increasing evidence that size of the ovarian reserve is negatively impacted by maternal environment during gestation. The size of the ovarian reserve is also associated with fertility, but the mechanisms whereby the inherent high variation in follicle numbers negatively impact ovarian function, endometrial development and embryo survival are complex and poorly understood. These findings highlight (i) the importance of the maternal environment during gestation in determining the size of the ovarian reserve in their offspring, (ii) the contribution of the ovarian reserve to subsequent fertility in cattle and (iii) the importance of future studies to unravel the factors and associated mechanisms that regulate not only size of the ovarian reserve, but also the linkage between the inherently high variation in numbers of follicles in the ovarian reserve with subsequent ovarian and uterine function and optimal fertility.
Acknowledgements
The authors’ research is funded by Science Foundation Ireland (07/SRC/B1156), the Department of Agriculture Food and the Marine, Ireland (RSF 06328), National Research Initiative Competitive Grants (2004–35203–14781; 2007–35203–18178) from the USDA National Institute of Food and Agriculture and funds from the Michigan Agriculture Experiment Station.
Conflicts of interest
The authors have no conflicts of interest in publishing this review.




