Infertile Boars with Knobbed and Immotile Short-tail Sperm Defects in the Finnish Yorkshire Breed
Contents
In the period 1996–2006 two specific sperm defects, the knobbed acrosome (KA) defect and the immotile short-tail sperm (ISTS) defect, showed a strong negative association with fertility in Finnish breeding boars. In this study, we examined the incidence of these two sperm defects in two pig breeds, their effects on fertility and their associations with sperm morphology and testicular histology. Semen samples from 2048 (1097 Yorkshire, 951 Landrace) boars were collected. None of the Landrace boars revealed either the KA defect or the ISTS defect. Of the Yorkshire boars, 0.8% were afflicted with the KA defect and 7.6% with the ISTS defect. Boars diagnosed with the ISTS defect produced no litters. Fertility data were available from two artificially inseminated (AI) boars and six farm breeding boars affected with the KA defect. Breeding boars with 45–81% knobbed spermatozoa (n = 6) did not produce any litters out of 71 sows bred. AI boars with 25–30% knobbed spermatozoa had a poor non-return rate (on average 47% compared with 85% for normal control boars) and produced small litters, on average 2.5 piglets less than other boars of the same breed. Morphometry of testicular tissue and distribution of different cells in the seminiferous tubules were examined in nine boars. Boars with the KA defect had a smaller diameter of the seminiferous tubules (p < 0.05) and a lower number of Sertoli cells (p < 0.05) than controls. ISTS boars, in turn, had a significantly lower number of elongated spermatids (p < 0.05), and they also produced on average only 12% of the spermatozoa of normal boars. The ISTS defect is a manifestation of an autosomal recessive disease caused by an insertion in the KPL2 gene in porcine chromosome 16. Although we tried to map the KA defect, its aetiology remains unclear.
Introduction
Specific sperm defects have been associated with male infertility (Donald and Hancock 1953; Blom 1976). Inherited or presumably inherited specific sperm defects include globozoospermia in humans where spermatozoa lack an acrosome (Aitken et al. 1990; Moretti et al. 2005), the knobbed acrosome (KA) defect in bulls, boars, stallions, rams and dogs (Hurtgen and Johnson 1982; Toyama and Itoh 1993; Soderquist 1998; Chenoweth 2005; Santos et al. 2006) and the immotile cilia syndrome in humans where dynein arms are affected (Afzelius 1976). Phenotypically different sperm tail defects affecting the length of flagella have been reported in spermatozoa of humans, bulls, stallions and boars (Ross et al. 1973; Vierula et al. 1987; Hrudka et al. 1991; Andersson et al. 2000; Sukura et al. 2002). Identification of inherited sperm defects affecting fertility in artificial insemination (AI) and farm breeding boars is of great importance for maintaining high-quality semen and good prolificacy.
In the period 1996–2006, two specific sperm defects with strong negative associations with fertility were observed in Finnish breeding boars. These defects are the KA defect (mainly type 2) (Revell and Chasey 1988; Santos et al. 2006) and the immotile short-tail sperm (ISTS) defect. KA in boars is characterized by a large cystic swelling anterior to the apical part of the acrosome in many of the affected spermatozoa. Although the KA defect in boars has been described before (Alanko 1985; Revell and Chasey 1988; Toyama and Itoh 1993), to our knowledge, no attempt to map the defect in boars has been made so far. Morphologically typical features of ISTS are a markedly reduced sperm tail length and severely affected midpiece microtubular components (Andersson et al. 2000; Sukura et al. 2002). The affected KPL2 gene has been localized in chromosome 16 (Sironen et al. 2002, 2006). In this study, we examined the incidence of the two above-mentioned specific sperm defects in two pig breeds with almost equal numbers of breeding and AI boars. We focused on sperm defects, fertility and testicular histology.
Materials and Methods
Animals and semen collection
Semen samples from 2048 boars (1097 Yorkshire, 951 Landrace) were collected. The specimens, which originated mainly from two boar stations, were examined for both the KA defect and the ISTS defect. Most of the semen samples (88%) were from candidates for use in AI (total 1795 boars; 876 Yorkshire and 919 Landrace). A further 3% (n = 63) of sperm samples were from farm breeding boars associated with a possible boar-related fertility disturbance on the farm. The rest of the semen samples (9%, n = 190) were collected from boars at test stations. The age of the boars upon collection of the first semen sample for sperm morphology studies varied between 8 and 14 months.
Semen smears and semen evaluation
The semen samples were diluted 1 : 1 with MR-A extender (Kubus, Madrid, Spain) prior to making a thin semen smear on the object glass. The smears were air-dried overnight and stained with a Giemsa staining method (Watson 1975); 100 spermatozoa/smears were examined. In a few cases, the testicles and epididymides were sent from the slaughterhouse for further studies. Semen samples were collected from both cauda epididymides and were diluted 1 : 10 with MR-A extender prior to making a thin semen smear on the object glass. For morphological examination of ejaculated and epididymal smears, 100 spermatozoa were examined under a light microscope (1250× magnification) using the Blom (1983) classification. Boars with more than 20% specific sperm defects were considered to have these specific sperm defects as a small proportion of almost any sperm defect can be present in the ejaculate of any male (Chenoweth 2005). For boars with a specific sperm defect, a new semen smear was typically prepared 2 months later.
Testicular histology
Fresh testes were sent from the abattoir to the laboratory and histological specimens were taken immediately upon arrival of the material and fixed in Bouin’s fixative and embedded in paraffin. The samples were available for six boars with specific sperm defects (three boars with the KA defect and three boars with the ISTS defect) and three control boars. Sections (5 μm) were cut and stained with haematoxylin and eosin (HE). For morphometry, 20 randomly chosen tissue sections from each boar were used. The total tissue area and the areas of the seminiferous tubules and of the interstitium were recorded.
To analyse the absolute cross-sectional value of each seminiferous tubule, the area as well as the shortest and longest axis of the tubule were recorded. Spermatogenic and Sertoli cells were identified according to Garcia-Gil et al. (2002), and their numbers were assessed per tubule area in groups of spermatogonia, spermatocytes, round spermatids, elongated spermatids and Sertoli cells. Data are presented as numbers of nuclei per total tubule area.
The morphometry of spermatozoa and testicular tissue was analysed using digitalized light microscopy views with imaging software (3.0 Image Analysis Software; Soft Imaging Systems GmbH, Muenster, Germany) coupled to a digital camera (Color View 12; Soft Imaging Systems GmbH). Morphological parameters of spermatozoa and testicular tissue were manually measured from the images with the software.
Genetic studies
The average inbreeding coefficient for the Yorkshire boars during the 10 year study period was counted. Experimental materials for the genome scan of DNA of boars with the KA defect were collected. Boars possessing the ISTS defect were not included in the genetic study as the aetiology of the defect has been investigated earlier (Sironen et al. 2002, 2006).
Eight Finnish Yorkshire boars affected with the KA defect and eight unaffected and unrelated control boars were used. All affected boars were clinically examined and showed symptoms typical of the syndrome. Six of the affected boars were closely related and inbred. The KA defect was assumed to be inherited as an autosomal recessive defect (Alanko 1985), and therefore a strategy of homozygosity mapping was adopted (see Sironen et al. 2002), where the affected individuals were expected to show increased homozygosity at marker loci near the defect locus.
Semen samples were collected from the 16 boars and DNA was derived from their sperm following phenol/chloroform extraction. Samples were diluted to 10 ng/μl in 1m Tris-HCl + 0.5m EDTA, pH8 (TE) buffer and used as templates in control polymerase chain reactions (PCRs) to confirm equal amplification prior to pooling. Two DNA pools were formed from equivalent amounts of DNA from eight affected and eight control individuals and used as templates for PCR amplification.
Whole genome screening was performed using 242 fluorescently labelled microsatellite markers for autosomal and X chromosomes from the US Pig Genome Coordination Program with an average spacing of 10 cM. Genome coverage of these markers is 91%, and three gaps between markers are longer than 30 cM. Information on PCR product sizes and genetic distances between markers were obtained from the US Pig Gene Mapping Coordination Program web site (http://www.genome.iastate.edu/pig). PCR amplification contained 50 ng of DNA, 1 U of Dynazyme DNA polymerase (Finnzymes, http://www.finnzymes.fi) in buffer supplied by the enzyme manufacturer, 0.2 mm of each dNTP and 10 pmol of each oligonucleotide primer in a final volume of 30 μl. Samples were subjected to 30 amplification cycles (94°C, 1 min; 58°C, 1 min; 72°C, 1 min) with some marker-specific changes in annealing temperature. PCR products were separated by electrophoresis using 5% denaturing polyacrylamide gels (7 m urea) using a MegaBace DNA sequencer (Amersham Biosciences, http://www4.amershambiosciences.com). Allele sizes for all samples were defined by Fragment Profiler, with respect to the TAMRA GeneScan 350 size standard. Markers that displayed increased homozygosity in pooled DNA of affected individuals as compared with unaffected individuals were analysed further by genotyping of individual DNA samples.
Statistical analysis
Data were analysed using SPSS 13.0 for Windows. Differences between control boars and ISTS boars and between control boars and KA boars in morphometry, semen analysis and fertility parameters were analysed with the Mann–Whitney U-test. Results are expressed as means or percentages (±SEM). Differences were considered significant at p < 0.05. For statistical analysis of marker association with the KA defect a chi-squared test was used, with a Bonferroni correction for 242 repeated tests (number of markers).
Results
None of the 951 Landrace boars possessed either the KA defect or the ISTS defect. Of the 1097 Yorkshire boars, nine boars (0.8%) were afflicted with the KA defect and 83 (7.6%) with the ISTS defect. Of the 876 Yorkshire boars selected for use in AI and transported to one of two AI stations for semen evaluation prior to use, three (0.3%) were afflicted with the KA defect and 19 (2.2%) with the ISTS defect.
The distribution of boars with the KA defect and the proportion of this defect in the ejaculate of affected boars are shown in Table 1. Fertility data were available for two AI boars and six farm breeding boars affected with the KA defect. Breeding boars with 45–81% knobbed spermatozoa did not produce any litters from the 71 sows bred. AI boars with 26% or 27% knobbed spermatozoa had poor non-return rates (44% and 50% compared with 85% for normal boars) and produced small litters (Table 1). Fertility data were available for 40 ISTS-affected farm breeding boars. All of these 40 boars were bred to several sows, but produced no litters.
| Number of boars with KA defect | Non-return rate (60 days) | Litter size (number of litters) | % Spermatozoa with the KA defect | |
|---|---|---|---|---|
| Boars selected for use in AI | 3 | |||
| Boar 1 | 44% (209 AIs) | 9.5 (37) | 27 | |
| Boar 2 | 50% (240 AIs) | 8.3 (35) | 26 | |
| Boar 3 | Not used in AI | Not used in AI | 47 | |
| Farm breeding boars | 6 | |||
| Boar 4 | No pregnanciesa | No litters born | 45 | |
| Boar 5 | No pregnancies | No litters born | 65 | |
| Boar 6 | No pregnancies | No litters born | 64 | |
| Boar 7 | No pregnancies | No litters born | 81 | |
| Boar 8 | No pregnancies | No litters born | 70 | |
| Boar 9 | No pregnancies | No litters born | 60 | |
| Total number of affected boars | 9 (Y = 9) |
- AI, artificial insemination; Y, Yorkshire boars.
- a5–37 sows bred/boar.
Morphometry of testicular tissue and distribution of different cell types in seminiferous tubules in the three normal, three KA and three ISTS boars are shown in Table 2. Testicular weights of boars with KA and ISTS did not differ from those of control boars.
| Control boars (n = 3) | ISTS boars (n = 3) | KA boars (n = 3) | |
|---|---|---|---|
| Testicular weight (g/testis) | 438 (±36) | 391 (±32) | 366 (±13) |
| Age (months) | 13 (±1.6) | 13 (±1.6) | 13 (±1.6) |
| Total sperm count in ejaculate | 70 (±5) × 109 | 8.2 (± 1) × 109* | 52 × 109a |
| Proportion of seminiferous tubules in testicular slides | 80 (±4) | 67 (±1)%* | 74 (±2)% |
| Minimum tubular diameter (μm) | 216 (±10) | 208 (±4) | 177 (±9)* |
| Sertoli cells/mm2 | 383 (±17) | 351 (±19) | 204 (±13)* |
| Spermatogonia/mm2 | 650 (±97) | 936 (±191) | 919 (±109) |
| Spermatocytes/mm2 | 1150 (±238) | 1481 (±197) | 1471 (±125) |
| Round spermatids/mm2 | 2031 (±53) | 2275 (±283) | 2266 (±292) |
| Elongated spermatids/mm2 | 2063 (±82) | 980 (±77)* | 2546 (±125) |
| Semen analysis and fertility | |||
| % Normal spermatozoa | 90 (±4) | 0.33* | 11 (±5)* |
| % Sperm motility | 80 (±2) | 0* | 70 |
| % Non-return rate | 86 (±1) | 0* | 0* |
| Litter size (piglets) | 11.9 (±0) | No litters* | No litters* |
| % Specific sperm defects | – | 100* | 65 (±6)* |
- Values are presented as mean (±SEM).
- aOne boar.
- *p < 0.05.
Boars afflicted with the KA defect had a smaller seminiferous tubule diameter (p < 0.05) and lower number of Sertoli cells (p < 0.05) compared with the control boars. Boars with ISTS had a significantly lower number of elongated spermatids (p < 0.05) than control boars (Table 2) and a reduction in the number of sperm. Boars with ISTS produced on average only 12% of the sperm of normal boars. The sperm defects described in this study are shown in Fig. 1. Light microscope views of the seminiferous epithelium are shown in Fig. 2.
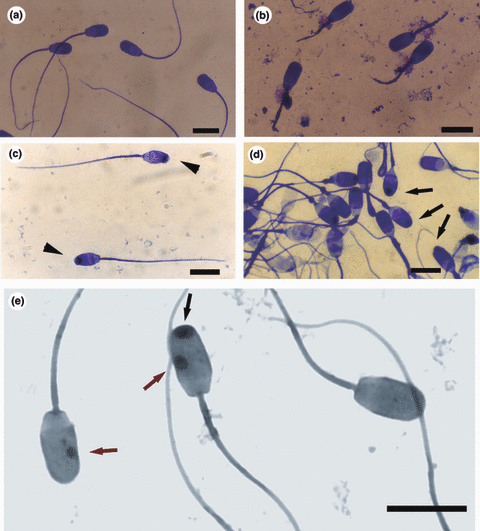
Different types of sperm defects in boars. Length of the sperm head is approximately 9 μm. Giemsa staining. Scale bars 10 μm. (a) Normal boar spermatozoa. (b) Short-tail spermatozoa from a boar affected with the immotile short-tail sperm (ISTS) defect. (c) Spermatozoa from a boar affected with the knobbed acrosome (KA) defect. Two spermatozoa with the KA defect (type 2); showing a marked cystic swelling anterior to the apical ridge of the acrosome (black arrowheads). (d) Phenocopy of the KA defect. Three spermatozoa affected with the KA defect (type 1) without cystic swelling anterior to the apical ridge of the acrosome (black arrows). A temporary disturbance in spermiogenesis. Two months later, the sperm morphology of this boar improved and spermatozoa with knobbed acrosomes disappeared from the ejaculate. (e) Spermatozoa from a boar affected with a rare type of sperm defect, the SME defect, containing type 1 knobbed acrosomes (black arrow) and in Giemsa staining hyperchromatic circular bodies (red arrows)
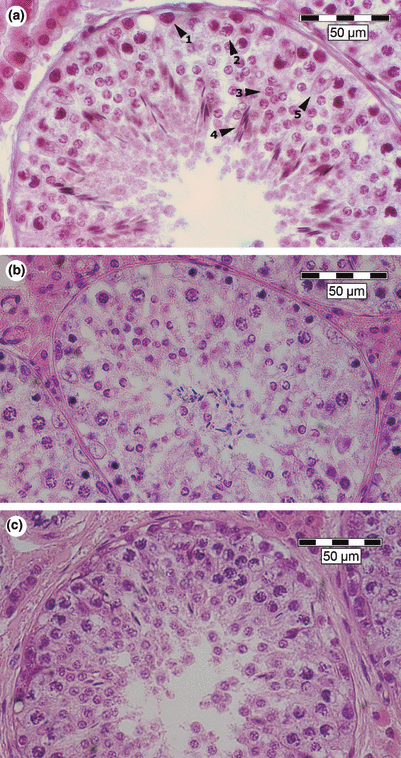
Light microscopic views of the seminiferous epithelium. Haematoxylin–eosin-stained testicular tissue sections. Scale bars 50 μm. (a) Normal control boar. 1: spermatogonia; 2: spermatocytes; 3: round spermatids; 4: elongated spermatids; 5: Sertoli cells). (b) Boar with knobbed acrosome. (c) Boar with immotile short-tail sperm
Genetic studies of the KA defect
The average inbreeding coefficient for the boars afflicted with the KA defect was higher (7.6–18.1%) compared with the average inbreeding coefficients of the Yorkshire boar population in the birth years; 1998: 5.4%; 1999: 6.2%; 2000: 6.6%. Generally two common boars were found in the pedigree of the boars with the KA defect.
Analysis of 242 markers in the screening of autosomal and X chromosomes identified several markers that exhibited a slight reduction in the number of alleles amplified from the DNA pool of affected boars as compared with unaffected boars. Markers showing a shift towards homozygosity were detected on chromosomes 3 (SW2618, SW1443, S0165 and SW487), 8 (S0098), 14 (SW2515 and SWC27), 15 (S0004 and SW2608) and X (SW2456). These were investigated in more detail by amplifying individual DNA samples and computing actual allele frequencies. Markers SW2618, SWC27 and S0098 showed only nominally significant difference in allele distribution between affected and control boars, but the difference was not significant after the Bonferroni correction for the total number of markers tested. Furthermore, available additional markers in the vicinity of these three markers were genotyped and showed no differences between affected and control boars.
Discussion
Two relatively well-defined specific sperm defects were observed at a frequency exceeding 20% in individual semen smears. These two specific sperm defects were present in Finnish Yorkshire boars but not in Finnish Landrace boars. The sperm defects occurring only in Yorkshire boars strongly indicate that these sperm defects are inherited.
The pedigrees of affected boars (Alanko 1984, 1985; Sironen et al. 2002) indicate that both defects are transmitted in a recessive manner. Sironen et al. (2006) recently showed that the ISTS defect in Yorkshire boars is caused by an insertion within an intron in the KPL2 gene in pig chromosome 16, leading mainly to a deletion of exon 30 in the mRNA. Homozygous boars with this insertion in the KPL2 gene are all affected with the specific sperm defect. All Finnish Yorkshire AI boars are now studied by a gene test for the presence of an insertion in the KPL2 gene. Alanko (1985) suggested that the KA defect is also inherited in a recessive pattern in Finnish Yorkshire boars. Although the results of homozygosity mapping of the KA defect in the present study were not statistically significant after the Bonferroni correction, they may indicate the most probable positions of the KA defect-associated chromosomal segments. The statistical insignificance of the results may be due to advanced stage of the disease-causing mutation in the population, which would have led to a narrowing down of the disease-associated region by recombination. In that case, the marker density used would not have been able to detect a statistically significant increase in homozygosity close to the defect locus. A denser marker map and more number of affected boars are needed for reliable association studies of the KA defect. In addition, it needs to be reckoned that the defect might be inherited in a more complex manner.
In bulls, the sterilizing KA defect is known to be caused by a single autosomal recessive gene (Hancock 1953; Hafez and Hafez 2000). In a recent study, the KA defect described in four closely related and significantly inbred dogs was taken as evidence of genetic transmission (Santos et al. 2006).
Interpretation of the results of sperm morphology investigations can sometimes be complicated because of phenocopies. Phenocopies are caused by environmental conditions that mimic a sperm defect produced by a gene. A phenocopy can be induced by a stressful environment, fever, elevated scrotal temperature or, such diseases as cancer. Between 1996 and 2005 one phenocopy for the KA defect was observed in one Yorkshire boar. The AI boar initially had normal sperm morphology, with sperm motility and morphology subsequently deteriorating for almost 2 months. Thereafter, sperm motility and morphology returned to normal. The boar had an acquired transient KA defect for the 2 months that was morphologically different (KA defect, type 1 = less protruding) from the defect of other boars (KA defect, type 2 with mainly cystic acrosome granules).
In addition, three boars had an SME defect (Blom and Birch-Andersen 1975; Andersen and Filseth 1976; Blom and Jensen 1977) affecting 15–30% of their spermatozoa. With this defect, a relatively small dense area was visible in the acrosomal area at various localizations. These boars showed a slight decrease in the non-return rate, as reported also by Blom and Birch-Andersen (1975). These three boars were not included in this study due to the small number of affected boars and the limited influence of this defect on fertility.
In boars with ISTS, the reduced number of elongated spermatids is explained by a disturbance in spermiogenesis and flagella formation (Sukura et al. 2002). The reduced number of Sertoli cells in boars with the KA defect is an interesting finding. In humans, a linear relationship exists between the number of Sertoli cells and daily sperm production, and considerable variation is present in the number of Sertoli cells in humans (Sharpe et al. 2003). In boars, variation has also been shown in the Sertoli cell numbers between different breeds (Okwun et al. 1996).
We conclude that in boars of the Finnish Yorkshire breed the probably inherited KA defect and the inherited ISTS defect were important reasons for infertility during the years 1996–2005. During the same period no boars of the Finnish Landrace breed were found affected with either of these two specific sperm defects.




