Comparative Immunolocalization of GLUTs 1, 2, 3 and 5 in Boar, Stallion and Dog Spermatozoa
Contents
Spermatozoa, as other eukaryotic cells, need hexoses to produce energy to maintain membrane homeostasis, to move along the female genital tract and to carry the male genome to the female gamete. GLUTs are a family of proteins that permit and improve the passive transport of hexoses inside cells. This study was aimed at investigating the presence and localization of GLUTs 1, 2, 3 and 5 in boar, stallion and dog spermatozoa by both immunofluorescence and western blotting. GLUTs exhibited a peculiar distribution along the sperm cell depending on the isoforms considered, the hexose they transport and the different species. The localization of GLUTs after capacitation and acrosome reaction highlighted the possible changes in their distribution because of the different functional moment. Only in dog spermatozoa changes in GLUTs distribution were demonstrated; these changes could be related to the different metabolic needs and modifications occurring in the sperm cell.
Introduction
Hexose and pentose transport through plasma membranes is essential for metabolic functions of all tissues, being these molecules the main substrate for metabolic processes as Krebs cycle and pentose phosphate shunt. The sugars’ ability to cross the lipidic bilayer is very low because of their polar and hydrophilic structure and cells need membrane carriers to bring them inside in a faster manner. Two different types of sugar transport may be distinguished: rapid protein-mediated passive facilitated transport and rapid active protein-mediated transport. GLUTs are a family of 14 transmembrane proteins (Scheepers et al. 2004) that transport glucose in a passive manner and according to a concentration gradient; they facilitate hexoses’ transport in an enzymatic-like manner, in that they can be saturated by a fixed concentration of their substrate (Carruthers 1990). On the basis of structural and functional characteristics, GLUT family members have been classified into three subclasses (Scheepers et al. 2004):
- •
Class I: GLUTs 1, 2, 3, 4, 14: proteins with particular tissue distribution;
- •
Class II: GLUTs 5, 7, 9, 11: GLUT 5 is a fructose transporter and the others are related proteins;
- •
Class III: GLUTs 6, 8, 10, 12, HMIT 1: proteins characterized by the translocation of the glycosilation site from the first to the ninth extracellular linker domain.
GLUT 1 is responsible for the basal uptake of glucose in all body tissues, particularly with a high expression in erythrocytes and brain (Mueckler et al. 1985; Birnbaum et al. 1986; Duhlmeier et al. 2007); GLUT 2 is a low-affinity glucose and fructose transporter, found in pancreatic β cells, liver, intestinal epithelium and kidney (Fukumoto et al. 1988; Thorens et al. 1988; Wood and Trayhurn 2003); GLUT 3 is a high affinity glucose transporter, abundant in brain and less in other tissues (Thorens et al. 1990; Haber et al. 1993; Maher and Simpson 1994); GLUT 4 exhibits an insulin dependent activity and is mainly expressed in insulin sensible tissues, such as skeletal muscle and adipocytes (Birnbaum and Clarkson 1989; Watson and Pessin 2001; Duhlmeier et al. 2007); GLUT 5 is a fructose transporter highly expressed in small intestine, kidney and muscle (Burant et al. 1992; Shepherd et al. 1992; Concha et al. 1997); GLUT 6 is expressed in leukocytes and brain (Doege et al. 2001a); GLUT 7 was found in small intestine and colon (Li et al. 2004); GLUT 8 is a glucose transporter widely expressed in a lot of tissues such as brain, testis, spermatozoa, liver and kidney (Doege et al. 2000; Schürmann et al. 2002; Gómez et al. 2006); GLUT 9 is a glucose transporter expressed in brain and leucocytes (Phay et al. 2000; Carayannopoulos et al. 2004); GLUTs 10 and 11 are glucose transporters of heart and skeletal muscle (Doege et al. 2001b,c; McVie-Wylie et al. 2001); GLUT 12 has a tissue distribution similar to GLUT 4 and has been also discovered in rat mammary gland (Rogers et al. 2002; Macheda et al. 2003); GLUT 14, a novel member of GLUTs’ family, is very close to GLUT 3 structure and is expressed in human testis (Wu and Freeze 2002).
GLUTs 3 and 5 are known to transport glucose and fructose both in human and rat spermatozoa (Burant et al. 1992; Haber et al. 1993); GLUTs 1, 2, 3, 4 and 5 have been demonstrated in human, bull and rat spermatozoa, in which they have been specifically localized along the sperm membrane (Angulo et al. 1998). Finally, GLUTs 3 and 5 have been found and localized also in dog (Rigau et al. 2002) and boar spermatozoa (Medrano et al. 2006; Sancho et al. 2007).
On the basis of all these information, this study was aimed at investigating the presence and comparing the distribution of GLUTs 1, 2, 3 and 5 in boar, dog and horse spermatozoa by immunofluorescence and western blot analysis. In addition, we investigated the possible relocation of these GLUTs in the three species in different functional moments as capacitation (Cap) and acrosome reaction (AR) due to the highest energy requirement during these phases of sperm life.
Materials and Methods
All the reagents were obtained from Sigma Chemical Co (St Louis, MO, USA), unless otherwise specified.
Semen collection and preparation
Sperm rich fraction of at least seven ejaculates was obtained with the gloved-hand technique from mature boars (aging from 9 months to 3 years) and dogs (aging from 2 to 10 years), while stallion semen (average age 8 years) was collected by an artificial vagina. Boars and stallions samples were obtained every week from different animals, while dog semen wasn’t collected in fixed time intervals.
Semen was brought to the laboratory within 30 min and it was diluted with an equal part of specific extender: Androhep™ for boar sperm (Minitüb, Tiefanbach, Germany), Tyrode for stallion (Rathi et al. 2001) and Tris glucose for dog. After that, aliquots of these diluted samples were immediately fixated for immunofluorescence technique, others were used for western blot analysis, induction of Cap and AR.
Induction of in vitro capacitation and AR
Boar spermatozoa were washed in phosphate-buffered saline (PBS) combined with 0.4% bovine serum albumin (BSA), and washed twice at 800 × g for 3 min. The pellet was resuspended in 2 ml of Brackett and Oliphant’s medium supplemented with 12% foetal calf serum (FCS) (Invitrogen srl, San Giuliano Milanese, MI, Italy) and 0.7 mg/ml caffeine at a final concentration of 100 × 106 spermatozoa/ml and incubated at 39°C in a humidified atmosphere of 5% CO2 for 3 h in order to induce in vitro capacitation.
A modified Tyrode medium added with bicarbonate (Tyr + bic) was used for inducing Cap in stallion spermatozoa (Christensen et al. 1996). Two millilitre of stallion sperm were transferred into a 15 ml tube, mixed with 6 ml of Tyr (without bicarbonate) and centrifuged at 900 × g for 10 min to allow the removal of seminal plasma and washed twice. The final pellet was resuspended in Tyr + bic to a final concentration of 25 × 106 spermatozoa/ml and then incubated at 37°C for 4 h.
Canine spermatozoa were suspended in Tris and washed twice by two successive centrifugations at 200 × g for 10 min. The resulting pellet was resuspended in the capacitating medium (1-CCM) at a final concentration of 60–80 × 106 spermatozoa/ml and incubated for 4 h at 38.5°C in a 5% CO2 atmosphere (Rota et al. 1999).
After different times of incubation in capacitating medium (1.5 h for boar, 3.5 h in stallion and 3 h and 45 min in dog), aliquots of semen were incubated in presence of 10 μm calcium ionophore A23187 for 30 min (horse and boar) and 10 min (dog) in order to induce AR.
Sperm evaluation
Sperm plasma membrane integrity
Sperm viability was evaluated by incubating 25 μl of semen with 2 μl of a 300 μm propidium iodide (PI) stock solution and 2 μl of a 10 μm SYBR GREEN-14 stock solution, both obtained from the live/dead sperm viability kit (Molecular Probes, Inc, Eugene, OR, USA), for 5 min at 37°C in the dark. After incubating for 3–5 min, 10 μl of sperm suspension, after mounting, were analysed with a Nikon epifluorescence microscope. The spermatozoa with green or red fluorescence on the head were considered live or dead, respectively.
Sperm capacitation
The degree of capacitation was assessed by different methods depending on the species: boar spermatozoa on the basis of Hsp-70 immunolocalization (Spinaci et al. 2005), stallion and dog sperm by chlortetracycline (CTC) staining.
Indirect immunofluorescence was performed in boar spermatozoa as previously described (Spinaci et al. 2005). During capacitation and, further on, AR, Hsp70 undergoes a redistribution with typical patterns that have been demonstrated to be strictly related to the functional status of sperm. Hsp70 immunoreactivity in uncapacitated spermatozoa is confined to a well-defined triangular-shaped area in the equatorial segment (uncapacitated pattern) while capacitated spermatozoa exhibit the reactivity in the equatorial line sometimes associated with a fainter triangular signal and/or a semicircular line on the anterior boundary of the equatorial segment (capacitated pattern).
For dog and stallion spermatozoa, CTC method was carried out as follows. Briefly, 50 μl of semen suspension was mixed with the same amount of CTC solution (750 μm CTC in a buffer of 20 mm Tris–HCl, 130 nm NaCl, 5 mm l-cysteine); after 30 sec, 10 μl of glutaraldehyde were added and then 10 μl of semen were placed onto a slide in a drop of Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). After mounting, the slides were analysed using an UV set filter. Spermatozoa were considered as capacitated not only when fluorescence appeared on both acrosome and tail, but also with a fluorescence free band on the post-acrosomal region (Mattioli et al. 1995).
Acrosome reaction
The occurrence of AR was confirmed with fluorescein isothiocyanate (FITC)-conjugated agglutinin derived from Pisum sativum (FITC-PSA) staining. Briefly, spermatozoa were fixed for at least 30 min at −20°C in 95% ethanol and dried onto a microscope slide, then incubated with FITC-PSA solution (1 mg PSA–FITC/10 ml H2O) for 15 min under dark conditions at room temperature. The slides were observed with the above described epifluorescence microscope. Spermatozoa with intact acrosome were considered non-reacted, while those with total or partial loss of acrosomal fluorescence were considered as reacted (Cross and Watson 1994).
Immunocytochemistry
All the procedures were carried out at room temperature unless otherwise specified. Sperm cells were spotted onto a poly-L-lysine-coated slide, fixed with absolute methanol at −20°C for 5 min and then with acetone for 30 s. The slides were washed with PBS, dried and then blocked with 10% (v/v) FCS in PBS for 30 min.
Rabbit anti-human GLUTs 1, 3 and 5 polyclonal antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and goat anti-human GLUT 2 polyclonal antibody (Santa Cruz Biotechnology) were added at the proper dilution in PBS 10% FCS. The incubation was carried out overnight at 4°C in humid chambers. After extensive washing, sperm cells were incubated with a goat anti-rabbit (GLUTs 1, 3 and 5; dilution 1 : 2200) and a rabbit anti goat (GLUT 2, dilution 1 : 800) FITC-conjugated secondary antibodies for 1 h under dark conditions at room temperature. Slides were then washed extensively with PBS and mounted with Vectashield mounting medium with PI. Control cells were treated similarly with the omission of the primary antiserum (data not shown). Images were obtained using a Nikon digital camera installed on a Nikon epifluorescence microscope.
Western blot analysis
Protein concentration in supernatants was measured according to Lowry et al. (1951). BSA was used as a standard. Each sample was tested at 750 nm in triplicate using 96-well microtiter plates in a MultisKan EX spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Western blotting analysis was carried out only for stallion and boar fresh spermatozoa, due to a relative difficulty to get dog semen. For protein analysis, spermatozoa (at a concentration of 100 millions/ml) were washed three times in PBS. The pellet was resuspended in PBS and divided into two aliquots: the first one was centrifuged at 800 × g for 5 min and the pellet was resuspended in PBS, while the supernatant was kept in another eppendorf. The second aliquot was sonicated for 2 min and then centrifuged at 10 000 × g for 10 min at room temperature. The supernatant was separated from the pellet that was resuspended in PBS. Each fraction was added with SIGMA anti-protease cocktail and freezed at −80°C until use.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis was performed using 10% Bis–Tris gels with 3-(N-morpholino) propane sulfonic acid as running buffer, under reducing conditions. Appropriate volumes of cytosolic extracts to obtain 35 μg protein/lane were loaded onto each well. After electrophoresis, proteins were transferred to nitrocellulose membranes for 1 h in an Invitrogen Xcell SureLock Blot Module using transfer buffer, pH 7.2. Blots were briefly washed in PBS and the non-specific protein binding was blocked with 4.5% milk powder in PBS–T20 (PBS 0.1% Tween 20) for 3 h at room temperature.
Membranes were then incubated with polyclonal rabbit anti-human GLUT 1, 3 and 5 antibodies (Santa Cruz Biotechnology) and goat anti-human GLUT 2 antibody (Santa Cruz) diluted 1 : 500 in Tris-buffered saline T-20 (20 mm Tris–HCl, pH 7.4, 500 mm NaCl, 0.1% Tween 20) overnight at 4°C. After several washings with PBS–T20 the membranes were incubated at room temperature with 1 : 20 000 biotin-conjugated anti-rabbit secondary antibody (Stressgen Bioreagents, Ann Arbor, MI, USA) for GLUTs 1, 3 and 5 and 1 : 10 000 anti goat biotin-conjugated secondary antibody (BioFix Laboratories, Owing Mills, MD, USA) for GLUT 2. After several washings the membranes were incubated with 1 : 1000 dilution of anti-biotin antibody, horseradish peroxidase (HRP)- conjugated (Cell Signalling Technology, Danvers, MA, USA).
The western blots were developed using Super Signal West Pico (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s instructions.
Results
Sperm plasma membrane integrity
Viability of fresh sperm cells assessed by SYBR-GREEN14-PI method was 86.4 ± 1.8%, 83.8 ± 2.3% and 80.6 ± 2.9% for boar, stallion and dog, respectively.
Sperm capacitation
The capacitation rate, assessed with Hsp70 immunolocalization in boar semen, showed a capacitation pattern in 88.5% sperm cells; this high rate is due to the high sensibility of the test, that can also detect the very early changes in sperm membrane. As for stallion and dog, the capacitation pattern, observed with CTC method, was 21.9 ± 1.9 and 32.7 ± 5% respectively.
Acrosome reaction
The acrosome reacted spermatozoa were 94.9 ± 0.1% in stallion, 87.2 ± 2% in boar and 83.1 ± 3% in dog.
Immunolocalization of GLUT 1
The localization of this transporter was similar in boar and stallion spermatozoa. In both species the signal was localized along the whole sperm tail and, with a spotted pattern, in the acrosomal membrane. In all stallion spermatozoa, a strong spotted positivity in the neck of the tail was observed. In canine spermatozoa, a very strong signal was evident at the top of the external membrane of the acrosome, while the rest of the acrosomal membrane was negative; the tail showed a very faint signal in all its parts (Fig. 1).
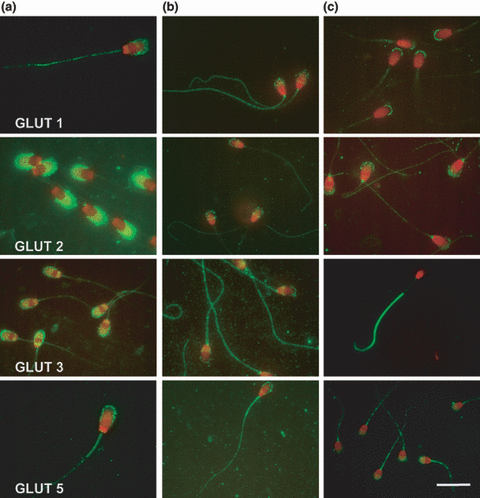
Representative photographs of the distribution of GLUTs 1, 2, 3 and 5 in boar, stallion and dog fresh semen. (a) Boar; (b) Stallion; (c) Dog spermatozoa (Bar 10 μm) 125 × 137mm (500 × 500 DPI)
After capacitation, boar and stallion spermatozoa didn’t show any difference in either intensity or localization of the signal, while dog spermatozoa expressed a different localization of GLUT 1, especially evident in the acrosomal membrane, in which the positivity spread all over the acrosome (Fig. 2).
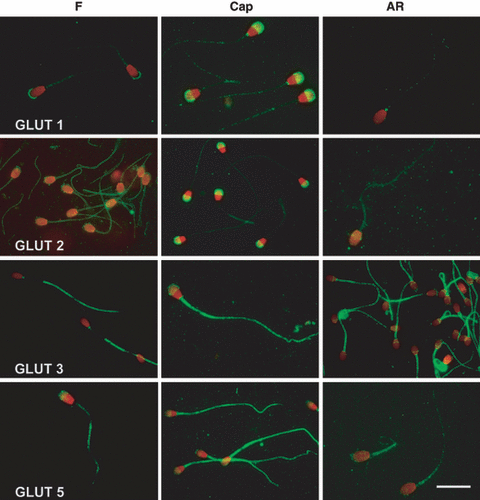
Representative samples of GLUTs 1, 2, 3 and 5 distribution in fresh (F), Capacitated (Cap) and acrosome-reacted (AR) dog spermatozoa. Note the redistribution of the positivity after capacitation (Bar 10 μm) 124 x 135mm (500 x 500 DPI)
Acrosomal reaction didn’t seem to have any effect on sperm localization of GLUT 1, in all the species, except for the disappearance of the positivity in the acrosomal region, due to the removal of the acrosome.
Immunolocalization of GLUT 2
The localization of this low-affinity glucose/fructose transporter was similar in dog and stallion, but not in boar, spermatozoa. In fact, an evident positivity in the acrosomal membrane, similar but stronger than that of GLUT 1, was observed in boar sperm. In stallion and dog the positive signal was present in the acrosome and in the tail; in this last species, a positive spot, localized at the beginning of the tail, was also shown (Fig. 1).
As reported for GLUT 1, no differences were recorded in boar and stallion spermatozoa after capacitation. In dog, the positivity changed a little bit: the positivity in the mid-piece disappeared, while the acrosomal positivity increased (Fig. 2). As for the acrosome-reacted spermatozoa, no changes were recorded in any species, except for the disappearance of the acrosomal positivity.
Immunolocalization of GLUT 3
The localization was different in the three species examined. In boar spermatozoa, the positivity was evident in the acrosome and in a subequatorial band of the sperm head, while a faint immunoreactivity was present in the tail. In stallion sperm cells, a strong signal was evident in the tail, with a particular highlighted neck spot, while acrosome and equatorial band immunoreactivity was fainter. In dog spermatozoa, the positive signal was present only in the principal and end-piece of the tail, with the exclusion of the mid-piece (Fig. 1).
As already seen for GLUTs 1 and 2, the capacitation induced modifications in GLUT 3 localization only in dog’s spermatozoa. In fact, GLUT 3 positivity of capacitated dog spermatozoa was localized in the acrosome and, with a vivid and homogeneous signal, in the whole tail (Fig. 2). Acrosome reaction never caused changes in either intensity or distribution of GLUT 3 signal.
Immunolocalization of GLUT 5
The fructose transporter showed the most constant localization among the three species, in all of which it was localized in the acrosome membrane and in the tail. In most of stallion sperm cells an equatorial line was also present (Fig. 1).
After capacitation, a difference was detected only in canine spermatozoa: the intensity of tail positivity increased, while that of acrosome became fainter (Fig. 2). Also in the case of this GLUT, no significant modifications were observed in the three species when AR was induced, except for the disappearance of the acrosomal positivity.
Western blot
Our investigations demonstrated the presence of immunoreactive bands in non-sonicated pellets (Fig. 3) and in sonicated supernatants; no bands in non-sonicated supernatant and only very weak bands in sonicated pellet, thus confirming the localization of GLUTs in sperm membrane.
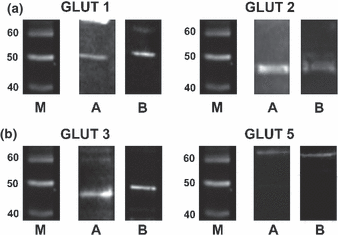
Representative western blottings of the different four GLUTs in boar and horse semen. M, molecular weight markers (kDa); (a) Boar samples; (b) Horse samples 108 x 84 mm (500 x 500 DPI)
Bands of the expected molecular weight for GLUTs 1, 2 and 3 in both boar and stallion spermatozoa were evident. In particular, GLUT 1 showed a reactive band approximately 50 kDa, GLUT 2 at approximately 45 kDa and a second reactive band at approximately 90 kDa, which can represent a dimer of this transporter; GLUT 3 was reactive in a band of approximately 45 kDa in pig and present in horse as a band of approximately 50 kDa. GLUT 5 showed a positive band of more than 60 kDa, both in stallion and boar sperm.
Discussion
In this study, we determined both presence and cellular distribution of the passive-facilitative hexoses transporters GLUTs 1, 2, 3 and 5 in boar, stallion and dog fresh spermatozoa by immunofluorescence; in addition, we carried out western blot analysis on boar and stallion samples to confirm their presence. Finally, the localization of these proteins was determined, for the first time, in capacitated- and acrosome-reacted sperm samples from the three species.
Our findings demonstrate that these GLUTs present a peculiar distribution in the sperm cell, depending on the species and on the difference between the various isoforms. In particular, GLUTs 3 and 5 exhibit stronger positive signals than GLUTs 1 and 2.
Angulo et al. (1998) demonstrated GLUT 1 expression in human, rat and bull spermatozoa and showed a cellular localization, similar to the horse and boar ones described in this study, at the level of the acrosome and in the principal piece of the sperm tail with the exclusion (total in rat and bull, partial in man) of the midpiece. Our findings clearly show other differences: the acrosomal positivity in boar and stallion is not diffuse, but it is distributed in a spotted pattern and horse’s cells present an evident equatorial line.
As for GLUT 2, our results show that its distribution pattern is similar in boar and rat (Angulo et al. 1998), and in man, dog and stallion, respectively (Angulo et al. 1998), while bull is different, being its positivity evident in the sperm head and mid-piece. We could find some similarities in GLUT 3 distribution only between man, horse and boar spermatozoa, while rat ones are immunoreactive only in the tail, and bull ones are positive in the sperm head and mid-piece (Angulo et al. 1998).
As for boar spermatozoa, our findings are consistent with those by Medrano et al. (2006), who confirmed the peculiar aspect of the acrosomal positivity as a cluster of immunoreactive complexes; the physiological role of this distribution is not clear yet, even if others (Carruthers 1990) report a tendency of glucose transporters to form self-associations. Furthermore, GLUT 3 position is strongly correlated to hexokinase distribution in cytoplasm (Medrano et al. 2006): being that glycolytic enzyme binds to tail’s fibrous sheath in mouse sperm cells (Krifalusi et al. 2006), we may hypothesize that GLUT 3 distribution is strictly related to enzymes involved in glycolytic chain, particularly as concerning their localization in the sperm tail. This hypothesis is strengthened by the results by Medrano et al. (2006), demonstrating the importance of hexokinase I as a regulatory factor for glycolysis in boar sperm cells, together with the presence of any hexose transporters. According to this hypothesis, we may suppose a different role played by the different GLUTs in regulating the entrance of hexoses in the sperm cell, with particular attention to fructose and hexose transporters, and the metabolic pathways these substrates undergo in sperm cells of different mammalian species (Fernandez-Novell et al. 2004).
As for immunolocalization of GLUT 3 in dog fresh semen, our results differ from those by others (Rigau et al. 2002), who described a mid-piece immunoreactivity with exclusion of the end-piece. This discrepancy could be the consequence of the fixation technique utilized.
Medrano et al. (2006) demonstrated the presence of GLUT 3 not only on the sperm external acrosomal membrane, but also in some internal structures and suggested it could undergo an exposure on the cell membrane, similar to what GLUT 4 does in insulin sensitive tissues (Watson and Pessin 2001). Our western blot results confirm this possible intracellular localization, as we recorded a positive band in sonicated pellets, containing nuclei as well as other organelles of the sperm cell.
GLUT 5, the fructose transporter, is the most stable in localization among the three species; also the comparison in bull, rat and human spermatozoa localization described by Angulo et al. (1998) strengthens the hypothesis of a well conserved distribution of this transporter into sperm cells: only bull shows a very thick positivity in the end-piece of the tail and rat evidences a poor acrosomal positivity.
This is the first report on the localization of the different hexoses transporters in capacitated and acrosome-reacted sperm cells; these studies were aimed at evidencing a possible functional relocation of GLUTs due to the different functional and metabolic status. As it is well-known, a rearrangement of the acrosomal membrane takes place after Cap, involving both protein content and status and lipid distribution; in addition, sperm cells undergo a different motility pattern, the hyper-activated motility, that can require more energy.
Our results about GLUTs’ relocation in dog spermatozoa could be explained by two different hypotheses: a passive relocation of the proteins or their active relocation. Capacitation could induce the relocation of some proteins that could be important for the next stages of sperm cell life, such as acrosomal reaction and penetration into the zona pellucida, and the translocation of the different GLUTs could be a passive consequence of the active movement of these proteins. Alternatively, GLUTs could actively move depending on the position of the different metabolic pathways the hexoses undergo.
The differences between dog and boar hexose metabolism, described by others (Marin et al. 2003; Albarracìn et al. 2004; Fernandez-Novell et al. 2004) could represent an explanation of the presence of an active relocation of GLUTs in dog but not in boar and stallion spermatozoa. In fact, in dog sperm cells, the use of hexoses not only for a mere energy obtainment (Albarracìn et al. 2004), the presence of a high-km hexokinase (Fernandez-Novell et al. 2004) and the different catabolism of hexose (Marin et al. 2003) are important peculiarities that could permit these spermatozoa to adapt to very diverse metabolic and functional situations as capacitation, and the relocation of GLUTs could be an aspect of this metabolic system. Furthermore, dog spermatozoa remain in the female genital tract for a longer period than other species’ sperm cells and this could lead to the necessity of a different metabolic strategy in energy management that could explained the relocation of GLUTs after capacitation.
Acrosomal-reacted spermatozoa do not present any difference in GLUTs position if compared with capacitated spermatozoa, except for a lack of positivity after external acrosomal membrane removal. This is consistent with an effective localization of the main part of different GLUTs in the external acrosomal membrane.
In conclusion, this study demonstrates the presence of GLUTs 1, 2, 3 and 5 in dog, boar and stallion spermatozoa, describing and underlining the differences in the three species. In particular we found out that each GLUT has a peculiar distribution in the cell and that each species differs in some little particulars from the others. What’s more our data show is that in boar and stallion spermatozoa no changes are detectable after capacitation and AR, while dog sperm exhibit a visible relocation of these proteins after capacitation; this could represent either an adaptation to the peculiar metabolic status of the cell or a passive translocation due to the rearrangement of the sperm membrane.
Acknowledgements
Work supported by a RFO (ex 60%) grant and by “Fondazione del Monte di Bologna e Ravenna”.
Author contributions
All the authors contributed equally to the manuscript.




