Endometrial Population of Oestrogen Receptors Alpha and Beta and Progesterone Receptors A and B During the Different Phases of the Follicular Wave of Llamas (Lama glama)
Contents
The aim of this study was to characterize the distribution of oestrogen receptor (ER)α and ERβ as well as both progesterone receptors isoforms progesterone receptor (PR) A and PRB in the luminal and glandular epithelia and stroma of the endometrium during the different phases of the follicular wave in llamas. Six llamas were examined by transrectal ultrasonography, and a transcervical biopsy was obtained when a follicle at the growing, plateau and regressing phase was recorded. Blood samples were collected at the time of biopsy for hormone determinations. An immunohistochemical technique was used to study receptor populations. Total positive area was evaluated in the different cell types by Image Analysis. Mean diameter measurements of the largest follicle were 6.9, 8.5 and 5.1 mm (p < 0.001) and mean plasma oestradiol-17β concentrations were 27.9 ± 3.26; 30.0 ± 2.79 and 24.0 ± 1.78 pmol/l (p = 0.32) during the growing, plateau and regressing phases, respectively. Immunostaining of ERα was higher in the luminal epithelium during the plateau and regressing phases (p < 0.05) than during the growing phase. More positive cells to ERβ were observed in the glandular epithelium of the growing and plateau phases (p < 0.05) than during the regressing phase. A higher percentage of cells positive to PRB was recorded in the luminal and glandular epithelia during the plateau phase (p < 0.05), while the PRA immunostaining was similar among phases. In brief, this study showed an increased population of ERα and PRB in the luminal epithelium, and only of PRB in the glandular epithelium at the time when an ovulatory follicle is present. The physiological importance of these changes in llamas remains to be elucidated.
Introduction
Ovarian steroid hormones, oestrogen and progesterone, are the main regulators of uterine function. The effects of both hormones on target cells are mediated through their respective nuclear oestrogen (ERs) and progesterone receptors (PRs). Two subtypes of ERs have been described: ERα and ERβ, which have been widely reported in female reproductive tissues of numerous mammalian species (Pelletier et al. 2000; Wang et al. 2000; Cárdenas and Pope 2005). It is well documented that ERα is the pre-dominant subtype in the uterus of most species (Hiroi et al. 1999; Pelletier and El-Alfy 2000; Meikle et al. 2004). Similarly, PR exists in two major isoforms, A and B, and it has been shown that both PRs are expressed in the epithelial and stromal cells of the endometrium in humans and rats (Mote et al. 1999; Sahlin et al. 2006).
Oestrogen and progesterone receptors populations are thought to be critical in determining cell responsiveness to steroids, and thus receptor regulation in endometrium has been studied extensively. Moreover, using immunohistochemical procedures, the regulation of steroid hormone receptor expression has been described to be cell type specific in many species: ewes (Spencer and Bazer 1995; Meikle et al. 2000), monkeys (Brenner et al. 1990), rats (Wang et al. 1999), mares (Watson et al. 1992), cows (Boos et al. 1996; Robinson et al. 2001), sows (Sukjumlong et al. 2003, 2005) and llamas (Bianchi et al. 2007). The results of these immunohistochemical studies indicate that the various cell types present in the endometrium exhibit different patterns of immunoreactivity for ER and PR (Boos et al. 1996), and thus the overall uterine response to steroid hormones depends on the combined responses of the various cell types (Cherny et al. 1991).
Steroid hormone receptor populations in the endometrium change during the oestrous cycle in several species, e.g. ewes, cows and mares. These changes alter the responsiveness of the target tissues to circulating hormones (Spencer and Bazer 1995; Robinson et al. 2001; Hartt et al. 2005). Several studies have demonstrated that oestrogen up-regulates the expression of both receptors in uterus, while progesterone generally has the opposite effect (Wathes et al. 1996). In most mammals, the pre-ovulatory surge of oestrogen increases the expression of ERα (Bergman et al. 1992; Robinson et al. 2001; Hartt et al. 2005) and PR (Hartt et al. 2005; Sukjumlong et al. 2005). Furthermore, PR is one of the most well-known oestrogen regulated genes and is therefore considered to be an indicator of oestrogen action (Graham and Clarke 1997; Kurita et al. 2000).
Llamas are induced ovulators, requiring copulation at the time when an ovulatory follicle is present, to trigger the ovulatory process (San-Martín et al. 1968; Fernández-Baca et al. 1970a; Bravo et al. 1990a; Aba et al. 1995). Many studies have reported that in the absence of an ovulatory stimulus, female camelids undergo successive follicular waves, with lack of ovulation and displaying prolonged periods of behavioural oestrous of at least 30 days, interrupted by brief periods of male rejection (San-Martín et al. 1968; England et al. 1971). During each follicular wave, one follicle becomes dominant, grows to maturity and finally regresses (Adams et al. 1990; Bravo et al. 1990b; Chaves et al. 2002). Thus, follicular waves may be divided into phases of growth, plateau and regression (Chaves et al. 2002; Vaughan and Tibary 2006). These waves tend to overlap and alternate between ovaries in 65% of the cases (Chaves et al. 2002). The pattern of follicular waves is positively correlated with plasma oestrogen concentrations, as these concentrations are supposed to be responsible for the prolonged periods of oestrous usually observed (Bravo et al. 1990b; Chaves et al. 2002). Consequently, instead of the typical oestrous cycle observed in other ruminants, with a follicular phase dominated by high plasma oestrogen concentrations followed by ovulation and a subsequent period characterized by high progesterone concentrations, non-mated llamas exhibit a prolonged period of high oestrogen concentrations and absence of progesterone.
The aim of this study was to characterize the distribution of ERα and ERβ as well as both PR isoforms (PRA and PRB) in the luminal and glandular epithelia and stroma of the endometrium during the different phases (i.e. growing, plateau and regressing) of the follicular waves in llamas. Moreover, using an antibody developed to recognize both PR isoforms, PRA + PRB (PRAB), total PR immunostaining was also evaluated.
Materials and Methods
Experimental design
Field studies were performed in compliance with regulations set by the Faculty of Veterinary Sciences, UNCPBA, where activities were carried out. Facilities are located in Tandil, Argentina, at 37°S, 60°W. Six adult non-pregnant and non-lactating llamas were kept in pens isolated from males and fed pasture hay and water ad libitum. Animals were examined every second day by transrectal ultrasonography to asses ovarian status (Pie Medical 100 vet with 5.0/7.5 variable traducer probe, Maastricht, Netherland).
Tissue samples were obtained by transcervical biopsies as previously described in cows elsewhere (Basu et al. 1988). Briefly, after emptying the rectum, the perianal area was washed with water and povidone iodine. A 45 cm long and 0.5 cm wide uterine biopsy forcep, specially designed for llamas, was used. The instrument was introduced transcervically approximately 30 cm into the uterus while directed by rectal manipulation and placed into the left uterine horn. An endometrial specimen weighing approximately 0.5–1 g was collected from a location about 5 cm from the bifurcation of the uterus.
A biopsy was obtained from each animal when a follicle in the growing, plateau and regressing phases was detected by ultrasonography. According to Chaves et al. 2002, follicles with at least two increasing consecutive measurements from 3 mm to 7 mm were included in the growing phase. Follicles with at least two similar diameters around 8 mm were considered being in the plateau phase, and those exhibiting two consecutive decreasing values were defined as regressing follicles. Depending on the animal, the number of ultrasound examinations varied between two to five.
Considering that 99% of the female camelids become pregnant in the left horn (Fernández-Baca et al. 1970b), all endometrial samples were obtained from the middle of this uterine horn to avoid any possible difference in receptor expression between horns. Immediately after collection, tissue samples were fixed in 4% paraformaldehyde and then embedded in paraffin until analysis. No clinical signs of uterus inflammation were observed in any of the animals in response to the biopsy.
Blood samples for progesterone and oestradiol-17β determinations were collected by jugular venipuncture at the time of biopsy. Samples were centrifuged and plasma was stored at −20°C until hormone assays were performed.
Hormone determinations
Progesterone was measured using a RIA kit (Diagnostic Products Corporation, Los Angeles, CA, USA) validated for use in llama plasma (Bianchi et al. 2007). The sensitivity of the assay was 0.3 nmol/l and the intra-assay coefficient of variation was below 13% for concentrations between 0.4 and 128 nmol/l. Oestradiol-17β was determined using a RIA kit (Diagnostic Products Corporation) reported for use in bovine plasma (Sirois and Fortune 1990) and validated for use with llama plasma after minor modifications (Aba et al. 1995). The sensitivity of the assay was 5.6 pmol/l and the intra-assay coefficient was below 11% for concentrations between 5.6 and 180 pmol/l. All samples were measured in duplicates and in one single assay for each hormone. Hormone concentrations are expressed in SI units. To convert from nmol/l to ng/ml and from pmol/l to pg/ml, the following factors should be used: progesterone: 3.2 and oestradiol-17β: 3.7.
Immunohistochemistry
An immunohistochemical technique (avidin-biotin-peroxidase) previously described (Sahlin et al. 2006; Bianchi et al. 2007) was, after minor modifications, used to visualize ERα, ERβ, PRAB, PRA and PRB immunostaining. After the paraffin tissue sections (5 μm) were dewaxed and rehydrated, an antigen retrieval procedure was performed. Sections were pre-treated in a microwave oven at 700 W power, in 0.01 m sodium citrate buffer (pH 6.0) for 10 min, and then allowed to cool for a further 20 min. After washing in buffer, non-specific endogenous peroxidase activity was blocked by treatment with 3% hydrogen peroxide in methanol for 10 min at room temperature (RT). After a 10-min wash in buffer, sections were exposed to a 30-min non-immunoblock using diluted Normal Horse Serum (NHS, Vectastain®; Vector Laboratories, Burlingame, CA, USA) in buffer in a humidified chamber at RT. The antibodies used for detection of ERα, ERβ, PRAB and PRA were all monoclonal mouse anti-human antibodies (Zymed Laboratories, South San Francisco, CA, USA; Cat No. 08-1149, Serotec Cat No. MCA 1974, Zymed Laboratories Cat No. 18-0172 and Novocastra Laboratories Ltd, Newcastle, UK; Cat No. NCL-PGR-312, respectively), and for PRB a monoclonal mouse anti-chicken antibody was used (Affinity Bioreagents, Rockford, IL, USA; Cat No. ABR-MA1-411). The antibodies for ERα and ERβ were diluted 1 : 15 and 1 : 20 respectively, and both incubated on sections at 4°C overnight. For PRAB, the antibody was diluted 1 : 200, for PRB 1 : 250 and for PRA 1 : 700 and incubated on sections at RT for 60 min. Negative controls for each receptor were obtained by replacing the primary antibody with normal mouse IgG at equivalent concentration (Serotec Cat No. MCA 929). After primary antibody binding, the sections were incubated for 30 min for ERα, for 60 min for ERβ and for 45 min for the other receptors, at RT with a biotinylated horse anti-mouse IgG (Vectastain®, Vector Laboratories Cat No. BA-2000) diluted in NHS. Thereafter, the tissue sections were incubated for 60 min for PRB and for 30 min for the other receptors at RT with a horseradish peroxidase-avidin-biotin complex (Vectastain® Elite ABC-kit, Vector Laboratories Cat No. PK-6100). The site of the bound enzyme was visualized by the application of 3,3′-diaminobenzidine (DAB kit; Dako cytomation, Glostrup, Denmark; Cat No. K3466), a chromogen that produces a brown, insoluble precipitate when incubated with the enzyme. The sections were counterstained with haematoxylin and dehydrated before they were mounted with Pertex (Histolab, Gothenburg, Sweden).
For all receptor determinations, washing buffer was (phosphate-buffered saline , 0.1 m; pH = 7.4) except for ERβ where (tris-buffered saline, 0.1 m; pH = 7.6) was used.
Three to six sections from each biopsy (depending on the size of each specimen) were mounted per slide, and a positive and a negative control from human and llama tissues were always included for each assay. In most cases, the biopsy samples contained luminal and glandular epithelia and stroma, even though some of the specimens did not have luminal epithelium. For each receptor, samples were divided into two separate assays but all tissues of the same animal were processed simultaneously to avoid any systemic error.
Image analysis
After a general inspection of each slide, a Leica microscope connected to a computer using Colorvision software (Leica Imaging System Ltd., Cambridge, England, UK) was used to assess immunostaining quantitatively for ERα and PRs by a computer image analysis system as previously reported (Sahlin et al. 2006). Quantification of immunostaining for all receptors was performed on the digitalized images of randomly selected fields of stroma, luminal and glandular epithelia and analysed separately. Ten fields of stroma and glandular epithelium and all luminal epithelium were measured separately in each tissue section. For each compartment, proper area was selected and all elements not belonging to the cells under study (e.g. blood vessels) were excluded interactively. By using colour discrimination software, the total area of positively stained cell nuclei (brown reaction product) was measured, and expressed as a ratio of the total area of cell nuclei (brown reaction product + blue haematoxylin).
Manual scoring
As the staining of ERβ was faint in all cell types, manual scoring was used to properly evaluate ERβ immunostaining. Evaluation was done independently by two observers blinded to the group of animals. The staining was evaluated semiquantitatively using a grading system. The immunostaining intensity was graded on a scale of: 0, no staining; +, weak staining; ++, moderate staining; and +++, strong staining. As not all cells stained positively, the proportion of positive to negative cells was also considered.
Statistical analysis
Data from follicular diameters was analysed by one-way analysis of variance (anova) to determine differences between means, and the Tukey-Kramer Multiple Comparison Test was performed for evaluation of significance. anova test was also applied to detect variations in plasma hormone concentrations due to the different phases of the follicular wave (growing vs plateau vs regressing). Results are expressed as mean ± SEM.
Statistical analysis for receptor population was performed using the Statistical Analysis System (SAS, Institute Inc., Cary, NC, USA). Nuclear staining was analysed by the mixed procedure, and the statistical model includes the effects of the different phases of the follicular wave, cell types (luminal and glandular epithelia and stroma) and the interactions between them. Data are presented as least square means ± pooled standard errors and the level of significance was always p < 0.05.
Results
Follicular size and hormone determinations
The occurrence of repetitive waves of ovarian follicular growth and regression was observed. Although different wave patterns were observed between individual llamas, it was possible to clearly divide all waves into phases of growth, plateau and regression by ultrasonography. In addition, overlapping of the follicular waves was observed.
Mean diameter of the largest follicle was 6.8 ± 0.02; 8.5 ± 0.01 and 5.1 ± 0.03 mm, at the time when biopsies were obtained during the growing, plateau and regressing phases respectively (p < 0.001). During the regressing phase, follicular growth in the contralateral ovary was recorded with the mean diameter of the new follicle being 4.4 ± 0.05 mm. During the growing and plateau phases, the follicular diameter in the contralateral ovary was always below 4 mm.
Mean plasma progesterone concentrations were between 0.4 and 1.5 nmol/l in all phases.
Mean plasma oestradiol-17β concentrations were 27.9 ± 3.26; 30.0 ± 2.79 and 24.0 ± 1.78 pmol/l during the growing, plateau and regressing phases respectively (p = 0.32) (Table 1).
| Phase | Mean follicular diameter (Mean ± SEM) | Mean oestradiol concentrations (pmol/l−1) | Mean progesterone concentrations (nmol/l−1) |
|---|---|---|---|
| Growth | 6.8 ± 0.02 | 27.9 ± 3.26 | 0.88 ± 0.22 |
| Plateau | 8.5 ± 0.01 | 30.0 ± 2.79 | 0.54 ± 0.21 |
| Regression | 5.1 ± 0.03 | 24.0 ± 1.78 | 0.74 ± 0.25 |
Endometrial receptors
Oestrogen and PRs were confined exclusively to the nuclei of all endometrial cell types studied. When specific monoclonal antibodies were substituted with a normal mouse IgG, the absence of staining confirmed the high specificity of immunostaining in all receptors (1, 2).
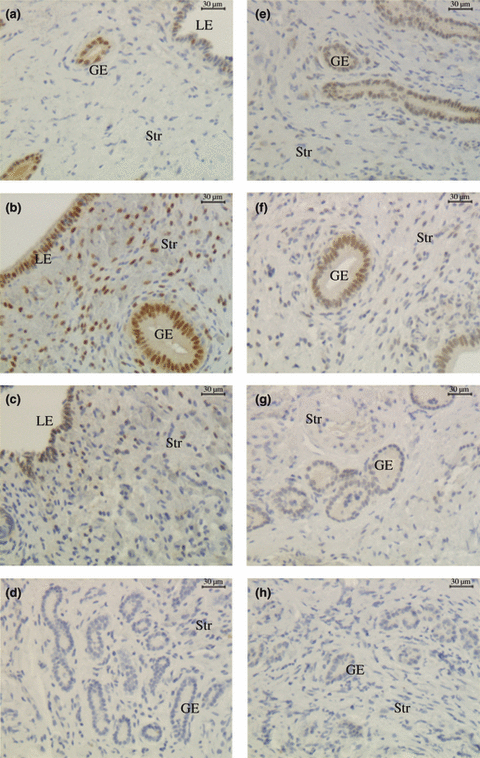
Immunohistochemical localization of oestrogen receptor (ER)α and ERβ in endometrium of llamas during the growing (a, e), plateau (b, f) and regressing (c, g) phases of the follicular wave (LE, luminal epithelium; GE, glandular epithelium; Str, Stroma) (200x). (a) ERα, growing phase; (b) ERα, plateau phase; (c) ERα, regressing phase; (d) ERα, negative control; (e) ERβ, growing phase; (f) ERβ, plateau phase; (g) ERβ, regressing phase; (h) ERβ, negative control
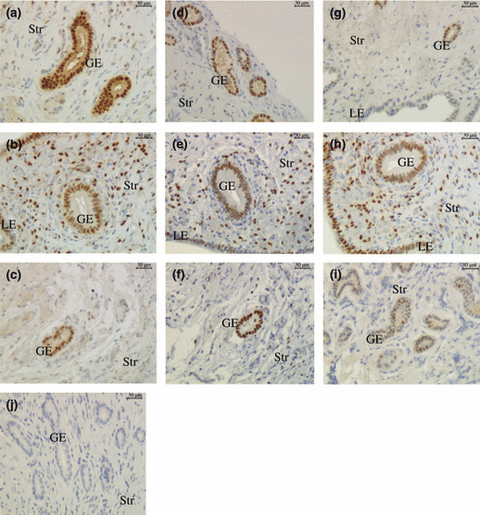
Immunohistochemical localization of PRAB, PRA and PRB in endometrium of llamas during the growing (a, d, g), plateau (b, e, h) and regressing (c, f, i) phases of the follicular wave (LE, luminal epithelium; GE, glandular epithelium; Str, Stroma) (200x). (a) PRAB, growing phase; (b) PRAB, plateau phase; (c) PRAB, regressing phase; (d) PRA, growing phase; (e) PRA, plateau phase; (f) PRA, regressing phase; (g) PRB, growing phase; (h) PRB, plateau phase; (i) PRB, regressing phase; (j) negative control
Oestrogen receptors
Oestrogen receptors alpha was detected in the nuclei of the three endometrial cell types, i.e. luminal epithelium, glandular epithelium and stroma during the three phases of the follicular wave. In the luminal epithelium, the percentage of positive cells was higher during the plateau and regressing phases than that during the growing phase (p < 0.05). No significant differences were recorded in the mean positive area of glandular epithelium and stroma during the different phases of the follicular wave (1, 3). For all phases, epithelia (luminal and glandular) displayed more positive cells than stroma (p < 0.0001).
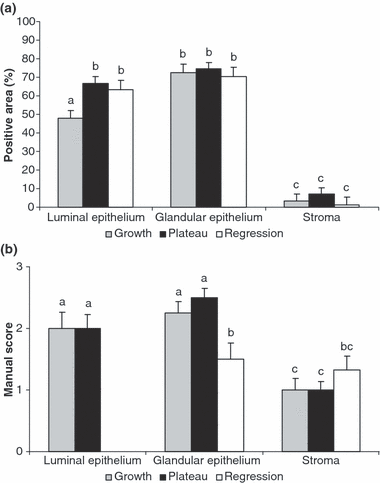
Positive area for oestrogen receptor (ERα) (a) and manual scoring of ERβ (b) during the growing (grey bars) plateau (black bars) and regressing (white bars) phases of the follicular wave in llamas. Bars with different letters are significantly different (p < 0.05)
Oestrogen receptors beta immunoreactivity was also detected in the nuclei of all cell types studied during the growing and plateau phases. During the regressing phase, ERβ was just evaluated in the glandular epithelium and stroma. Due to limitations in the amount of tissue sample collected, it was not possible to evaluate ERβ in the luminal epithelium of the regressing phase. The immunostaining of ERβ was higher in the glandular epithelium of the growing and plateau phases (p < 0.05) than that in the regressing phase (1, 3). As observed for ERα, ERβ was fainter in the stroma than in the epithelia (p < 0.05), except during the regressing phase when the staining was similar between the glandular epithelium and the stroma.
Progesterone receptors
Progesterone receptors immunostaining was visualized in the nuclei of the different cell types during all phases of the follicular wave.
When the percentage of positive cells was evaluated using an antibody with the capacity to bind both isoforms, no significant differences were observed between the different phases (2, 4). However, as it was previously recorded for ERs, the percentage of positive area was greater in epithelia than that in the stroma (p < 0.05).
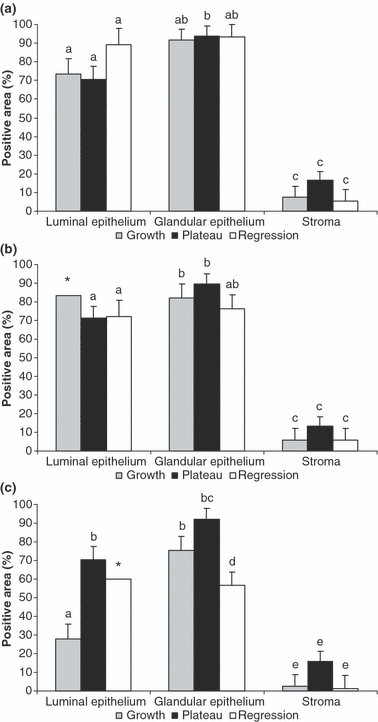
Positive area for PRAB (a), PRA (b) and PRB (c) during the growing (grey bars) plateau (black bars) and regressing (white bars) phases of the follicular wave in llamas. Bars with different letters are significantly different (p < 0.05; except Fig. 4c. b vs d p = 0.067; * no statistical significance is reported due to the low amount of tissue available for these groups)
When both PRA and PRB were evaluated separately, it was observed that the immunostaining of PRA in all cell types was similar between the different phases of the follicular wave (2, 4).
Conversely, significant differences were demonstrated in the immunostaining of PRB. A lesser positive area was detected in the luminal epithelium during the growing phase than during the plateau phase (p = 0.0005) (2, 4). The percentage of positive cells for PRB in the luminal epithelium of the regressing phase could not be evaluated in a sufficient amount of tissue. In the samples in which luminal epithelium was observed, the percentage of positive cells was similar to that recorded during the plateau phase. The immunostaining of the glandular epithelium was significantly greater (p = 0.0006) during the plateau phase and tended (p = 0.067) to be higher during the growing phase than during the regressing phase (2, 4).
Discussion
To our knowledge, this is the first report where the populations of ERs and PRs are studied at the same time, and where each ER subtype and PR isoform are determined in the endometrium of a ruminant species and particularly in llamas. Moreover, this is the first study evaluating the population of ERs and PRs during the phases of follicular growth, plateau and regression in llamas.
The fact that plasma progesterone concentrations remained below 1.5 nmol/l during the study clearly indicates the absence of functional corpora lutea (Adams et al. 1991; Aba et al. 1995). Meanwhile, the observation that plasma oestradiol-17β concentrations were not statistically different between the different phases was somehow unexpected. The overlapping of follicular waves occurring in llamas during which the mature follicle starts to regress when another follicle is growing and therefore releases oestradiol (Bravo et al. 1990b) may account for the similar oestradiol-17β levels observed between the different phases.
The higher immunostaining of ERα in the luminal epithelium during the plateau phase is in agreement with the expression of ERα in ewes and cows, reported to be higher around ovulation (Spencer and Bazer 1995; Robinson et al. 2001). At this time, an ovulatory follicle, bigger than 7.0 mm (i.e., plateau phase), is present and plasma oestradiol-17β concentrations have been suggested to be higher in llamas (Chaves et al. 2002).
The increase in the immunostaining of ERβ in the glandular epithelium during the growing and plateau phases recorded in this study somehow resembles previous reports in humans. Lecce et al. 2001; demonstrated that the expression of ERβ varies along the menstrual cycle. Thus, ERβ labelling peaks in the peri-ovulatory period in epithelial cells in humans is comparable with the increase recorded during the growing and plateau phases in llamas. (Lecce et al. 2001). Previous reports have shown an absence of ERβ RNAm during the follicular phase in llamas (Powell et al. 2007). The disclosure may be related to the fact that in the latter study no attempts to identify ERβ population were carried out, as well as there is no mention of the stage of the follicular development at the time of sample collection. In rats, it has been suggested that ERβ is not involved in any of the major effects of oestrogens on the uterus (Wang et al. 1999). Although very few reports are available describing the expression of ERβ in the endometrium of other ruminants (Walther et al. 1999; Whitley et al. 2000), it is believed that mainly ERα mediates the classical oestrogen actions on the uterus in these species (Meikle et al. 2004). While the importance of ERβ in llamas remains unknown, the specific cell type regulation during the follicular phase observed in this study and the greater expression of ERβ RNAm in uterus of pregnant llamas, previously reported (Powell et al. 2007), could suggest a possible role of this receptor during pregnancy in llamas.
Early reports in humans demonstrated that the population of the individual PR isoforms A and B change throughout the menstrual cycle in the different cell types (Mangal et al. 1997). More specifically, it was reported that the concentrations of PRB vary considerably, while PRA tends to be relatively constant. It was reported that PRB starts to increase during the late follicular phase when oestrogen level rises to its peak, and both isoforms are highest during the peri-ovulatory phase. It was also suggested that oestrogen induces expression of PRB protein while the level of PRA is much less sensitive to hormonal manipulation in humans (Mangal et al. 1997). These results agree with the patterns hereby presented in llamas, where an increase in the immunostaining of PRB was observed in the luminal and glandular epithelia during the plateau phase, while the PRA level was constant during all phases. Although the importance of the different PR isoforms in llamas is unknown, the relative levels of the uterine PRs can be expected to play an important role in the responsiveness to progesterone (Sahlin et al. 2006). Studies in knockout mice have revealed that PRA and PRB have distinct functions in the uterus. It has been suggested that PRA have the capacity to mediate anti-proliferative and implantation responses to progesterone, while PRB is associated with some proliferative effects of progesterone on the endometrium (Conneely et al. 2002; Mulac-Jericevic and Conneely 2004). It could be speculated that the constant PRA level might be in relation to its anti-proliferative effects, while the relatively high expression of both receptors in the epithelia during the plateau phase (around 70% and 90% of positive cells in the luminal and glandular epithelia respectively) could be necessary to prepare the endometrium for a possible pregnancy. Previous reports have proposed that the up-regulation of PR genes by the pre-ovulatory surge of oestradiol in the endometrial glands, would allow them to fully respond to progesterone to secrete products that will be necessary for the survival of the embryo prior to placentation in the ewes (Ing and Zhang 2004).
The observation that receptor immunostaining varied according to cell type gives further support to the hypothesis that endometrial steroid hormone receptors are regulated in a specific manner depending on cell type (Cherny et al. 1991; Spencer and Bazer 1995; Meikle et al. 2000; Sosa et al. 2006). Stroma presented less immunostaining for all receptors and the population of the different receptors in the stromal cells was constant during the different phases of the follicular wave. These observations are in agreement with previous results in llamas (Bianchi et al. 2007) and sheep (Sosa et al. 2004, 2006), showing that stroma is less sensitive to oestradiol than epithelial cells (Meikle et al. 2000). Oestradiol is required for epithelial DNA synthesis and mitosis, and it has been demonstrated that epithelial ERs alone are neither necessary nor sufficient for uterine epithelial responses to oestradiol. Thus, it has been established that the presence of ERα in the stroma is required for the epithelial proliferation induced by oestradiol (Cooke et al. 1997).
In summary, the results of this study show that ERs and PRs fluctuate during the follicular wave of the llama according to each cell type, supporting the hypothesis that the regulation of reproductive steroid receptors is cell type specific in this species. Moreover, at the time when a mature follicle capable to ovulate is present, an increased population of ERα and PRB was observed in the luminal epithelium, whereas in the glandular epithelium only PRB showed increased immunostaining. Although the physiological importance of the different receptors remains to be elucidated, the changes observed during the different stages in the different cell types could be related to their possible roles in the reproductive process in llamas.
Acknowledgements
This study received financial support from The Swedish Research Council (project 73X – 20137 LS), The Swedish Institute (CB) and Karolinska Institutet. This research was also supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (PICT 08-14231).
Author contributions
Bianchi, Carolina Paula, has contributed to the design of the experiment. She has carried out the experiments, set up the immunohistochemical technique for the determination of each receptor and analysed the staining of each receptor. She has collected and analysed the data, and has written the article. Sahlin, Lena has contributed to setting up the immunohistochemical technique to measure each receptor, evaluating the staining, analysing data and revising critically the content of the article. Meikle, Ana has contributed with the design of the experiment. She has contributed to the statistical analysis and its interpretation, and the revision of the article. Masironi, Britt has contributed to setting up the immunohistochemical technique, evaluating the staining of the receptors, collaborating with the interpretation of the statistical analysis and revising the contents of the article. Cavilla, María Verónica has contributed in carrying out the experiment and with the contents of the article. Aba, Marcelo Alfredo as the director of the doctoral project of Miss Bianchi, Carolina, has designed the experiment. He has contributed in carrying out the experiment, with the analysis of the data and revising critically the contents of the article.




