Exogenous DNA Uptake of Boar Spermatozoa by a Magnetic Nanoparticle Vector System
Contents
The sperm-mediated gene transfer method is applicable to transgenesis in many species that use spermatozoa for reproduction recently, which has been shown various results. In the current study, we show that transgenic porcine embryos can be efficiently produced by employing a simple transfection method that uses magnetic nanoparticles (MNPs). The complexes formed between plasmid DNA and MNPs were bounded on ejaculated boar spermatozoa at a higher efficiency compared to methods using DNA alone or lipofection. Using confocal microscopy, rhodamine fluorophore-labelled MNPs were detected on external surfaces of the spermatozoa membrane, which were bounded on zona pellucida of in vitro maturated oocyte during in vitro fertilization. Electron microscopy revealed that clusters of MNPs were detected in inside of plasma membrane and nucleus of the spermatozoa head. Additionally, we found that magnetofected boar spermatozoa could be fertilized with oocytes in vitro and that the resulting gene of green fluorescent protein was detected in fertilized eggs by genomic PCR analysis. Taken together, these results suggest that MNPs can be used to efficiently introduce a transgene into embryo via spermatozoa.
Introduction
The previous methods for producing the transgenic animal have a limitation such as the high cost of a manipulator, the need for skilled researchers and low efficiency. For more efficient and simple transgenesis in animals, mammalian spermatozoa have been proposed as a carrier for the introduction of foreign DNA into an egg genome during the fertilization process (Lavitrano et al. 1997). The sperm-mediated gene transfer (SMGT) method is applicable to transgenesis in many species that use spermatozoa for reproduction. The exogenous DNA interacts with DNA-binding proteins (DBPs) that are present on the surface of spermatozoa head (Zani et al. 1995). On the contrary, inhibitory factor I isolated from mammalian seminal fluids could block the binding of exogenous DNA to the surface of the spermatozoa (Zani et al. 1995). Based upon the complexity of spermatozoa–DNA interactions, the SMGT method using naked DNA is thought to be not as efficient for transgenesis. Therefore, it is necessary to find another technique that is capable of introducing foreign DNA into spermatozoa.
To improve the efficiency in the formation of DNA-spermatozoa complexes, many researchers have investigated the possibility of other approaches, such as lipofection (Bachiller et al. 1991), electroporation (Gagne et al. 1991) and use of Triton-X or DMSO-treated spermatozoa (Kuznetsov and Kuznetsova 1995; Perry et al. 1999). Although the quantity of exogenous DNA introduced into spermatozoa by these other methods is higher when compared with the use of naked DNAs, the generation of transgenic offspring is low (Coward et al. 2007; Spadafora 2007). In addition, it fails to fully integrate into the egg genome resulting in rare or transient expression. Therefore, new strategies with regard to the SMGT technique must be developed to resolve these problems.
Magnetofection techniques have been studied extensively for the purpose of delivering drugs or genes into target cells or tissues (Scherer et al. 2002). The basic principle of magnetofection is to associate magnetic nanoparticles (MNPs) with a transgene or virus, so that the complexes can be delivered into intracellular spaces using a magnetic field. This approach has the advantage of significantly increasing the expression of a transgene into the target cell, while reducing overall cell damage.
This present study investigated whether magnetofection technique could be developed that would allow for binding and integration of exogenous DNA on boar spermatozoa by MNPs and for efficiently introducing a transgene into embryo via transfected spermatozoa.
Materials and Methods
Preparation of exogenous DNA
To analyze the exogenous DNA uptake of spermatozoa, the transgene construct, pCX-EGFP/Neo (Miyoshi et al. 2000), contains the enhanced green fluorescent protein (EGFP) reporter gene, cytomegalovirus enhancer, chicken β-actin promoter and rabbit β-globin poly A signal. After pCX-EGFP/Neo was digested with both SalI and PstI, linear DNA fragments were then labelled with 32P – dCTP by the random primer method (Amersham Pharmacia Biotech, Piscataway, NJ, USA) according to standard protocol.
Optimal conditions of exogenous DNA binding on boar spermatozoa by magnetofection
In standard magnetofection, the working solutions (150 μl) were composed of radioactively labelled DNA fragments (80 ng) and 0.5% (v/v) MNPs (PolyMag, OZ Biosciences, Marseille, France) and were incubated in a 96-well plate at room temperature for 30 min. The spermatozoa (107) from Duroc boars in 50 μl of a swine fertilization medium (SFM; Manzini et al. 2002) was mixed in working solution on a 96-well plate, and the plate was then placed on the magnetic field. We changed conditions of standard magnetofection based on the incubation time on the magnetic field, total number of spermatozoa, MNPs (approximately 20 ∼ 30 × 109 particles/ml), and quantity of radioactively labelled DNA fragments, respectively.
To compare efficiency of magnetofection to the other common methods such as only DNA solution and liposome with or without MNPs, the spermatozoa (107) were washed in SFM by brief centrifugation and were then incubated in treatments containing exogenous DNA solution and/or liposome and/or MNPs (Fig. 1e). The treatment prepared that a final volume of 200 μl of SFM contains radioactively labelled exogenous DNA fragments (160 ng), radioactively labelled DNA fragments (160 ng) + 0.5 μl of liposome [0.5% (v/v); SuperFect, Qiagen, Valencia, CA, USA], radioactively labelled DNA fragments (160 ng) + 1 μl of MNPs [0.5% (v/v)], radioactively labelled DNA fragments (160 ng) + 0.5 μl of liposome (0.5% (v/v) + 1 μl of MNPs (0.5% (v/v). Before mixing the spermatozoa, liposome and MNPs were pre-incubated with radioactively labelled DNA fragments for 90 and 30 min, respectively. The samples were washed three times by centrifuging in SFM. And then, the pellet was re-suspended with either 20 μl of SFM or DNase (15 U) solution. The DNase treatment was used to remove the surface binding exogenous DNA on the spermatozoa. Treated spermatozoa were then washed by centrifugation and resuspended in 20 μl of SFM. The radioactivity of each fraction was measured using a liquid scintillation counter

Optimal conditions of DNA binding on spermatozoa by magnetofection. Boar spermatozoa were treated under different conditions such as incubation time on the magnetic field (a), number of spermatozoa (b), and quantity of magnetic particles (c) and DNA fragments (d). (e) DNA binding on spermatozoa by magnetofection under optimal conditions was compared to that by liposome and both of them with (white bar) or without DNase I (15 U) (black bar). Different superscripts (a, b and c) indicate significant differences (p < 0.05) as determined by anova followed by a Fisher’s PLSD as a multiple comparison test. The results were from six-independent experiments
In vitro production of embryos with treated spermatozoa magnetofection
In vitro maturated porcine oocytes were then transferred into a 50-μl drop of fertilization medium (mTBM) containing 0.4% (w/v) BSA covered with mineral oil. For IVF, spermatozoa (107) were washed in SFM by centrifugation and incubated in 200 μl of solution composed of 160 ng of pCX-EGFP/Neo and 0.5% (v/v) of MNPs on the magnetic field for 90 min. Magnetofected spermatozoa were resuspended with mTBM containing 2 mm caffeine to give a concentration of 4 × 105 cells/ml, and 50 μl of spermatozoa suspension were added to 50 μl of the fertilization drops containing the oocytes. After insemination for 6 h, eggs were removed from the fertilization medium and cultured in 100 μl of porcine zygote medium-3 (PZM-3) containing 0.3 mg/ml fatty – acid free BSA.
Confocal laser scanning microscopy
After incubation in mTBM both rhodamine fluorophore-labelled nanoparticles on the spermatozoa and in vitro maturated oocyte for 6 h, putative zygotes were washed in PZM-3 and repetitive pipetted to remove loosely bound spermatozoa. Putative zygotes were then placed into 50 μl drops of Hepes-buffered TALP medium containing 0.1 (v/v) polyvinylalcohol (H-TL-PVA) containing Hoechest 33342 (bis-Benzamide; 1.3 mg/ml) and incubated for 30 min at 39°C, 5% CO2 in air. Putative zygotes were then washed twice in 300 μl of H-TL-PVA, mounted and tightly bound spermatozoa on zona pellucida of zygote were analyzed with CLSM with ultraviolet illumination (excitation at 330–380 nm, emission at 420 nm).
Transmission electron microscopy
Treated spermatozoa MNPs were fixed with 4% glutaraldehyde in 0.1 m phosphate buffer (pH 7.4). The fixed spermatozoa were post-fixed in 1% osmium tetroxide in 0.1 m cacodylate buffer for 1 h at 4°C. The spermatozoa were then washed briefly in 0.1 m cacodylate buffer, dehydrated through a graded ethanol series, infiltrated using propylene oxide and EPON epoxy resin (Structure Probe, West Chester, PA, USA), and finally embedded in Beam capsules. The spermatozoa were polymerized at 60°C for 48 h. Thin sections were cut with a diamond knife on an ULTRACUT UCT ultramicrotome (Leica, Vienna, Austria) and mounted on formvar-coated slot grids. Unstained sections were observed under a Tecnai G2 Spirit Twin transmission electron microscope (FEI Company, Hillsboro, OR, USA) and a JEM ARM 1300S high-voltage electron microscope (JEOL, Tokyo, Japan).
Genomic PCR analysis of embryos
The embryos were analyzed with fluorescent microscope with GFP filter, and then GFP expressing embryo was used in PCR analysis. Briefly, the zona pellucida of embryos was removed by repetitive pipetting, and the cytoplasm of the embryos were washed with PBS and transferred into PCR tubes with 10 μl of deionized water. The cytoplasm was incubated at 100°C for 5 min, and 10 pmol of each primer were added to a PCR mixture tube (AccuPower PCR PreMix; Bioneer). PCR amplification was performed according to standard protocol using forward primer 5′-TGA ACC GCA TCG AGC TGA AGG G-3′ and reverse primer 5′-TCC AGC GGA CCA TGT GAT CGC-3′. The primers were designed to amplify a 307-bp fragment from the pCX-EGFP plasmid (GenBank Accession #: U55763). The PCR reaction conditions consisted of denaturation at 94°C for 1 min, followed by 35 amplification cycles: denaturation at 94°C for 30 s; annealing at 55°C for 30 s; extension at 72°C for 30 s. Cycle 35 contained an additional extension at 72°C for 5 min. Normal embryo and DNA fragments were used as a negative and a positive control, respectively.
Statistical analysis
Values of DNA uptake of spermatozoa are presented as means ± standard error of the mean (SEM) from three replications and were analyzed by anova followed by a Fisher’s PLSD as a multiple comparison test in a Statistical Analysis Systems (sas, Cary, NC, USA) program to determine differences between initial groups and treatments. Significant differences among the treatments were determined when the p value was less than 0.05.
Results
Optimal conditions of DNA binding on spermatozoa by magnetofection
To establish the optimal conditions of DNA binding on boar spermatozoa by magnetofection, we analyzed DNA binding on spermatozoa using varying conditions such as incubation time on the magnetic field, number of spermatozoa and quantity of MNPs and exogenous DNA, respectively. DNA binding c.p.m. of spermatozoa at 90 and 120 min of incubation was significantly (p < 0.05) higher than at 15 min of incubation (Fig. 1a). Moreover, the DNA binding c.p.m of spermatozoa in 106, 107 and 108 spermatozoa were significantly (p < 0.05) higher than that of 105 spermatozoa (Fig. 1b). To determine the optimal effect of DNA binding by spermatozoa with regard to the quantity of MNPs, DNA binding c.p.m. of spermatozoa at MNP concentrations of 0.5% and 1.0% (v/v) was significantly (p < 0.05) higher than those at MNP concentrations of 0.125% and 0.25% (Fig. 1c). We next determined the effect of DNA binding of spermatozoa by manipulating the quantity of exogenous DNA. Increasing exogenous DNA gradually increased the amount of DNA binding of spermatozoa (Fig. 1d). The DNA binding c.p.m. of spermatozoa using 320 or 160 ng of DNA fragments were significantly (p < 0.05) higher than that found at 20, 40 and 80 ng. Based upon these data, we used the 160 ng concentration of exogenous DNA as the optimal condition, because DNA binding of spermatozoa at 160 ng was significantly (p < 0.05) higher than that at 80 ng, and radioactivity (c.p.m.) also gradually decreased at an increasing rate from concentrations over 160 ng. In summary, we determined the following optimal conditions for magnetofection of boar spermatozoa: total spermatozoa (107), 0.5% (v/v) of MNPs, 160 ng of exogenous DNA and 90 min incubation time on the magnetic field. Both MNPs and exogenous DNA concentrations that were used exhibited minimal risk of inducing damage to spermatozoa.
We compared magnetofection to the other common methods such as DNA solution and liposome with or without MNPs. We found that the DNA binding radioactivity (c.p.m.) of spermatozoa by magnetofection and by complexes of magnetofection and liposome was significantly higher compared to those of DNA fragment and liposome with or without DNase I (Fig. 1e). Although DNA binding of spermatozoa by MNPs was not reduced, that by liposome/MNP complexes was significantly (p < 0.05) reduced by DNase I treatment. Therefore, this result implies that DNA uptake on spermatozoa by the liposome method is not as efficient compared to SMGT.
Localization of MNPs on spermatozoa by CLSM and transmission electron microscopy
The spermatozoa head (blue color) and MNPs labelled with a rhodamine fluorophore (red color) were co-localized (Fig. 2a), which also bound on the zona pellucid of oocyte (Fig. 2b). Additional visualization studies using electron microscopy were performed to determine whether MNPs can be transferred into the plasma membrane of the spermatozoa head. Clusters of MNPs were localized inside of the plasma membrane or nucleus of the spermatozoa head (Fig. 2c,d).
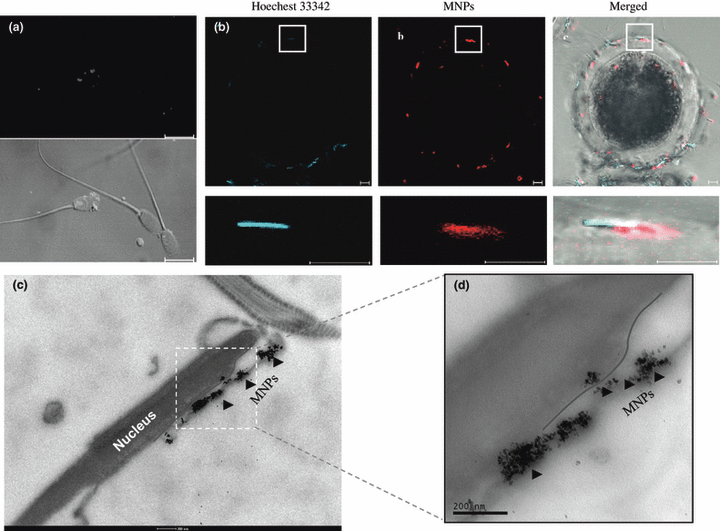
Localization of MNPs in the spermatozoa plasma membrane. MNPs labelled with a rhodamine fluorophore (red color) were shown by CLSM (a,b) with the fluorescence image (excitation, 550 nm; emission, 580 nm). The nucleic of spermatozoa were stained with Hoechest 33258 (blue color). Electron microscopy image of MNPs in spermatozoa head (c) and high magnification (d). MNPs are indicated by dark arrowheads, and plasma membrane is indicated red line. Bar = 10 μm (a,b), 200 nm (c,d)
Detection of transgene of the embryo by genomic PCR
The GFP expressing embryos by fluorescent microscope with GFP filter compared with fertilized embryo with normal spermatozoa as control were analyzed with genomic PCR analysis. As a result, 21 embryos were detected EGFP transgene (Fig. 3).
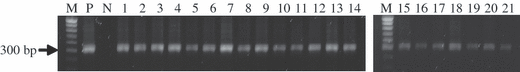
Detection of transgene (EGFP) in the transfected embryo. Genomic DNA of blastocyst and morulae staged embryos were subjected to genomic PCR amplification using a specific primer set for EGFP. M; DNA ladder, P; DNA fragments (positive control), N; Normal porcine embryo (negative control), Number 1-21; blastocyst or morulae staged embryos
Discussion
A major goal of this research was to investigate the potential for using exogenous DNA uptake of spermatozoa by MNP-mediated gene delivery as a novel means of creating transgenic animals. We first demonstrated the optimal conditions for exogenous DNA binding on boar spermatozoa by magnetofection. The quantity of exogenous DNA binding the spermatozoa was gradually increased according to the increasing incubation times, number of spermatozoa and quantity of MNPs and exogenous DNA (Fig. 1). Specifically, the total number of spermatozoa and quantity of exogenous DNA significantly affected the efficiency of exogenous DNA binding (Fig. 1b,d). This result is similar to expression studies in which a dose-dependent DNA response was reported for the transgene luciferase by magnetofection in NIH3T3 cells (Scherer et al. 2002). Our results show that efficiency of exogenous DNA binding of spermatozoa increased more than two-fold following magnetofection under optimal conditions compared to that of spermatozoa incubated with DNA alone or liposome (Fig. 1e). Our findings are consistent with reported higher transgene expression levels found using NIH3T3 cells by magnetofection compared to the use of Lipofectamine (Scherer et al. 2002). Exogenous DNA binding on spermatozoa by solutions composed of both liposome and MNPs was significantly reduced by DNase I compared to MNPs (Fig. 1e). Magnetic nanoparticles that are bound to exogenous DNA localize to either the inside of the plasma membrane or to the plasma membrane itself, while most liposome-bound exogenous DNA localizes to the plasma membrane. These data indicate that exogenous DNA binding on spermatozoa by magnetofection was much more efficient and stable.
Autoradiography using radioactive material (Francolini et al. 1993) and confocal microscopy using rhodamine-labelled DNA fragments (Anzar and Buhr 2006) has been the primary techniques used to investigate binding and localization of exogenous DNA on spermatozoa. However, these techniques are both complex and labour intensive. Our research directly shows that MNPs on spermatozoa can be visualized by fluorescence microscopy techniques using nanoparticles labelled with a rhodamine fluorophore without complications. Our results show that MNPs predominantly bound to the head and tail regions of spermatozoa (Fig. 2a), which was located in zona pellucida of oocyte (Fig. 2b). This data demonstrate that the treated spermatozoa with MNP may be possible fertilization with maturated oocyte or insemination. In the previous studies, DNA molecules have been shown to bind to post-acrosomal regions of the spermatozoa head (Horan et al. 1992), since DBPs found on the spermatozoa surface interact with exogenous DNA. The results of our study show that DNA uptake of spermatozoa by magnetofection is more efficient compared to that of DNA alone, because MNPs can bind over the entire surface of the spermatozoa, while exogenous DNA only binds the DBPs on the spermatozoa head.
In this study, nanoparticles localized in inside the plasma membrane and/or nucleus of the spermatozoa head (Fig. 2c,d). Interestingly, after acrosomal exocytosis by spermatozoa, a single MNP was detected that continually stayed in the nucleus and plasma membrane (data not shown). It is likely that the acrosomal reaction has no negative influence on the ability of MNPs to bind porcine spermatozoa, which is important for gene transfer into an oocyte. Because the acrosomal reaction of mammalian spermatozoa is an exocytotic event that occurs prior to or during spermatozoa penetration of the zona pellucida around the oocyte (Barros et al. 1967), exogenous DNA weakly bound to or inserted onto the spermatozoa membrane during the acrosome reaction may undergo little or no release before fertilization. These results imply that large amounts of exogenous DNA localize to the spermatozoa head.
In this present study, we also investigated the EGFP expression in embryos inseminated with magnetofected spermatozoa. The EGFP was successfully detected in the cytoplasm of embryos at the morula stage of development by CLSM analysis (data not shown). In addition, the GFP positive embryos confirmed to insert transgene by genomic PCR analysis (Fig. 3). These results imply that the exogenous DNA could be transferred into fertilized oocyte by magnetofected spermatozoa.
Taken together, our results clearly demonstrate that MNPs containing exogenous DNA can be transferred into the plasma membrane of spermatozoa, and the exogenous DNA can be delivered into the oocyte through IVF. Magnetofection technique could help improve the efficiency of SMGT in order to livestock transgenic applications.
Acknowledgements
This work was supported by a grant (Code# 20070401034015) from BioGreen21 Program, Rural Development Administration and by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. R01-2008-000-21076-0) Republic of Korea. We thank Seung Hae Kwon from the Korea Basic Science Institute Chuncheon Center for his help with fluorescence imaging by CLSM.
Author contributions
TS Kim, GT Gang, MY Shin: Experiment and design SH Lee, SU Kim: analyzed data DB Ko, CK Park, DS Lee: drafted paper




