Expression of Genes in the Canine Pre-implantation Uterus and Embryo: Implications for an Active Role of the Embryo Before and During Invasion
Contents
The aim of the present study was to assess genes expressed in maternal uterine tissue and pre-implantation embryos which are presumably involved in maternal recognition and establishment of canine pregnancy. For this purpose, 10 pregnant bitches were ovariohysterectomized between days 10 and 12 after mating. Four non-pregnant bitches served as controls. Early pregnancy was verified by flushing the uterine horns with PBS solution. The collected embryos (n = 60) were stored deep-frozen (−80°C). Uterine tissue was excised, snaps frozen in liquid nitrogen and homogenized using TRI Reagent. All embryos from one litter were thawed together and also homogenized in TRI Reagent. RT-PCR was performed to prove mRNA expression of progesterone receptor, key enzymes of the prostaglandin synthesis pathway, selected growth factors, cytokines, immune cell receptors, major histocompatibility complex (MHC) and matrix-metalloproteinases (MMP). Only pregnant uteri revealed the presence of mRNA for interferon (IFN)-γ, IL-4 and CD-8, which resembles the milieu in humans and other mammalians. Similarly, in day 10 embryos, mRNA for transforming growth factor-β, insulin-like growth factor-1,-2, hepatocyte growth factor, leukaemia inhibitor factor, tumour necrosis factor-α, interleukin-1β,-6,-8, cyclooxygenase-2, CD4+ cells, and MMP-2 and -9 were detected, but not MHC-I or -II. We therefore suppose that the canine embryo, like its human counterpart, actively initiates measures to prevent attacks from the maternal immune system to prepare its own adhesion, nidation, growth and further development.
Introduction
During early mammalian gestation, both maternal and embryonic factors contribute to the success or failure of establishment of pregnancy. In many species, factors that contribute to maternal recognition and maintenance of pregnancy are well known and have substantially advanced preventive medicine in humans during recent years. However, in canine species, knowledge about the intrauterine immunological milieu during early pregnancy and foeto-maternal interactions leading to successful establishment of pregnancy is generally deficient. Almost no information exists about whether the uterus is an immunologically privileged site or not. If so, what distinguishes this special site from other body compartments? Little is known about trophoblast derived proteins. In canine species, no early pregnancy factor has been detected short time after fertilization, as has been in other species (Koch and Ellendorf 1986; Knotek 1990; Bose 1997; Cavanagh 1997; Lash and Legge 1997). The synthesis and immunolocalization of several proteins (cP5, cP6, RBP, CUPD) in dog endometrium and embryonic membranes has been investigated, but no differences between pregnant and non-pregnant animals could be detected comparing different days of dioestrus with corresponding days after mating (Buhi et al. 1992, 1993, 1995).
However, it was proven that recognition and maintenance of pregnancy is different within the domestic species and dependant on multiple factors. In ruminants (Capon et al. 1985; Bazer 1989; Gnatek et al. 1989; Homeida and Al-Afaleq 1994), pigs (Perry et al. 1973; Bazer 1989; Harney and Bazer 1989) and horses (McDowell et al. 1988), prevention of luteolysis of the cyclic corpus luteum by species-specific signals from the pre-implantation embryos is an essential pre-requisite. In all mammals, progesterone (P4) is essential for the maintenance of pregnancy. However, in most animals, development of normal pregnancy involves a precise, cytokine-regulated interaction between maternal and foetal tissues, in many cases initiated by the pre-implantation embryo, as well as control of the timely and locally developing adhesion and vascularization processes during the course of pregnancy. Thus, the aim of the present study was to investigate the immunological milieu inside the early pregnant canine uterus and foeto-maternal interactions which support the development of physiological pregnancy. For this purpose, the main pathways important for embryo-maternal signalling and maintenance of pregnancy and embryo development in other species were tested for their presence in pre-implantation canine embryos as well in pre-implantation uterine tissue by means of RT-PCR. Thus, within the frame of this study, the expression of (i) progesterone receptor (PR); (ii) key enzymes of the prostaglandin synthesis pathway: cyclooxygenase (COX)-1, COX-2; (iii) growth factors: transforming growth factor (TGF)-β, tumour necrosis factor (TNF)-α, granulocyte macrophage-colony stimulating factor (GM-CSF), insulin-like growth factor (IGF)-1,-2, hepatocyte growth factor (HGF), (iv) cytokines: IL-1β, -2, -4, -6, -8, -10, -12, leptin, leukaemia inhibitor factor (LIF), IFN-γ, (v) immune cell receptors: CD-4 and -8, (vi) major histocompatibility complex (MHC)-I,-II and (vii) matrix-metalloproteinases (MMP)-2 and -9 in pre-implantation maternal and embryonic tissues was analysed. The qualitative expression of these factors was compared between embryonic and maternal tissues. From the results, we got new insight into the physiology of early canine pregnancy as well as suggestions for new in vitro fertilization and embryo culture protocols.
Materials and Methods
Ten bitches of different breeds and ages, brought by their owners for routine castration, were identified as early pregnant (day 10–12 after mating). Four bitches that proved to be non-pregnant served as controls.
The bitches were spayed under general anaesthesia. Spaying was performed as a contraceptive measure or for termination of possible pregnancy. Early pregnancy was verified by flushing the uterine horns immediately after ovariohysterectomy with PBS solution (Tsutsui et al. 2001). Embryos were washed in PBS and stored deep-frozen (−80°C). Uterine tissue was excised from the middle of the left horn both from pregnant and non-pregnant animals and snap frozen in liquid nitrogen after embedding in Tissue Tec® (Sanova Pharma GmbH, Vienna, Austria).
For qualitative RT-PCR, pieces of uterine tissue (30 mg) were homogenized in a sterile glass homogenizer using 1 ml of TRI Reagent (# T 9424; Sigma-Aldrich, Vienna, Austria) and subsequently treated as recommended by the manufacturer. With TRI Reagent, it is possible to isolate total RNA, protein and DNA at the same time and from the same sample. All embryos from one bitch were thawed together and their morphology and developmental stage evaluated immediately afterwards. After adding 0.5 ml of TRI reagent, the embryos were homogenized in a sterile glass homogenizer. After extracting RNA, no DNA digest was performed with these samples. Embryos from one litter were analysed together to provide sufficient material and to be able to compare the data obtained with that obtained from the uterine tissue of the mother. As a negative control, for each gene investigated RNase free water was used instead of the template in the RT-PCR reaction. For positive controls, DNase digested total RNA from ovarian tissue, oocytes and white blood cells were used.
For all genes investigated, primers for qualitative RT-PCR were designed by means of the GCG Wisconsin package using canine sequences available from the database. If no canine sequences of the genes of interest were available, sequences from closely related species were aligned and primers designed at conserved regions of the coding sequence using GCG Wisconsin package (Devereux 1989a–c). All primers were designed using canine sequences except for IGF-1 and IGF-2, where equine primers were used. The products were visualized after electrophoresis on agarose gels (1.5%) containing ethidium bromide. Bands of the appropriate size were cut out of the agarose gel and sent to a sequencing service (IBL, Vienna, Austria) using the amplification primers. The sequence obtained was compared with the databases using blast analysis. The primers used in this study are listed in Table 1.
| Primer | Sequences (5’–3’) sense and antisense | Length of amplicon | Reference | Accession no. (Genbank) |
|---|---|---|---|---|
| IL-1β S | TCC AAT GTG AAG TGC TGC TGC | 323 bp | Markus et al. (2002) | Z70047 |
| IL-1 β AS | TAT GAG TTA GAC AGC ACC AGG | |||
| IL-2 S | AGA TGG AGC AAT TAC TGC TGG | 366 bp | Markus et al. (2002) | D30710 |
| IL-2 AS | AAG TCA GTG TTG AGA AGA TGC | |||
| IL-4 S | CAC CTC CCA ACT GAT TCC AAC | 373 bp | Van der Kaaij et al.(1999) | AF054833 |
| IL-4 AS | CTT CTG CAT GAT CAC TTT TAG CC | |||
| IL-4 S | ACT CAC CAG CAC CTT TGT CC | 248 bp | AF187322 | |
| IL-4 AS | TGC TGC TGA GGT TCC TGT AG | |||
| IL-6 S | GCA AGG AGG CAC TGG CAG AA | 102 bp | Markus et al. (2002) | U12234 |
| IL-6 AS | TTC TTG TCA AGC AGG TCT CC | |||
| IL-8 S | ACT TCC AAG CTG GCT GTT GC | 172 bp | Markus et al. (2002) | U10308 |
| IL-8 AS | GGC CAC TGT CAA TCA CTC TC | |||
| IL-10 S | CCT GGG TTG CCA AGC CCT GTC | 212 bp | Gröne et al. (1999) | U33843 |
| IL-10 AS | ATG CGC TCT TCA CCT GCT CC | |||
| IL-12 p40 S | CAC CTG CCA TAC CCC TGA AG | 452 bp | Markus et al. (2002) | U49100 |
| IL-12 p40 AS | CAC TGC CTT CCT GAC ACT CC | |||
| IFN g S | GCA AGT AAT CCA GAT GTA TCG | 283 bp | Markus et al. (2002) | S41201 |
| IFN g AS | TTA TCG CCT TGC GCT GGA CC | |||
| IFN g S | TTC AGC TTT GCG TGA TTT TG | 438 bp | – | AF126247 |
| IFN g AS | GAC TCC TTT TCC GCT TCC TT | |||
| P4R S | CAG GTG TAC CAG CCG TAC CT | 618 bp | Lantinga-van Leeuwen et al. (2000) | AF177470 |
| P4R AS | ATT TCG AAA ACC TGG CAA TG | |||
| TNF a S | CCA AGT GAC AAG CCA GTA GC | 274 bp | Markus et al. (2002) | Z70046 |
| TNF a AS | TCT TGA TGG CAG AGA GTA GG | |||
| TGF- β S | TTC CTG CTC CTC ATG GCC AC | 393 bp | Markus et al. (2002) | L34956 |
| TGF- β AS | GCA GGA GCG CAC GAT CAT GT | |||
| IGF-1 S | AAA ATC AGC CAG TCT TCC AAC CC | 199 bp | – | U28070 |
| IGF-1 AS | TGT CTC CAC ACA CGA ACT GAA G | |||
| IGF-2 S | GCA TCG TGG AAG AGT GCT GT | 158 bp | – | AF097586 |
| IGF-2 AS | CGT TCG CGA AGG TCA TGT TG | |||
| COX-1 S | ACA GCT ATG AGC AGT TCC | 375 bp | – | NM_001003023 |
| COX-1 AS | CCC CAA TTT CTA TCA TAC TC | |||
| COX-2 S | TCA ATA ACA TCC CCT TCC | 479 bp | Kowalewski et al. (2006) | AY044905 |
| COX-2 AS | CAC CTC TCC ATC AAT TAC C | |||
| GM-CSF S | GCA GAA CCT GCT TTT CTT GG | 408 bp | Nash et al. (1991) | NM_001003245 |
| GM-CSF AS | CAG CAG TCA AAG GGG ATG TT | |||
| Leptin S | TCC GAA AAG TCC AGG ATG AC | 380 bp | Iwase et al. (2000) | AB020986 |
| Leptin AS | CAG TCT GTT CAG AGC CAC CA | |||
| CD4 S | ACA CAG ACA AGA GGC AAG | 577 bp | – | L06130 |
| CD4 AS | ATT CCA GAA CCA GCA TAC | |||
| CD8 S | GCG CAG TGA AAA CAA GTG | 196 bp | – | L14287 |
| CD8 AS | ACA TAT TTC TCT GAA GGA CTG G | |||
| MMP-2 S | CCA ACT ACA ACT TCT TCC C | 362 bp | Muir et al. (2005) | AF177217 |
| MMP-2 AS | GGC ATT CCC ATA CTT CAC | |||
| MMP-9 S | TCG GTG GAA CAG ATG TAC CC | 169 bp | – | AF169244 |
| MMP-9 AS | GGC ACT GCA AAA TGT CAA AG | |||
| MHC-1 S | CTC CCA CTC CCT GAG GTA TT | 487 bp | DQ469801 | |
| MHC-1 AS | CGT CGT CTC CAG GTA GTT CC | |||
| MHC-2 S | TGA CTG TGC TCT CAA ACA CCC | 357 bp | – | NM_001011723 |
| MHC-2 AS | TAA TGA TGC CCA CCA GAC CC | |||
| HGF S | ATG GGG AAT GAG AAA TGC AG | 210 bp | – | AB090353 |
| HGF AS | AAA AAT GCC AGG ACG ATT TG | |||
| LIF S | GAC AGA CTT CCC ACC ATT CC | 127 bp | – | AF479881 |
| LIF AS | GGG ATT GAG GAC CTT CTG GT | |||
- S, sense primer; AS, anti-sense primer; bp, base pairs; CD, cluster of differentiation; COX, cyclooxygenase; GM-CSF, granulocyte macrophage colony stimulating factor; HGF, hepatocyte growth factor; IFN, interferon, IGF, insulin like growth factor; IL, interleukin; LIF, leukaemia inhibitor factor; MHC, major histocompatibility complex; MMP, matrix metalloproteinase; P4R, progesterone receptor; TGF, transforming growth factor; TNF, tumour necrosis factor.
Results
After flushing the uteri, 10 bitches proved to be in early pregnancy and 60 embryos were collected. Four other bitches which proved to be non-pregnant (early dioestrus stage) served as controls. Between 10 and 12 days after mating, blastocysts could never be identified, only earlier developmental stages between the 4-cell and morula stage were observed. For RT-PCR, more than two embryos have been pooled for homogenization, otherwise the mRNA yield was not sufficient. An overview of the genes detected is provided in Table 2 and the raw data is shown in 1-9 as supplementary information.
| Preimplantation uterus day 10 | Preimplantation embryos day 10 | Early dioestrus uterus day 10 | |
|---|---|---|---|
| Receptors | |||
| P4-receptor | + | − | + |
| PG synthesis | |||
| COX-1 | + | − | + |
| COX-2 | − | + | − |
| Growth factors | |||
| TGF-β | + | + | + |
| TNF-α | − | + | + |
| GM-CSF | − | − | − |
| IGF-1 | + | + | + |
| IGF-2 | − | + | − |
| HGF | + | + | + |
| Cytokines | |||
| IL-1β | − | + | − |
| IL-2 | + | − | + |
| IL-4 | + | − | − |
| IL-6 | − | + | + |
| IL-8 | − | + | − |
| IL-10 | + | − | + |
| IL-12 | − | − | − |
| Leptin | − | − | − |
| LIF | + | + | + |
| IFN-γ | + | − | − |
| Immune cell receptors | |||
| CD-4 | − | + | + |
| CD-8 | + | − | − |
| MHC | |||
| MHC-I | + | − | + |
| MHC-II | + | − | + |
| MMPs | |||
| MMP-2 | + | + | + |
| MMP-9 | + | + | + |
- +, detected; −, non-detected; CD, cluster of differentiation; COX, cyclooxygenase; GM-CSF, granulocyte macrophage colony stimulating factor; HGF, hepatocyte growth factor; IFN, interferon; IGF, insulin like growth factor; IL, interleukin; LIF, leukaemia inhibitor factor; MHC, major histocompatibility complex; MMP, matrix metalloproteinase; P4R, progesterone receptor; TGF, transforming growth factor; TNF, tumour necrosis factor.
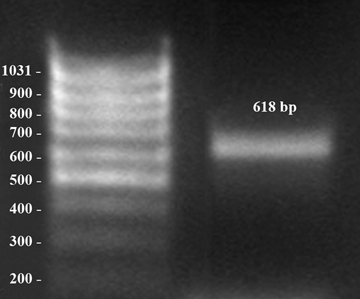
Amplification of progesterone receptor. Early dioestrus, middle of the horn. bp = base pairs
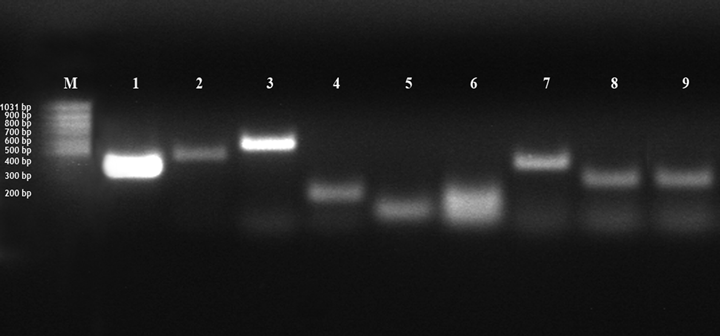
Amplification of COX-1,-2; CD-4,-8; IL-6,-8; TGF-β; TNF-a Mixed sample: amplification of β -actin (band 1; 100 bp), COX-1 (band 2, 375 bp), COX-2 (band 3, 479 bp), CD-4 (band 4, 577 bp), CD-8 (band 5, 577 bp), IL-6 (band 6, 102 bp), IL-8 (band 7, 172 bp), TGF- β (band 8, 393 bp), TNF-a (band 9, 274 bp); bp = base pairs; M = 100 bp marker
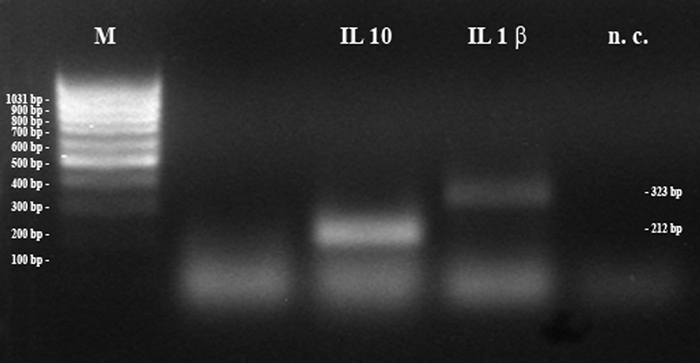
Amplification of IL-1β and IL-10. Two samples: five embryos (IL-1β) and non-pregnant uterine tissue (IL-10); bp = base pairs; M = 100 bp marker; n.c. = negative control
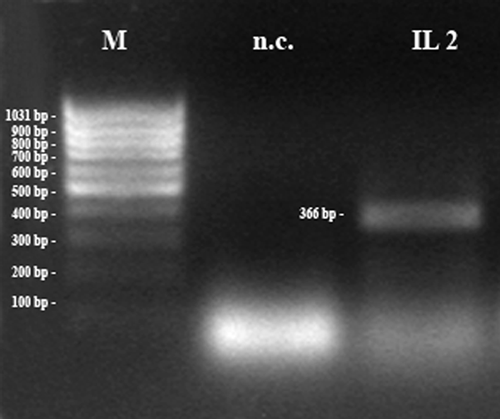
Amplification of IL-2. Pregnant uterine tissue. bp = base pairs; M = 100 bp marker; n.c. = negative control

Amplification of IL-4 and leukaemia inhibitor factor (LIF). Pregnant uterine tissue: amplification of IL-4 (248 bp) and LIF (127 bp); bp = base pairs; M = 100 bp marker, n.c. = negative control
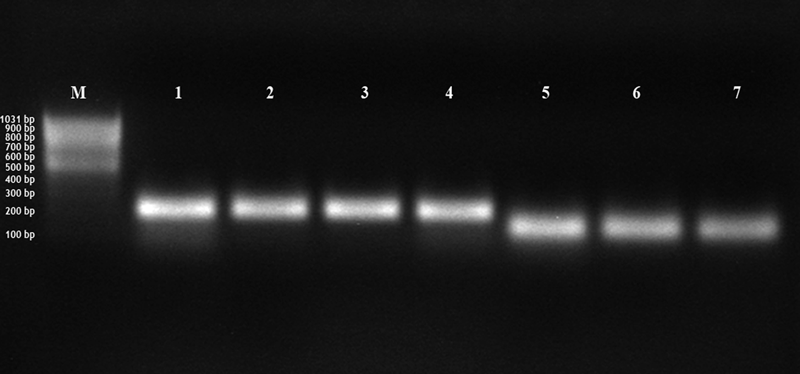
Amplification of hepatocyte growth factor (HGF) and leukaemia inhibitor factor (LIF). Pregnant uterine tissue: amplification of HGF (band 1–4, 210 bp) and LIF (band 5–7, 127 bp); bp = base pairs; M = 100 bp marker
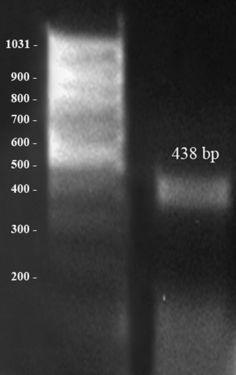
Amplification of IFN-γ. Pregnant uterine tissue: amplification of IFN-γ (438 bp); bp = base pairs
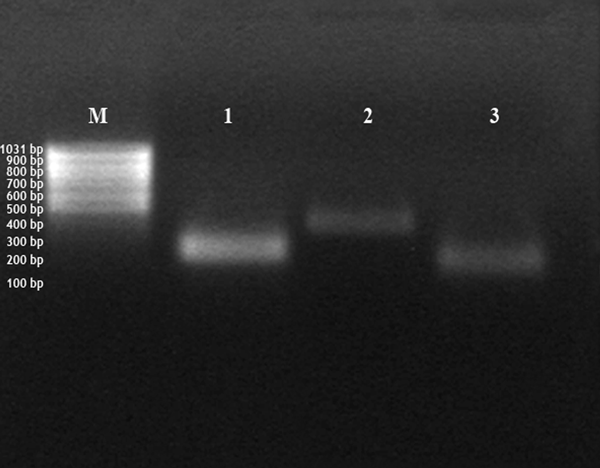
Amplification of insulin-like growth factor (IGF), matrix-metalloproteinases (MMP)-2 and -9. Pregnant uterine tissue: amplification of IGF-1 (band 1, 199 bp), MMP-2 (band 2, 362 bp) and MMP-9 (band 3, 169); bp = base pairs; M = 100 bp marker
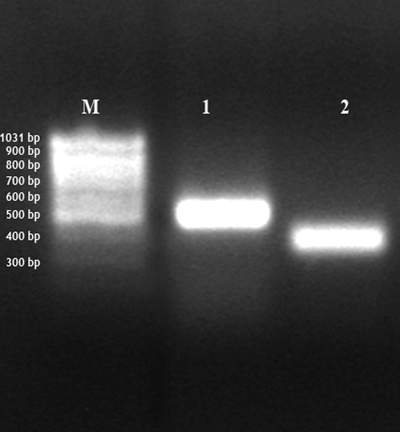
Amplification of major histocompatibility complex (MHC)-I and –II. Early dioestrus, middle of the horn: amplification of MHC-I (band 1, 487 bp) and MHC-II (band 2, 357 bp); bp = base pairs; M = 100 bp marker
Factors exclusively detected in the pre-implantation uterus were mRNA for IFN-γ, CD-8 and IL-4. PR, COX-1, growth factors TGF-γ, HGF and IGF-1, cytokines IL-2, IL-10 and LIF, MHC-1 and -2 as well as MMP-2 and -9 were detectable in all uterine tissues. Expression of TNF-α was absent in the pre-implantation uterus, however it was expressed in the early dioestrus uterus and day 10 embryos. Interleukins (IL)-6 and -8 were not expressed in the early pregnant uterus, but IL-6 appeared in the dioestrus tissue. Interleukin-1β, -6 and -8 together with MMP-2 and -9 as well as HGF and LIF were detectable in embryonic tissue (see Table 2). In day 10 embryos, IL-1β, IL-8, IGF-2 and COX-2 were exclusively expressed.
Granulocyte macrophage-colony stimulating factor was not detectable in any tissue which was assessed; the same was the case for the cytokines leptin and IL-12.
The dioestrus uterus did not differ markedly from the early pregnant uterus, except for the additional expression of TNF-α, IL-6 and CD-4.
Discussion
In the bitch, P4 is essential for the establishment and maintenance of pregnancy (Concannon et al. 1990). The biological effect is mediated by the presence of PR, which was not detected in day 10 canine embryos. This study therefore reveals for the first time that a direct effect of P4 on the pre-implantation embryo can be excluded, at least between the 4-cell and morula stage.
An interesting result is the strong expression of COX-2 in day 10 pre-implantation embryos, which indicates activation of prostaglandin synthesis, possibly important for the regulation of embryonic and endometrial cytokines. The human trophoblast has been found to secrete both PGE and PGF, the equine conceptus both PGE2 and PGF2α (Stout and Allen 2002).
At the uterine level, many factors contribute to foetal tolerance, partly alone, partly in interaction with each other. In humans, TGF-β is an inhibitor of lymphocyte proliferation and up-regulates the endometrial expression of LIF (Meisser et al. 1999). TNF-α has manifold effects; in low concentrations, as probably secreted by the pre-implantation embryos, it supports the growth of endothelial cells (Fajardo et al. 1992). It also up-regulates the expression of MHC-I in various cells except trophoblast cells and up-regulates protein expression of adhesion molecules (for review see Hunt et al. 1996). In the pre-implantation canine uterus, no mRNA for TNF-α was detected, which might be beneficial, as TNF-α promotes Fas expression and sensitivity of trophoblast cells and may lead to increased apoptosis and trophoblast rejection in humans (Agarwal et al. 2000; Aschkenazi et al. 2002). In this study, HGF, also known as scatter factor, was detected in all tissues investigated. The HGF participates in degradation and reorganization of tissues and is reported to regulate the depth of human cytotrophoblast invasion (Nasu et al. 2000). Leukaemia inhibitory factor and its receptor (LIFR) have been detected in rabbit pre-implantation uteri and blastocysts, so it is therefore thought to participate in foeto-maternal interactions in this species (Lei et al. 2004). LIF and LIFR were also detected in pre-implantation mouse embryos (Nichols et al. 1996), bovine embryos (Eckert and Niemann 1998) and human embryos (Chen et al. 1999). In human species, the secretion of LIF and the presence of LIFR on trophoblast cells is important for trophoblast growth and differentiation, and for a shift towards the intrauterine predominance of T helper (Th2) cells (Kojima et al. 1995; Piccinni et al. 1998), which is important for the survival of the embryos (for review see Kwak-Kim et al. 2005). In this study, we detected mRNA for LIF and IL-4 in the pre-implantation uterus and for LIF in the corresponding embryos. Interleukin-4 is a typical Th2 cell cytokine which is important for the regulation of B-cell proliferation and isotype secretion; it may additionally stimulate the expression of MHC-II on B cells and macrophages.
Further cytokines, namely IL-2 and -10, were detected in the pre-implantation canine uterus, however not IL-6, -8, -12 or leptin. As IL-10 is an anti-inflammatory cytokine, one function in the early pregnant uterus could be the regulation of the embryo induced local inflammatory reaction (Pomini et al. 1999; Hanna et al. 2006). It could also function as a regulator of MMP-9 activity as has been shown in early pregnant women (Meisser et al. 1999).
Other genes, detected in pre-implantation canine embryos, are expressed by human embryos as well. Some increase the gelatinolysis of maternal tissue, such as TNF-α and IL-6, while others like TGF-β and LIF inhibit it, thus contributing to a balanced degradation of basal membranes during embryonic invasion. IL-6 was additionally detected in pre-implantation embryos from sheep, pigs and cattle and is supposed to support the developing embryo and to participate in placentation (Mathialagan et al. 1992).
In contrast to earlier findings (Schäfer-Somi et al. 2006), we now detected mRNA for IFN-γ in the pre-implantation uterus, however, the function is still unclear. IFN-γ might regulate gestational endometrial angiogenesis, as has been shown in pigs (Tayade et al. 2006) and mice (Ashkar et al. 2000). IFN-γ might also induce an up regulation of MHC genes, as in vitro, treatment with IFN-γ leads to an increased expression of mRNA for HLA-G (Lefebvre et al. 1999). The interaction between HLA-G and its receptor inhibits T-cell proliferation and induces the production of immunosuppressive CD4+ cells, which is important for the maintenance of pregnancy (Riteau et al. 1999; Bainbridge et al. 2000; LeMaoult et al. 2004). Whether the expression of IFN-γ is initiated by the embryo itself or by intrauterine natural killer cells as in mice (Ashkar et al. 2000) cannot be answered in this study; it could also be enhanced by CD8+ lymphocytes, as has been shown in some cases of human bacterial disease (Raziuddin et al. 1995).
In the early pregnant uterus, we detected mRNA for CD-8 but not for CD-4 so far. It is thought that CD-8 surface molecules mainly exert regulatory and suppressor functions, which would closely resemble the circumstances in humans and other species. In day 10 embryos and early metestrus uterine tissue, only mRNA for CD-4 was detected, which is consistent with the mixed profiles of Th1 and Th2 type cytokines detected here. In the human pre-implantation embryo, CD-4 is supposed to be a receptor with regulatory functions. Several complement regulatory CD molecules such as CD55, CD59 and CD46 have been found to be present, and some seem to play an important role in cell-to-cell interaction during fertilization and/or implantation. These regulatory proteins together with the lack of expression of MHC-I are thought to protect the embryos from complement-mediated damage during their travel through the female genital tract (for review see Fenichel et al. 1995). Additionally, in the canine species, the embryonic CD-4 molecules could interact with immune cells from the maternal immune system to inactivate or divert their attacks.
In mammals, the MHC antigen mainly acts as T-cell recognition molecule (Felsburg 1998; Debenham et al. 2005); however, the human leukocyte antigen (HLA)-G binds to inhibitory receptors of natural killer cells, which inhibits their cytolytic activity (Rouas-Freiss et al. 1997). In addition, HLA-G1 suppresses T-cell proliferation (Bainbridge et al. 2000). In early pregnant women, only the invading extra villous trophoblast expresses MHC-I (Tripathi et al. 2004) and it has been suggested that silencing of MHC-II on trophoblast cells is pivotal for the prevention of immune rejection (Murphy et al. 2004). In this study, mRNA for MHC-I and -II was detected in the pre-implantation and early metestrus uterus, but not in the pre-implantation embryos. Our most recent investigations reveal that MHC-II is not expressed on invading canine trophoblast cells (unpublished data), which points towards species-specific conditions in dogs.
Matrix-metalloproteinases are essential for the preparation of the maternal tissue for the invasive growth of the cytotrophoblast. Recently we found out that before and during implantation, the activity of MMP-2 was significantly elevated in the serum of pregnant bitches in comparison to that of non-pregnant ones (Schäfer-Somi et al. 2005a). In another study, we demonstrated that MMP-2 activity was higher in the endometrium and myometrium of pregnant bitches compared with those of non-pregnant animals (Beceriklisoy et al. 2005; Schäfer-Somi et al. 2005b). In this study, we detected mRNA for IL-1β, and -6 as well as MMP-2 and -9 in pre-implantation canine embryos. As cytotrophoblast derived IL-1β and -6 up-regulate and activate MMP-2 and -9 in humans (Librach et al. 1994; Meisser et al. 1999; Karmakar and Das 2002, 2004), we suppose that in the pregnant bitch, the enzyme activation is similarly affected by these embryo secreted factors.
All these results considered together suggest an active role of the early canine embryo in nidation and implantation.
Conclusions
In the present study, we examined transcriptional expression of genes important for embryonic establishment. In the early pregnant uterus, we verified mRNA for IFN-γ, IL-4 and CD-8, which was the main difference in comparison to the non-pregnant, early dioestrus uterus. This specific immunological milieu in the pre-implantation uterus is supposed to be directly or indirectly initiated by the pre-implantation embryos. In day 10 embryos, we could prove the expression of mRNAs, inducing the up-regulation of adhesion molecules in maternal endometrial tissue and later promoting the degradation of basal membranes during embryonic invasion, as well as supporting angiogenesis and foetal growth and development. The question remains what initiates the expression of these growth factors, enzymes and surface molecules; one hypothesis would be that selected contact with maternal immune cells or with secretion products from the oviduct might be a necessary step for activation of transcriptions of these specific genes. The question remains to be clarified which of the factors assessed here are necessarily important for establishment and further development of the canine embryo. In addition, the presence and distribution of cytokine and hormone receptors in the course of pregnancy remain to be investigated. Follow-up studies should thus assess the regulation of selected factors and their receptors in uterine and embryonic tissue, as well as the presence and distribution of lymphocytes and natural killer cells inside the pregnant uterus during different stages of gestation.




