Follicular, Oocyte and Embryo Features Related to Metabolic Status in Primiparous Lactating does Fed with High-Fibre Rearing Diets
Contents
Fertility of primiparous lactating does in the early postpartum (pp) period is very low mainly due to pronounced deficient energy intake, influencing oocyte and embryo developmental competence. The hypothesis used in this work was that high-lignin fibre diet supplied during the rearing period could increase feed intake and, consequently, improve the reproductive physiology and metabolic status of primiparous does in the early pp period. Diets with high-lignin [HL: 15.8% dry matter (DM)] or standard-lignin content (SL: 4.9% DM) were supplied until parturition time. No diet effects in serum oestradiol, progesterone concentrations and follicle categories were found in the histological study. Metaphase II rate of in vitro-matured oocytes was significantly higher in the SL vs the HL group (p < 0.001). Cytoplasmically degenerated oocytes (in terms of abnormal distribution of cortical granules) and follicular atresia rate were significantly lower in the SL group than in the HL group (p < 0.05 and p < 0.005 respectively). In addition, HL-fed does showed lower number of viable embryos and higher rate of retarded in vivo-recovered embryos compared with the SL group (p < 0.05). Neither in vitro embryo development of viable embryos nor conception rate was significantly different between groups. Feed intake increased during the first pregnancy in the HL group (p < 0.05), but not during early lactation. Serum protein, non-esterified fatty acid and leptin concentrations, as well as estimated body composition were similar in does fed with both diets. In conclusion, the enhancement of reproductive management by using highly lignified products in rearing diets does not seem to report physiological reproductive benefits affecting oocyte maturation rate and embryo viability in primiparous lactating does.
Introduction
Fertility rate in high-yield primiparous rabbit does is especially low when they are inseminated shortly after kindling (Rebollar et al. 2006). At that moment, energy from feed intake is insufficient to cover the energy needs for lactation and growth of the primiparous mother (Parigi-Bini et al. 1992; Pascual et al. 2002). It is well known that this situation causes a negative energy balance (NEB) in the first lactation, when does are inseminated (Fortun-Lamothe 2006). Such NEB in the early postpartum (pp) period is associated with endocrine and metabolic changes, as reflected in the environment of the growing and maturing female gametes. This results in the ovulation of developmental incompetent oocytes (Leroy et al. 2008), presumably affecting embryo survival, as previously reported (bovine: Leroy et al. 2005; gilt: Ferguson et al. 2006).
Much nutritional management strategies have been driven to increase diet energy level before insemination in several species (ewe: O′Callaghan et al. 2000; gilt: Ferguson et al. 2003; rabbit: Pascual et al. 2003). In rabbits, alternative feeding strategies based on diets with fibre-rich content during the rearing period have been suggested to increase feed ingestion capacity in the first lactation (Xiccato et al. 1999; Pascual et al. 2002; Arias-Álvarez et al. 2009). In this sense, fibre is one of the main constituents of commercial diets for intensively reared rabbits (De Blas et al. 1999). Particularly, indigestible and inexpensive lignin increases voluntary feed intake and gut content during this period (García et al. 1999; Nicodemus et al. 1999; Gidenne 2003). Therefore, lignin supplementation during rearing should enhance energy intake after the first parity, when a more concentrated feed will be furnished, reducing lactation energy deficit (Nizza et al. 1997; Xiccato et al. 1999; Pascual et al. 2002). However, the reproductive results of this type of strategies are scarce and controversial.
Using different feeding strategies and analysing their influence on reproductive physiology are crucial to understand the complexity of nutritional treatments to enhance reproductive managements in industrial rabbit breeding. Therefore, the aim of this study was to determine the long-term effects on ovarian status, embryo development and conception rate of a high-lignin fibre diet supplied during the rearing period, and their relationship to serum endocrine and metabolic parameters, body composition and feed intake during the early pp in primiparous does submitted to a semi-intensive reproductive rhythm of production (inseminated at day 11 pp).
Materials and Methods
Unless otherwise stated, all chemicals were purchased from Sigma® Chemical Company (St. Louis, MO, USA). Experimental procedures were approved by the Animal Ethics Committee of the Polytechnic University of Madrid (Spain) and were in compliance with the Spanish guidelines for care and use of animals in research (BOE, 2005).
Animals and experimental design
New Zealand × California white rabbit does (Oryctolagus cuniculus) were housed in the experimental farm of the Animal Production Department, Polytechnic University of Madrid (Spain), in individual flat-deck cages under a constant photoperiod of 16 h of light per day, a temperature of 18–22°C and a relative humidity of 60–75% maintained by a forced ventilation system.
The experimental design is shown in Fig. 1. Briefly, a total of 95 nulliparous does were randomly assigned to be fed ad libitum with the standard-lignin diet (SL group, n = 47) or with the high-lignin diet (HL group, n = 48) during the rearing period until the first parturition. The SL diet was formulated to contain a standard proportion of lignified fibre [40.9% neutral detergent fibre (NDF) and 4.9% acid detergent lignin (ADL) on dry matter basis (DM)]. The HL diet was formulated to contain a high proportion of lignified fibre (49.6% NDF and 15.8% ADL on DM basis) (Table 1). The fibre level and lignin content of the SL diet were similar to a commercial diet (Pascual et al. 2002) and to that used in previous works (Nizza et al. 1997; Xiccato et al. 1999; Arias-Álvarez et al. 2009). After parturition, primiparous lactating does were fed a commercial diet for lactating does (Cunimax A®; Cargill S.A., Zaragoza, Spain).
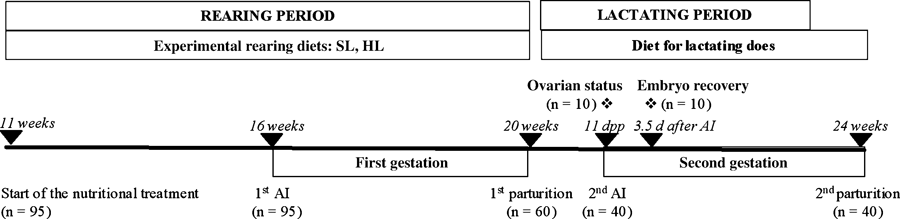
Schematic representation of the experimental design. AI: artificial insemination; dpp: days postpartum; *: euthanasia
| Rearing diets | ||
|---|---|---|
| Standard-lignin diet (SL) | High-lignin diet (HL) | |
| Ingredients (%) | ||
| Lucerne hay | 28.0 | 50.0 |
| Defatted grape seed meal | 0.0 | 19.0 |
| Wheat | 10.0 | 10.0 |
| Wheat bran | 12.5 | 12.5 |
| Sugarbeet pulp | 14.0 | 0.0 |
| Straw | 22.0 | 0.0 |
| Sunflower 30% | 7.4 | 7.4 |
| Soybean 44% | 5.0 | 0.0 |
| L-Threonine | <0.1 | <0.1 |
| Calcium Carbonate | 0.3 | 0.3 |
| Salt | 0.5 | 0.5 |
| Zinc Bacitracin | 0.2 | 0.2 |
| Correctora | 0.2 | 0.2 |
| Chemical analysis (DM %) | ||
| Dry matter | 90.6 | 90.5 |
| Ash | 8.2 | 8.6 |
| Crude protein | 16.8 | 16.6 |
| Starch | 17.9 | 8.9 |
| Acid detergent fibre | 20.3 | 33.9 |
| Neutral detergent fibre | 40.9 | 49.6 |
| Acid detergent lignin | 4.9 | 15.8 |
| Ether extract | 3.1 | 3.3 |
| Energy (MJ/kg) | 18.2 | 18.5 |
- aProvided by DSM Nutritional Products Iberia, S.A. Mineral and vitamin composition (mg/kg of complete diet): Mn: 15; Zn: 50; I: 0.8; Fe: 40; Cu: 8; Co: 0.30; Se: 0.05.; riboflavin: 3; calcium d-pantothenate: 10; nicotinic acid: 25; menadione: 1; α-tocopherol: 35; thiamine: 1; pyridoxine: 1.5; biotin: 0.05; folic acid: 1.5; cianocobalamin: 0.012; robenidine: 50; vitamin A: 10 000 IU/kg; vitamin D3: 900 IU/kg.
The first artificial insemination (AI) in all nulliparous does was performed at 16 weeks of age. The second AI was carried out at day 11 pp, according to a semi-intensive reproductive rhythm, in 60 primiparous does randomly assigned to both nutritional groups (HL group, n = 30 and SL group n = 30). Litter size of the mothers was equalized to eight kits 1 day after parturition, and weaning was performed at day 28 pp. Lactating does were oestrus-synchronized by transient doe-litter separation during 24 h before AI according to Alvariño et al. (1998). Insemination was carried out using a pool of fresh heterospermic semen between 20 and 25 million spermatozoa in 0.5 ml of commercial diluent (Magapor®, S.L., Zaragoza, Spain). Ovulation was induced by intramuscular injection of 1 μg buserelin (Suprafact®; Hoechst Marion Roussel, S.A., Madrid, Spain).
Daily feed intake (g/day) during the first pregnancy and early lactation was measured by weighing of the feeder and feed at the beginning and at the end of such period in all animals. Live body weight (LBW), estimated body composition and serum concentrations of total protein, non-esterified fatty acids (NEFA) and leptin were determined at parturition day and at day 11 pp (AI time). Ovarian status of primiparous does at insemination measured by serum concentrations of steroid hormones (oestradiol-17β and progesterone) was recorded. To further determine ovarian physiology, five animals in each nutritional group were euthanized with 30 mg/kg i.v. of pentobarbital sodium (Dolethal®, Vetoquinol, Spain) before AI at day 11 pp (G1 group), and the other five, 3.5 days after AI (G2 group). In the G1 group, both ovaries were weighted and one of them preserved for histological studies – follicular categorization and apoptosis assessment, whereas the other one was used for in vitro maturation assessment. In the G2 group, ovulation rate, embryo viability and in vitro embryo development were studied. Finally, conception rate (number of parturitions per number of inseminations × 100) of the remaining primiparous does in both the HL group (n = 20) and the SL group (n = 20) was recorded.
Blood sampling and serum analyses
Blood samples were collected from the margin ear vein into non-heparinized tubes at 9:00 h am to avoid circadian variations. Serum was obtained after centrifugation at 1200 × g for 10 min at 4°C and stored at −32°C until analysed.
Protein
Serum total protein was determined by the Biuret method, according to Tietz (1995). In alkaline media, cupric ions interact with protein peptide bonds, forming a coloured complex. This method is linear up to 13 g/dl.
Non-esterified fatty acids
Serum NEFA determinations were performed in duplicate samples using a two-reaction, enzymatic-based colorimetric assay from Wako Pure Chemical Industries®, Ltd. (Osaka, Japan), based on the ability of NEFA to acylate Coenzyme A in the presence of CoA synthetase. The method is linear over the range from 0.0 to 2.0 mmol/l.
Leptin
Serum leptin concentrations were determined in duplicate samples by a double antibody RIA using a multi-species leptin RIA kit® (LINCO Research Inc., St. Charles, MO, USA), as previously reported in rabbits (Depoortere et al. 2004; Brecchia et al. 2006). Intra- and inter-assay coefficients of variation were 3.1% and 7.3% respectively. The detection limit after adjusting the standard curve to rabbit values was 0.1 ng/ml Human Equivalent (HE).
Oestradiol-17β (E2) and Progesterone (P4)
Serum E2 and P4 concentrations were measured in duplicate samples by specific chemiluminiscence methods (CMIA®; Abbott laboratories, Abbott Park, IL, USA). For oestradiol detection, purified rabbit anti-oestradiol monoclonal antibodies were used. Progesterone detection was achieved by using rabbit anti-progesterone polyclonal antibodies. Intra- and inter-assay coefficients of variation were 6.6% and 7% for E2, and 5.8% and 6.3% for P4 respectively. The detection limit was 10 pg/ml for E2 and 0.2 ng/ml for P4.
Follicular classification
Number of pre-ovulatory follicles ≥1 mm on the ovarian surface was first recorded. Then ovaries were placed into a 4% buffered neutral paraformaldehyde solution (pH 7.2–7.4). All samples were gradually dehydrated with increasing concentrations of ethyl alcohol (50–100%). These dehydrated specimens were first embedded in paraffin, prepared by sectioning at 5 μm and stained with hematoxylin/eosin. To study follicle population, hystological sections of each half ovary were examined at light microscope (Olympus BX40). Rabbit ovarian follicles were categorized into four specific development stages related to the number of layers of granulosa cells according to Arias-Álvarez et al. (2007a) and Rebollar et al. (2008), namely, primordial, primary, secondary and antral follicles.
Follicular atresia assay
DNA strand breaks occurred during the cell apoptosis process were detected using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labelling (TUNEL; In Situ Cell Death Detection Kit, POD®; Roche Diagnostics S.L., Applied Science, Barcelona, Spain) as previously reported by García-García et al. (2009) with some modifications. Briefly, dewaxed, rehydrated sections by standard methods were first prepared. The slides were then pretreated with 20 μg/ml of proteinase K working solution for 30 min in a humidified dark chamber at 37°C. Incubation with the TUNEL reaction mixture took place in a humidified dark chamber at 37°C for 1 h. After each step of the procedure, sections were rinsed three times in phosphate-buffered saline (PBS). Finally, the slides were covered with Vectashield mounting medium with 4′,6-diamino-2-phenylindole® (DAPI) (Vector Laboratories Ltd., Peterborough, UK). Positive control sections were treated with DNase I® for 10 min at room temperature in a humidified chamber (Roche Diagnostics S.L., Applied Science, Barcelona, Spain) before incubation with the TUNEL reaction mixture. For negative controls, samples were just incubated with the label solution of the TUNEL reaction mixture without enzymatic solution. TUNEL-stained and DAPI-counterstained slides were observed under a fluorescent microscope (Leica, F550). Green fluorescence could be visualized only in TUNEL-positive cells (Fig. 2). Primordial follicular population was omitted. Follicles in medium or advanced stage of atresia were examined according to Kasuya (1995), and the percentage of apoptosis expressed is the number of TUNEL-positive follicles divided by the total number of recorded follicles.
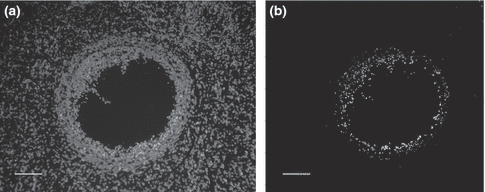
Follicular apoptosis measured by TUNEL in ovarian sections of primiparous rabbit does at insemination time (day 11 pp). (a) Control section showing ovarian cells stained with DAPI (magnification 400×); (b) Follicle in image (a), showing TUNEL staining. Labelled cells are apoptotic (magnification 400×); scale bar = 150 μm
Oocyte collection and in vitro maturation
The rest of the ovaries were placed in PBS at 37°C and transported to the laboratory. Cumulus–oocyte complexes (COC) were obtained from ovarian follicles ≥ 1 mm by aspiration with a 25 gauge needle. Compact cumulus cells were washed and placed in 500 μl of maturation medium in four-well dishes and cultured for 16 h at 38°C under an atmosphere of 5% CO2 in air with maximum humidity. The maturation medium consisted of tissue culture medium (TCM-199) with 2 mm l-glutamine, 0.1 mg/ml sodium pyruvate, 10% foetal calf serum (FCS), 10 ng/ml epidermal growth factor and 100 ng/ml insulin growth factor according to Lorenzo et al. (1997).
Oocyte maturation assessment by confocal microscopy
After the maturation period, COC were treated for the confocal study as previously reported by Arias-Álvarez et al. (2009). Briefly, cumulus cells were removed with 2 mm hyaluronidase by gentle pipetting. Then, oocytes were treated with 0.5% pronase to digest the zone pellucida, fixed for 30 min in PBS containing 4%-buffered neutral paraformaldehyde solution (pH 7.2–7.4) and stored in PBS. Oocytes were washed with permeabilizating solution (0.02% Triton X-100 in PBS) and treated for 40 min with blocking solution [7.5% bovine serum albumin (BSA) in PBS]. They were then incubated for 30 min at room temperature in PBS with 100 μg/ml fluorescein isothiocyanate of lens culinaris for cortical granule (CG) staining and 15 min at 39°C with 10 μg/ml Propidium Iodide for nuclear staining. After that, oocytes were mounted between a coverslip and a glass slide supported by columns of paraffin and examined under a confocal laser-scanning microscope (Leica, TCS SP5) (Fig. 3). Nuclear maturation was measured in terms of metaphase II rate. Cortical granule migration has been used as a criterion to assess oocyte cytoplasmic maturation as previously reported by other authors (Sun et al., 2003; Velilla et al. 2004). According to a previous work (Arias-Álvarez et al. 2007b), CG distribution was categorized as follows (Fig. 3): (a) peripheral: CG was adjacent to the plasma membrane, showing they were cytoplasmically matured; (b) cortical: most of the CG were scattered throughout the cortical area, being considered as partially matured; (c) homogeneous: CG were scattered throughout the cytoplasm, not showing cytoplasmic maturation; (d) non-homogeneous or abnormal: anomalous distribution of CG compatible with poor quality or degenerated oocytes.

Cytoplasmic oocyte maturation related to cortical granule (CG) migration: (a) peripheral CG distribution; (b) cortical CG distribution; (c) homogeneous CG distribution; (d) non-homogeneous or abnormal CG distribution. Oocyte diameter is approximately 80 μm; scale bar = 20 μm
Assessment of ovulation and embryo viability
The ovarian response in terms of ovulation rate (number of corpora lutea) and embryo recovery was assessed 3.5 days after AI. Embryos were recovered by flushing the reproductive tract with PBS + 0.1% BSA. Morphology of compact morulae and early blastocysts was evaluated immediately after recovery. Morphological viable embryos were in vitro cultured (IVC) and embryonic developmental competence was determined by assessing their developmental progression until expanded blastocyst stage during 72 h with a stereomicroscope (SMZ800; Nikon, Tokyo, Japan). Embryos were cultured in IVC medium, consisting of TCM-199 supplemented with 10% FCS incubated at 38°C under an atmosphere with 5% CO2 in air at maximum humidity. Embryos were classified on the basis of conventional morphological criteria according to their developmental stage following the guidelines of the International Embryo Transfer Society. Viability rate at recovery time, number of unfertilized oocytes, degenerated and retarded embryos (embryos whose stage of development did not correspond to time after ovulation, i.e.: zygotes, 2–4, 5–8, 9–16 cell embryos and early morulae) were referred to the total number of recovered oocytes/embryos. The viability rate after IVC was obtained by dividing the number of progressed embryos after IVC by the number of cultured embryos.
Analytical methods
Chemical analysis of diets was carried out using the method of Van Soest et al. (1991) and Mertens (2002) for NDF and the official method (973.18) of the Association of Official Analytical Chemists International (AOAC international, 2000) for acid detergent fibre (ADF) and ADL. Neutral detergent fibre, ADF and ADL were extracted successively using the filter bag system® (Ankom Technology, New York, NY, USA) and corrected by ash content of ADL residue. Procedures of the AOAC International (2000) were also used for DM (oven drying method: 930.15), ash (muffle furnace incineration: 923.03) and crude protein (CP; Dumas method: 968.06; FP-528 LECO, St. Joseph, MI, USA) determination. Gross energy was determined in an adiabatic calorimeter. Digestible energy (DE) was determined in a faecal digestibility trial according to the method of Pérez et al. (1995) using eight animals per diet. Body composition was determined by bioelectrical impedance according to multiple regression equations described by Pereda et al. (2007) to estimate water, protein, ash, fat and energy content (%) in relation to LBW.
Statistical analysis
Trial data were analysed as a completely randomized design using the sas (SAS/STAT User’s Guide, Release 8.2, Inst. Inc, Cary, NC, USA) and the spss program for Windows (SPSS 13.0, Inc, Chicago, IL, USA). Live body weight, estimated body condition and serum metabolic parameters were analysed with a MIXED procedure of the sas program according to an auto-regressive model to analyse repeated measures, including the effects of diet (HL and SL groups) and time (parturition and day 11 pp) as the main sources of variation in the early pp period and their interactions. Does were considered a nested random effect in the treatment. Mean values were compared using a protected Student’s t-test. Mean values of feed intake between nutritional groups were analysed with a GLM procedure of the sas program. Average serum oestradiol and progesterone concentrations, ovary weight and follicular categorization were compared using a protected Student’s t-test with the spss program, considering diet effect as the main source of variation. Chi-square test was carried out to compare the percentage of atretic follicles over the total number of follicles, the metaphase II rate and the peripheral, cortical, homogeneous and abnormal patterns of CG migration index of IVM oocytes. The index of viable, degenerated and unfertilized oocytes, as well as in vitro embryo development after culture and conception rate between nutritional groups were analysed by a chi-square test using the spss program. All the results are expressed as the mean ± SEM, and statistical significance was accepted for p < 0.05.
Results
Steroid concentrations, ovarian follicular categorization and atresia rate
The rearing diet did not exert a long-term effect on the average serum oestradiol and progesterone concentrations at AI time in primiparous does (Table 2).
| Rearing diets | |||
|---|---|---|---|
| Standard-lignin diet (SL) | High-lignin diet (HL) | p-value | |
| Ovary weight/doe (mg) | 329.9 ± 19.1 | 360.6 ± 22.7 | NS |
| Oestradiol 17β (pg/ml) | 21.6 ± 1.9 | 19.3 ± 1.7 | NS |
| Progesterone (ng/ml) | 3.6 ± 1.4 | 4.4 ± 1.7 | NS |
| Follicular population/ovary | |||
| Pre-ovulatory follicles | 10.5 ± 1.9 | 11.2 ± 1.2 | NS |
| Antral follicles | 8.7 ± 1.9 | 10.0 ± 2.7 | NS |
| Secondary follicles | 3.8 ± 0.8 | 3.3 ± 0.3 | NS |
| Primary follicles | 10.6 ± 5.0 | 5.0 ± 1.5 | NS |
| Primordial follicles | 58.7 ± 16.9 | 47.1 ± 7.1 | NS |
| Follicular apoptosis rate/ovary (%) | 20.8 ± 3.0 | 35.1 ± 6.7 | 0.001 |
| Oocyte maturation (n) | 69 | 97 | |
| MII rate (%) | 60.8 ± 6.9 | 29.1 ± 5.1 | 0.001 |
| CG migration rate (%) | |||
| Peripherycal distribution | 16.3 ± 5.3 | 14.5 ± 3.8 | NS |
| Cortical distribution | 16.3 ± 5.3 | 18.1 ± 4.2 | NS |
| Homogeneous distribution | 42.9 ± 7.1 | 20.5 ± 4.4 | 0.04 |
| Abnormal distribution | 24.5 ± 6.2 | 47.0 ± 5.5 | 0.04 |
- NS, non-significant differences.
Ovary weight and mean total of pre-ovulatory ≥1 mm follicles in the ovarian surface per ovary at AI were similar between nutritional groups. In addition, histologically categorized follicular population (n = 1206 follicles) was not affected by the nutritional treatment used.
To assess follicular atresia, a total of 499 follicles were studied for apoptosis signs (HL group, n = 250; SL group, n = 249) (Fig. 2). Does submitted to HL diet during the rearing period showed higher percentage of atretic follicles than the SL group at day 11 pp (p < 0.005; Table 2).
Oocyte in vitro maturation rate
A total of 166 COC were analysed, and significant differences were found for both nuclear and cytoplasmic oocyte in vitro maturation measured as metaphase II and CG migration rates (Table 2, Fig. 3). The SL group showed higher nuclear maturation rate compared with the HL group (p < 0.005). Regarding CG migration, the rate of cytoplasmically matured oocytes, measured as peripheral migration rate of CG, was similar between groups. The HL group presented higher rate of oocytes with abnormal movement or distribution of CG compared with the SL group (p < 0.05).
Ovulation rate, embryo viability and in vitro embryo developmental rate
Ovulation rate per female was similar between nutritional treatments (Table 3). A total of 115 embryos were studied. Mean embryonic viability rate at recovery time, evaluated by morphological criteria, was lower in the HL-fed rabbit does than in the SL-fed group (p < 0.005). The HL group also presented a higher number of retarded embryos (p < 0.05) compared to the SL group. The percentage of unfertilized oocytes and degenerated embryos recovered were similar in both nutritional groups, as well as mean embryo survival after IVC.
| Rearing diets | |||
|---|---|---|---|
| Standard-lignin diet (SL) | High-lignin diet (HL) | p-value | |
| Ovulation rate/doe (n) | 14.3 ± 1.3 | 14.1 ± 0.9 | NS |
| Embryo yield (n) | 66 | 49 | |
| Embryo viability at recovery time (%) | 65.4 ± 5.9 | 44.9 ± 7.1 | 0.001 |
| Unfertilized oocytes (%) | 30.3 ± 5.7 | 36.7 ± 6.9 | NS |
| Embryo degeneneration (%) | 4.5 ± 2.5 | 2.0 ± 2.0 | NS |
| Retarded embryos (%) | 3.0 ± 2.1 | 14.3 ± 5.0 | 0.04 |
| Embryo viability after IVC (%) | 67.4 ± 7.2 | 77.3 ± 9.1 | NS |
| Conception rate (%) | 63.6 ± 11.4 | 58.3 ± 14.8 | NS |
- NS, non-significant differences.
Conception rate
As depicted in Table 3, the average fertility achieved in primiparous does was not significantly different between both nutritional groups.
Feed intake
Digestible energy of the HL diet (Table 4) was lower than that of the SL diet (p < 0.005). Does receiving the HL diet showed a significantly higher daily feed intake (g/day) during pregnancy (p < 0.05) than those fed with the SL diet. A similar feed intake was observed at the beginning of the first lactation in both groups.
| Rearing diets | |||
|---|---|---|---|
| Standard-lignin diet (SL) | High-lignin diet (HL) | p-value | |
| Digestible Energy (MJ/kg)a | 11.3 ± 0.1 | 9.3 ± 0.1 | 0.001 |
| Feed intake (g/day) | |||
| Pregnancy | 156.0 ± 11.6 | 243.0 ± 11.4 | 0.04 |
| Early postpartum period | 267.3 ± 12.4 | 281.0 ± 12.7 | NS |
- aDigestible energy was determined by a faecal digestibility trial according to Pérez et al. (1995).
- NS, non-significant differences.
Metabolic parameters
Does fed with rearing diets did not show significant differences in metabolic parameters neither at parturition nor at day 11 pp (Fig. 4). Throughout the pp period, serum NEFA concentrations significantly decreased from parturition time to day 11 pp in both nutritional groups (HL, p < 0.005 and SL, p < 0.05), whereas the opposite situation was observed for serum protein (HL, p < 0.001 and SL, p < 0.005) and leptin (p < 0.05) concentrations.
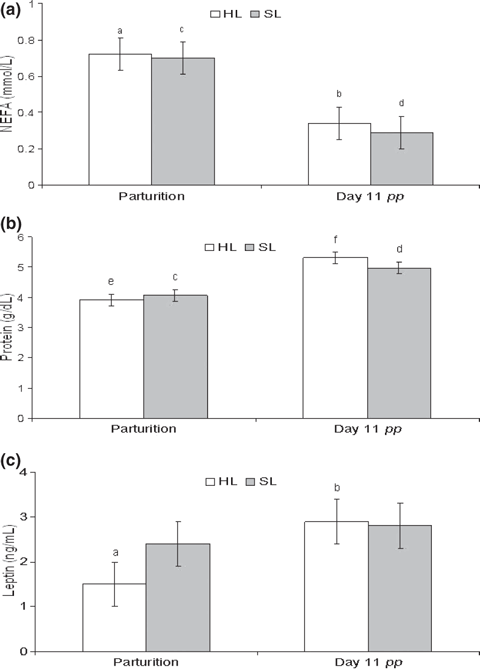
Serum metabolic parameters from parturition until insemination (day 11 pp) in rabbit primiparous does fed with different diets (HL and SL) during the rearing period. Different letters in the same column indicate significant differences: a,b(p < 0.05); c,d(p < 0.005); e,f (p < 0.001)
Live body weight and body composition
Doe LBW and estimated body composition are displayed in Table 5. There were no significant differences in LBW and estimated body composition neither at parturition nor at day of AI between nutritional groups. Live body weight did not increase in the early pp period. With respect to body composition, water content was significantly lower at day 11 pp (p < 0.005), whereas body protein (p < 0.005), lipid depots (p < 0.005) and energy content (p < 0.005) were significantly higher at day 11 pp compared with parturition time in both nutritional groups.
| Diet | Time | p-value | |||||
|---|---|---|---|---|---|---|---|
| High-lignin diet (HL) | Standard-lignin diet (SL) | Parturition | Day 11 pp | pdiet | ptime | p(diet × time) | |
| LBW (g) | 3813.7 ± 3.9 | 3798.1 ± 3.9 | 3834.7 ± 3.9 | 3777.1 ± 3.9 | NS | NS | NS |
| Body compositiona (%) | |||||||
| Water | 62.87 ± 0.2 | 62.39 ± 0.2 | 63.9 ± 0.2 | 61.3 ± 0.2 | NS | 0.001 | NS |
| Ash | 3.14 ± 0.05 | 3.14 ± 0.05 | 3.12 ± 0.05 | 3.16 ± 0.05 | NS | NS | NS |
| Proteins | 17.55 ± 0.1 | 17.68 ± 0.1 | 17.3 ± 0.1 | 17.9 ± 0.1 | NS | 0.001 | NS |
| Lipids | 13.22 ± 0.5 | 13.58 ± 0.5 | 12.5 ± 0.5 | 14.3 ± 0.5 | NS | 0.001 | NS |
| Energy (kJ/100 g) | 1024.5 ± 2.5 | 1042.8 ± 2.5 | 986.4 ± 2.5 | 1080.9 ± 2.5 | NS | 0.001 | NS |
- aBody composition was estimated using multiple regression equations as described in Pereda et al. (2007).
- NS, non-significant differences.
Discussion
In commercial farms, there is a need to improve energy balance in high-yield primiparous lactating does to enhance their reproductive outcome. In this sense, this study provides some reproductive, endocrine and metabolic physiological basis when nutritional management includes highly lignified products in the rearing period. This diet does not seem to enhance effectively reproductive physiology, energy intake or metabolic status of primiparous does in the subsequent pp period.
Controversial results have been described in the literature about the relationship between nutrition and their reproductive consequences. It has been reported that modifications in the composition of the pre-mating diet (high-fibre vs high-starch diets) lead to high in vitro developmental oocyte rate in animals fed with the fibre diet (cattle: McEvoy et al. 1997; gilt: Ferguson et al. 2006). Besides, long-term effects of feeding strategies have also been evidenced on reproductive function in gilts (Cia et al. 1998) and embryo quality in heifers (Mantovani et al. 1993). By contrast, some studies have shown no nutritional differences in ultrastructural oocyte morphology (O′Callaghan et al. 2000) and oocyte quality (Yaakub et al. 1999; Rizos et al. 2008; Arias-Álvarez et al. 2009). The impact of nutrition on follicle-enclosed oocytes and consequently on embryo survival (Zak et al. 1997; Ashworth et al. 1999; Lozano et al. 2003; Lonergan et al. 2006) could be mediated by changes in some metabolic and endocrine parameters (Fortun-Lamothe 2006; Chagas et al. 2007). Cia et al. (1998) showed that reproductive performance can be affected by reductions in body protein mass, as altered in vitro amino acid concentrations influence cytoplasmic maturation (Hong and Lee 2007). In addition, high NEFA concentrations seem to increase in vitro apoptosis of granulosa cells (Vanholder et al. 2005) and reduce the developmental potential of bovine oocytes (Leroy et al. 2005), thus reflecting the detrimental effect of NEB on reproduction (Walters et al. 2002). Body lipid composition and feed intake could also be reflected as changes in serum leptin concentrations. In turn, this hormone may modulate both hypothalamus-pituitary axis (Brecchia et al. 2006) and some aspects of ovarian physiology, such as follicular development (Brannian and Hansen 2002) and oocyte quality (Ryan et al. 2002; Kun et al. 2007; Van Tol et al. 2008).
In this study, serum metabolic parameters, E2 and P4 concentrations, antral follicular population or ovulation rate did not seem to be affected by the rearing diet. Yet, in vitro-matured oocytes from the group fed with the HL diet showed a decrease in nuclear maturation rate and higher number of oocytes with abnormal migration of CG (considered as degenerated or with reduced quality). This finding matches the increased levels of follicular atresia in the HL group. As follicular cells and oocytes are strongly coordinated and mutually dependent, certain follicular events, such as atresia, are thought to be related to oocyte quality (Mermillod et al. 2008). Ovarian follicular atresia is related to follicular health and associated with apoptotic cell death, mainly in granulosa cells (Tilly et al. 1991; Maillet et al. 2002; Hutt et al. 2006).
In agreement with our findings regarding follicular atresia and oocyte maturation, the viable embryo rate at recovery time in the HL group was lower than in the SL group. In this sense, developmental competence of the oocyte and steroidogenic capacity of the pre-ovulatory follicle, as well as the subsequent corpus luteum, seem to be determined by their endocrine and metabolic environment even in the long period prior to ovulation (Britt 1992). Therefore, oviduct microenvironment can be affected in the long term by nutrition and influence normal embryo development (Boland et al. 2001; McEvoy et al. 2001), especially when high-fibre diets are supplied (Adamiak et al. 2006; Ferguson et al. 2006, 2007). These results led us to suggest that (i) although oocyte CG migration rate was similar in both nutritional groups, the SL diet could be affecting other cytoplasmic mechanisms associated to oocyte quality that guarantee higher survival of the early embryo prior to embryonic genome activation, and (ii) there could be other metabolic and endocrine factors not measured in this study which could also mediate the negative long-term nutritional effect of the HL diet on follicular and oviduct environments. Thus, when good morphological embryos were IVC in the same culture medium, in vitro developmental rate was similar in both groups. This result is in agreement with observations reported in pigs fed with different rearing (Le Cozler et al. 1998) or pre-mating diets (Novak et al. 2003).
In this study, we found no differences between nutritional groups in serum leptin, as well as in NEFA and protein concentrations at day 11 pp. This agrees with the absence of differences in body lipid energy and protein contents at such time point and the comparable feed intake during early lactation as reported by others (Delavaud et al. 2002; Ferguson et al. 2003; Chilliard et al. 2005). On the other hand, during the first gestation, energy and protein requirements for the development of the gravid uterus and foetuses are increased (Young 1979; Fortun-Lamothe 2006). As a consequence, in this study, NEFA concentrations were higher at parturition than at day 11 pp in both nutritional groups; yet, serum leptin and protein concentrations were enhanced at day 11 pp in contrast to the results observed in cattle (Leroy et al. 2005) but in agreement with previous reports in rabbits (Fortun 1994). Consistently, we observed lower body lipid depot, energy and protein content at parturition time compared with day 11 pp. The HL diet did not improve enough the energy balance and reproductive physiology in the early pp period, leading to similar fertility rates between groups, according to previous results published by our group (Rebollar et al. 2006). This results support the knowledge about the relationship between body reserve status of high-yield females and their reproductive success (Butler and Smith 1989; Fortun-Lamothe 2006; Lucy 2007). Thus, further studies are required to establish how feeding strategies modify nutritional and metabolic factors affecting oocyte and embryo quality, even during post-implantation period.
Finally, the highly lignified diet used as a feeding strategy to improve nutrient intake in high-yield rabbit does increased feed intake during pregnancy according to previous reports (Gidenne and Perez 1994; García et al. 1999; Nicodemus et al. 1999; Gidenne 2003). This is probably due to the lower DE and the higher transit speed of the HL diet, which may have induced a compensatory high feed intake during this period. Yet, later on, in the early lactation, feed intake of the same commercial lactating diet was not improved in the HL group, and it did not reduce lactation energy deficit, contrarily to the findings reported by other authors (Nizza et al. 1997; Xiccato et al. 1999; Pascual et al. 2002). This is reflected in the similar metabolic parameters, LBW and estimated body composition observed in both nutritional groups in the early pp period.
In conclusion, the impact on follicular health, oocyte competence and early embryo development of a high-lignin-based diet during the rearing period does not lead to reproductive physiology enhancement in primiparous rabbit does. In addition, it does not enhance feed intake during lactation, metabolic status or body composition, although changes in these parameters were observed throughout the pp period studied. This is the first study to approach the possible mechanisms mediating the nutritional and metabolic effects of nutrition on follicular, oocyte and embryo parameters in primiparous lactating rabbit does. Future insights are required to find the most favourable management strategies based on the understanding of the reproductive physiology of these animals.
Acknowledgements
This work was supported by MEC projects AGL07–60168, PR1/07–14906 and the UCM research program (920249). MAA has been granted by CM and FSE, and RMGG was supported by the ‘Juan de la Cierva’ MEC Program. The authors wish to thank P. Bermejo-Álvarez and M. Cuadrado for their invaluable support in the experimental part of this work.
Author contributions
M Arias-Álvarez performed artificial insemination, oocyte collection and in vitro maturation, embryo recovery and culture, confocal study, TUNEL and statistical analysis. RM García-García performed oocyte collection and in vitro maturation, embryo recovery and culture, confocal study, TUNEL and statistical analysis. PG Rebollar involved in maintenance of experimental farm, bioelectrical impedance and statistical analysis. These three authors namely MA, RMG and PGR also contributed to conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content and final approval of the version to be published. N Nicodemus involved in formulation and analysis of the experimental diets and also contributed to conception and design, revising the article critically and final approval of the version to be published. P Millán involved in blood sampling and serum analyses, and revising the article critically and final approval of the version to be published. L Revuelta involved in blood sampling and serum analyses, bioelectrical impedance, and revising the article critically and final approval of the version to be published. PL Lorenzo involved in oocyte collection and in vitro maturation, embryo recovery and culture, confocal study and statistical analysis, also contributed to conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content and final approval of the version to be published.




