Effect of a Gonadotropin-releasing Hormone Vaccine (ImprovacTM) on Steroid Hormones, Boar Taint Compounds and Performance in Entire Male Pigs
Contents
The objective of this study, comprising two trials, was to evaluate the effect of a gonadotropin-releasing hormone (GnRH-vaccine ImprovacTM; Pfizer Ltd) in a sample of the Swedish pig population. The pigs (n = 120) were assigned to three groups: control (entire male pigs), surgical castration and immunization against GnRH. Surgically castrated pigs did not express detectable levels of either testosterone or estrone sulphate (E1S) in plasma, or androstenone in fat and had lower skatole and indole levels in fat than entire male pigs. Immunization significantly reduced testes weight and bulbourethral gland length, plasma levels of the testicular hormones testosterone and E1S, and fat levels of androstenone, skatole and indole. Skatole levels in plasma were significantly lower than in entire male pigs in the second trial, but not in the first due to overall low skatole levels. All immunized pigs and surgically castrated pigs expressed skatole concentrations in fat below the level of 0.2 μg/g, above which meat is regarded as tainted. In contrast, eight entire male pigs exceeded this level. Indole levels in plasma from immunized pigs were lower than those from entire male pigs. Surgical castration caused lower daily weight gain in the suckling period compared with piglets raised intact, whereas in the post-weaning period no difference was observed. Immunization resulted in higher feed intake and daily weight gain after the second injection. The estimated lean meat content was improved in comparison with the castrated pigs, but was lower than for entire male pigs. Dressing percentage was lower in immunized pigs than in surgically castrated and entire male pigs. The frequency of skin damage did not differ between immunized and entire male pigs or between immunized and surgically castrated pigs.
Introduction
An unpleasant odour called boar taint may occur in the meat of sexually mature male pigs. It is mainly caused by accumulation in fat of at least one of the two compounds: androstenone (5α-androst-16-en-3-one) and skatole (3-methylindole). To a lesser extent, indole has also been recognized as contributing to boar taint. Concentrations above 0.5–1.0 ppm for androstenone and 0.20–0.25 ppm for skatole has been shown to give consumer reactions (as reviewed by Walstra et al. 1999). Androstenone is synthesized in testicular Leydig cells simultaneously with other testicular steroids and its levels primarily depend on the stage of sexual maturity (Bonneau 1982). The amount of androstenone synthesized differs among breeds (Xue et al. 1996) and among individuals within the same breed (Bonneau 1987). Recently, a single nucleotide polymorphism in cytochrome b5 gene was shown to be responsible for low androstenone levels in fat (Lin et al. 2005). Skatole is produced from the amino acid tryptophan in the large intestine of pigs and its levels are influenced by sexual maturity (Zamaratskaia et al. 2005a,b) and genetic factors (Lundström et al. 1994; Doran et al. 2002a). Skatole metabolism may be influenced by genetic polymorphisms in the enzymes cytochrome P4502A6 (Lin et al. 2004a) and thermostable phenol sulphotransferase SULT1A1 (Lin et al. 2004b). In addition, skatole levels are strongly influenced by nutrition (Jensen et al. 1995; Andersson et al. 1997; Zamaratskaia et al. 2005c) and rearing conditions (Hansen et al. 1994). Indole, like skatole, is produced in the intestine of pigs. Although indole levels have not been extensively studied, there is evidence that like skatole, indole levels are regulated by nutrition (Zamaratskaia et al. 2006), sexual maturity and genetic factors (Babol et al. 2004).
Castration of male piglets is routinely performed in most countries to prevent the occurrence of boar taint in pig carcasses. However, avoiding castration provides benefits, because of improved feed conversion and leaner carcasses of entire males (Babol and Squires 1995; Andersson et al. 1997; Bonneau 1998). Castration may also negatively affect animal health and welfare. The number of chronic inflammations is markedly higher in castrated male pigs compared with entire males and female pigs (Kruijf and Welling 1988). Worldwide pressure to restrict surgical castration has drastically increased. Other effective methods to prevent boar taint are therefore required which should be easy for use on farms and effectively reduce taint.
An alternative to surgical castration is active immunization against gonadotropin-releasing hormone (GnRH), known as immunocastration. Gonadotropin-releasing hormone is a neuropeptide that is released in a pulsatile manner from the hypothalamus to stimulate the secretion of pituitary luteinizing hormone (LH) and follicle stimulating hormone (FSH), which subsequently control the production of testicular steroids. Immunological blocking of the signal from GnRH decreases the production of LH, FSH and testicular steroids. Immunization against GnRH reduces the concentrations of testicular steroids, including androstenone, the size of reproductive organs, sperm numbers and aggressive behaviour (Bonneau et al. 1994; Dunshea et al. 2001; Turkstra et al. 2002; Cronin et al. 2003; Metz and Claus 2003; Oliver et al. 2003; Jaros et al. 2005; Bowen et al. 2006). Skatole levels were shown to depend partially on the presence of testicular steroids (Doran et al. 2002b) and can therefore also be reduced by immunocastration (Dunshea et al. 2001; Metz et al. 2002). The effect of immunocastration on indole levels has not been previously studied. Growth performance of immunized pigs is comparable with (Bonneau et al. 1994; Jaros et al. 2005) or even better (Dunshea et al. 2001) than that of entire male pigs. Thus, immunocastration can be used to reduce the risk of high androstenone and skatole levels in fat and relieve unwanted aggression in male pigs without negatively affecting performance.
Advances in immunocastration techniques have been made: improved safety, reduced variability of response to vaccination and optimization of the age for vaccination. A potentially promising product in use in Australia, ImprovacTM (Pfizer Ltd, formerly CSL Limited, Parkville, Vic., Australia) is a vaccine that contains a modified form of GnRH in an aqueous adjuvant system. Vaccination is performed twice in the growing/finishing period, at least 4 weeks apart, with the second injection approximately 4 weeks prior to slaughter. This procedure reduces boar taint in entire male pigs but retains part of the production advantages of entire males vs castrated pigs (Dunshea et al. 2001; Jaros et al. 2005). Further work is required; however, to optimize its application in different pig populations because of between-country variations in breeds and pig farm management. Immunocastration is particularly relevant currently as there is increasing debate about the prohibition of surgical castration.
The objective of the present study was to evaluate the effectiveness of ImprovacTM in suppressing testicular activity in a sample of the Swedish pig population as well as reducing skatole, indole and androstenone concentrations. In addition, performance, carcass and meat quality traits were compared between entire male, surgically castrated and ImprovacTM treated pigs.
Materials and Methods
Animals and treatment
A total of 120 crossbred male pigs (Swedish Yorkshire dams × Swedish Landrace sires) were used in this study, comprising two trials. The sires used were randomly selected from the available AI sires. The first trial included 72 pigs. The piglets within litter were randomly allocated to treatments at birth. The growing/finishing period started when the pigs were at the average age of 69.3 ± 3.2 days (mean ± SD) and had an initial live weight (LW) of 25.9 ± 5.0 kg. The pigs were raised in single-sex pens with eight pigs in each. All pigs were fed the same commercial diet (12.4 MJ ME per kg, digestible CP 13.5%) twice a day according to appetite (semi ad libitum). Twenty-four male piglets were surgically castrated in the traditional way before the age of 1 week. Twenty-four male pigs received the vaccine ImprovacTM (Pfizer Ltd) containing a modified form of GnRH (200 μg GnRH-protein conjugate/ml) in an aqueous adjuvant system. Vaccination was performed twice, 4 weeks apart, with the first injection given 8 weeks prior to slaughter (age 111.2 ± 7.5 days; LW 57.8 ± 7.6 kg) and the second injection given 4 weeks prior to slaughter (LW 89.5 ± 9.5 kg). The remaining 24 male pigs were kept intact throughout the study and served as controls. Live weights of the pigs were recorded at start of the experiment, thereafter every second week and the day before slaughter. Feed consumption was recorded on a daily basis and feed conversion ratio was calculated pen-wise.
Blood samples were taken by jugular venipuncture from all pigs three times: prior to the first ImprovacTM injection (8 weeks prior to slaughter); prior to the second injection (4 weeks prior to slaughter) and 1 day prior to slaughter. To obtain plasma, blood samples were collected into vacutainer tubes with heparin, separated by centrifugation at 2000 × g and stored at −70°C prior to analysis. To obtain serum, blood samples were taken in vacutainer tubes without heparin, separated by centrifugation at 2000 × g and stored at −20°C until determination of anti-GnRH antibodies. Samples of adipose tissue were taken at slaughter from the neck region of the carcass and kept at −20°C prior to analysis.
All pigs from the first trial were slaughtered at an average age of 167.2 ± 7.5 days and an average LW of 123.7 ± 10.7 kg. Slaughter was performed on two occasions per pen. All pigs were mixed with unfamiliar pigs on the transport to the commercial slaughterhouse in Uppsala as well as at the abattoir, to simulate normal transport and slaughter procedure. All pigs were slaughtered after 2 h of lairage at the abattoir.
The second trial included 48 pigs, 24 entire male pigs and 24 immunized pigs and started when the pigs had an average age of 70.1 ± 2.9 days and initial LW of 29.6 ± 5.2 kg. Pigs were raised in conditions similar to those in first trial except that the first Improvac vaccination was given 8–11 weeks prior to slaughter (age 102.1 ± 3.5 days; LW, 55.2 ± 10.2 kg), to make management of the pigs more practicable. The earlier timing of the first Improvac vaccination in the second trial was consistent with the manufacturer's label recommendations for the timing of vaccination (D. Harrington, Pfizer, personal communication). Blood samples were taken from all pigs 1 day prior to slaughter and treated as above. Pigs from the second trial were slaughtered as described for the first trial but without mixing with unfamiliar pigs during transport and lairage at an average age of 169.0 ± 6.6 days and an average LW of 125.1 ± 11.3 kg.
The experiment was performed in accordance with Swedish regulations for the use of animals.
Chemical analysis
Skatole and indole levels in plasma were measured as described by Zamaratskaia et al. (2004), and androstenone, skatole and indole in fat as described by Chen et al. (2006).
Estrone sulphate (E1S) concentrations in plasma were measured with radioimmunoassay procedures (DSL-5400; DSL UK Ltd, Cherwell Innovation Centre, Upper Heyford, UK) in 100-μl plasma aliquots, according to manufacturer's instructions. The manufacturer reported a sensitivity of the assay of 0.01 ng/ml with intra-assay CVof 4.6–9.2% and inter-assay CV of 5.1–8.8%, depending on the E1S concentrations.
Total testosterone concentrations were measured with radioimmunoassay procedures (Diagnostic Products, Los Angeles, CA, USA) in 50-μl plasma aliquots, according to manufacturer's instructions. The manufacturer reported a sensitivity of this assay of 0.04 ng/ml with intra-assay CV of 4.0–18.0% and inter-assay CV of 5.9–12.0%, depending on the testosterone concentrations.
Antibodies against GnRH were measured by SGS Life Science Services, Wavre, Belgium, using an indirect non-competitive ELISA in accordance with a fully validated method described in SGS analytical protocol B105601. Briefly, plates were prepared by adding 100 μl stock GnRH solution (10 mg/ml diluted 1 : 1000 with 1m sodium carbonate buffer solution) to each well and incubating at 2–8°C overnight. Before use, plates and reagents were equilibrated to room temperature. Plates were washed five times with Phosphate Buffer Saline with Tween 20 (PBST) solution (8 g NaCl, 0.2 g KH2PO4, 0.2 g KCl, 1.15 g Na2HPO4 and 0.5 ml Tween20 per litre) and excess fluid drained after the final wash. Wells were blocked by adding 150 μl GnRH diluent (0.5 g thiomersal, 5.75 g Na2HPO4, 50 g Na casein, 40 g NaCl, 1 g KCl, 1 g KH2PO4 and 2.5 ml Tween20 per litre) to each well and incubating for 1 h at room temperature. Plates were washed five times with PBST. After preparation of plates, 100 μl each of standard (freeze-dried pig sera from a pig vaccinated with ImprovacTM, calibrated by the Victorian Institute of Animal Science, Australia, using radioimmunoassay in accordance with CSL SOP VIAS0825.1) and serially diluted test sera were added to plates and incubated for 1 h at room temperature. Plates were washed five times with PBST and excess fluid removed. One hundred microlitre conjugate (goat anti-swine IgG reconstituted with 1 ml 50% glycerol, diluted 1 : 16 000 in GnRH diluent) was added to each well and incubated for 1 h at room temperature. Plates were washed five times with PBST. One hundred microlitre TMB substrate (1 : 1 v/v 3, 3′5, 5′-tetramethylbenzidine, 0.02% H2O2 solution in citric acid buffer) was added to all wells and incubated for 15 min at room temperature. The colorimetric reaction was stopped by adding 100 μl stop solution (0.55 m H2SO4) to all wells and absorbance at 450 nm (A450) measured using a microplate reader (Spectra Max 384/340 PC; Molecular Devices, Sunnyvale, CA, USA).
All chemical analyses were performed in duplicate.
Parameters of reproductive organs
Reproductive organ measurements were performed at slaughter. Testes and bulbourethral (Cowper's) glands were removed from entire male and immunized pigs at slaughter and dissected from extraneous tissue. The length of both bulbourethral glands was measured to record the average length and testes were weighed as pairs.
Carcass and technological meat quality
Carcass characteristics were evaluated in all pigs. Before cooling, carcass weight was recorded and lean meat content was determined with the Hennessy Grading Probe. Ham from the right carcass half was weighed with skin and fat, defatted and reweighed to determine meat and bone. The proportion of lean and bone in ham was used for the estimation of lean meat percentage in the whole carcass (lean meat percentage = 0.729 × % meat and bone in ham; I. Hansson, personal communication). In the second trial, the amount of interstitial abdominal fat was recorded at slaughter.
In the first trial, technological meat quality was measured on samples of M. longissimus dorsi (LD) and M. biceps femoris (BF) approximately 24 h after slaughter. Ultimate pH was measured, using a portable pH-meter (Knick, Berlin, Germany) equipped with a combination gel electrode (SE104; Knick) calibrated to chilling room temperature. Internal reflectance (FOP) was measured, using a fibre optic probe (FOP, 900 nm; TBL Fibre Optics Group Ltd, Leeds, UK). Additionally, skin damages were recorded in the first trial at cutting using a 6-point scale (0: no visible skin damage; 5: very highly damaged skin).
Statistical analyses
Data were analysed with the Statistical Analysis System, version 9.1 (SAS Institute, Cary, NC, USA). The effect of treatment on testes weight, bulbourethral gland length, levels of androstenone, skatole and indole in adipose tissue, performance and carcass quality was evaluated on combined data from the two trials with procedure mixed. The model included the fixed factor of treatment (control, surgical castration and vaccination with ImprovacTM), and the random factors of trial, pen and litter. When analysing daily weight gain during suckling and post-weaning period in the first trial, surgically castrated pigs were compared with all entire pigs, i.e. also pigs intended for immunization. The model included the fixed factor of treatment (surgical castration vs entire pigs) and the random factor of litter. To evaluate the effects on variables measured repeatedly in plasma (E1S, testosterone, skatole and indole) and serum (anti-GnRH antibodies) in the first trial, the model included either the fixed effect of treatment within sample occasion or sample occasion within treatment and the random factors of pen and litter (for sampling occasion also the individual pig). In the second trial, only one plasma sample was taken the day before slaughter and values were evaluated using the model with the fixed factor of treatment and the random factors of pen and litter.
The levels of E1S, testosterone, skatole and indole in plasma, anti-GnRH antibodies in serum and androstenone, skatole and indole in fat were log-transformed to normalize the distribution. Back-transformed least-squares means (LS means) and 95% confidence interval were used in Results section.
Results
Anti-GnRH antibody titres
Only low anti-GnRH antibody titres were detected in the serum from entire and surgically castrated male pigs at all sampling occasions (Fig. 1). In immunized pigs, anti-GnRH antibody titres were higher compared with entire and surgically castrated male pigs after the first vaccination (second sampling occasion, range 21.7–192.6 units/ml; p < 0.001) and markedly increased after the second vaccination (third sampling occasion, range 194.0–1659.0 units/ml; p < 0.001). No non-responders among immunized pigs were observed.
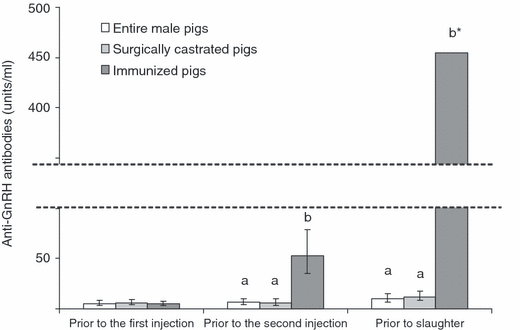
Anti-GnRH antibody titres of entire, surgically castrated and immunized male pigs prior to the first and second injections and prior to slaughter. Data are presented as least- squares means and 95% confidence interval after back-transformation to the original scale. Means with different superscripts (effect of treatment for each sampling occasion) differ at p < 0.05. *95% confidence interval for immunized pigs is from 280 to 740
Reproductive organs at slaughter and levels of androstenone, skatole and indole in adipose tissue
Immunization against GnRH significantly reduced testes weight and bulbourethral glands length (Table 1). The distribution of testes weight in entire male and immunized pigs is presented in Fig. 2, showing an overlap for the recorded weights. A similar pattern was found for the bulbourethral glands length (data not shown). Androstenone levels in adipose tissue from castrated and immunized pigs were below 0.1 μg/g and could not be quantified (Table 1). Entire male pigs expressed androstenone levels from 0.1 to 9.7 μg/g. In the first trial, 12 of 24 (50%) and in the second 18 of 22 (81.8%) entire male pigs expressed androstenone levels above 1.0 μg/g.
| Entire male pigs (n = 45) | Surgically castrated male pigs (n = 23) | Immunized male pigs (n = 47) | p-value, treatment | |
|---|---|---|---|---|
| Testis weight, pairs | 594a ± 16.0 | – | 279b ± 15.7 | 0.001 |
| Bulbourethral glands | 13.1a ± 0.50 | – | 8.7b ± 0.49 | 0.001 |
| Androstenone | 1.3a (0.82–2.20) | <0.1b | <0.1b | 0.001 |
| Skatole | 0.07a (0.050–0.116) | 0.04b (0.023–0.061) | 0.04b (0.029–0.067) | 0.001 |
| Indole | 0.04a (0.029–0.063) | 0.03b (0.017–0.043) | 0.02b (0.015–0.033) | 0.001 |
- Testis weight and bulbourethral gland length are presented as least squares means and standard error. Androstenone, skatole and indole levels are presented as least squares means and 95% confidence interval after back-transformation to the original scale. Least-squares means followed by different superscripts differ (p < 0.05).
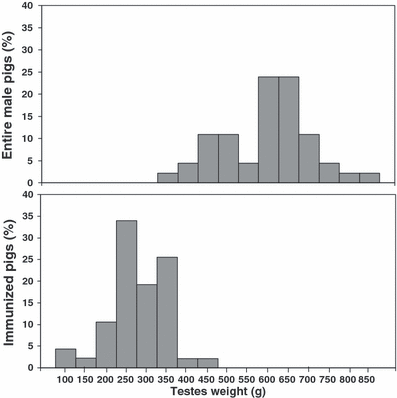
Distribution of testes weight in entire and immunized male pigs
Skatole and indole levels were lower in fat from surgically castrated and immunized pigs compared with those from entire male pigs (Table 1). Entire male, surgically castrated and immunized pigs expressed skatole levels from 0.01 to 1.28, 0.01 to 0.11 and 0.01 to 0.12 μg/g respectively. Only eight of 115 pigs produced skatole levels above 0.2 μg/g. Those eight pigs were raised intact (two of 23 and six of 22 in the first and second trial respectively). Both surgically castrated and immunized pigs expressed skatole levels far below 0.2 μg/g in fat.
Levels of estrone sulphate and testosterone in plasma
Estrone sulphate plasma levels in entire male pigs increased steadily with age in the first trial (Table 2). At first sampling, E1S levels were similar in entire male pigs and the pigs chosen for immunization (p = 0.253). E1S levels in immunized pigs at second sampling were slightly lower than those in entire male pigs (p = 0.056), and were undetectable at third sampling (p < 0.001).
| Sampling occasion | Entire male pigs (n1 = 23 and n2 = 22) | Surgically castrated male pigs (n = 23) | Immunized male pigs (n1 = 24 and n2 = 23) | p-value, treatment1 | ||
|---|---|---|---|---|---|---|
| Trial 1 | E1S | 1 | 1.0 a A (0.60–1.80) | <0.01 b | 0.7 a A (0.39–1.18) | 0.001 |
| 2 | 2.0a B (1.24–3.33) | <0.01 b | 1.0 a B (0.63–1.69) | 0.001 | ||
| 3 | 3.3a C (2.42–4.54) | <0.01b | <0.01b C | 0.001 | ||
| p-value, sampling occasion | 0.001 | 0.221 | 0.001 | |||
| Testosterone | 1 | 0.5 a A (0.29–0.86) | <0.04 b | 0.5 a A (0.28–0.80) | 0.001 | |
| 2 | 1.0a B (0.62–1.68) | <0.04 b | 0.9 a B (0.56–1.54) | 0.001 | ||
| 3 | 1.5a B (1.14–2.09) | <0.04b | <0.04b C | 0.001 | ||
| p-value, sampling occasion | 0.001 | Could not be calculated | 0.001 | |||
| Skatole | 1 | 2.7 (1.87–3.89) | 2.5 A (1.70–3.59) | 2.2 (1.53–3.20) | 0.716 | |
| 2 | 2.1 (1.26–3.38) | 1.7AB (1.04–2.80) | 1.5 (0.94–2.53) | 0.551 | ||
| 3 | 2.2 (1.41–3.30) | 1.2B (0.77–1.83) | 1.6 (1.06–2.50) | 0.093 | ||
| p-value, sampling occasion | 0.463 | 0.014 | 0.183 | |||
| Indole | 1 | 0.3 A (0.12–0.60) | 0.2A (0.11–0.56) | 0.2 A (0.11–0.54) | 0.980 | |
| 2 | 0.5a B (0.32–0.78) | 0.2b A (0.15–0.38) | 0.5 aB (0.31–0.77) | 0.018 | ||
| 3 | 0.8 a C (0.58–1.15) | 0.4b B (0.31–0.62) | 0.5 b B (0.34–0.67) | 0.002 | ||
| p-value, sampling occasion | 0.001 | 0.018 | 0.001 | |||
| Trial 2 | E1S | 8.7 a (5.22–14.59) | 0.1b (0.04–0.11) | 0.001 | ||
| Testosterone | 2.3 a (1.20–4.38) | <0.04b | 0.001 | |||
| Skatole | 5.8 a (3.52–9.45) | 2.3b (1.43–3.74) | 0.010 | |||
| Indole | 1.4 a (0.76- 2.63) | 0.5b (0.28–0.92) | 0.014 |
- 1p-value for treatment within sampling occasion for trial 1.
- Data are presented as least squares means and 95% confidence interval after back-transformation to the original scale. Means with different low-case superscripts within the rows (effect of treatment) differ at p < 0.05. Means with different capital superscripts within the columns (effect of sampling occasion) differ at p < 0.05.
Similar to E1S, testosterone plasma levels in entire male pigs increased with age in the first trial (Table 2). No differences in testosterone levels were observed between entire male pigs and the pigs chosen for immunization at first (p = 0.864) and second (p = 0.782) sampling. At slaughter, testosterone levels in immunized pigs were undetectable. Neither E1S nor testosterone were detected in surgically castrated pigs at any sampling (Table 2).
Results from the second trial confirmed that the levels of E1S and testosterone in plasma taken the day before slaughter were close to the detection limit in immunized pigs (Table 2).
Levels of skatole and indole in plasma
Skatole levels in plasma from entire male pigs were not affected by age in the first trial (p = 0.463, Table 2). In surgically castrated pigs, the levels were highest at first sampling and decreased afterwards (p = 0.014). Skatole levels in the pigs from the first trial were not affected by immunization with ImprovacTM; the levels did not differ from those of entire or surgically castrated pigs at any sampling.
Plasma indole levels in all pigs increased with age in the first trial (p = 0.001, 0.018 and 0.001 for entire, surgically castrated and immunized pigs respectively). This increase had a higher magnitude in entire pigs compared with surgically castrated and immunized pigs, thus causing a significant effect of treatment on indole levels at the second and third sampling (Table 2).
In the second trial, both skatole and indole levels in plasma taken the day before slaughter were lower in immunized pigs than in entire male pigs (p = 0.010 and 0.014 respectively; Table 2).
Performance, carcass and technological meat quality
Surgically castrated male piglets had significantly lower daily weight gain during the suckling period than entire piglets, but in the post-weaning period no difference was observed (Table 3). Daily weight gain from the start of the growing/finishing period to the time of second injection did not differ between entire male pigs, surgically castrated or immunized male pigs (Table 4). Immunization against GnRH resulted in a significantly higher daily weight gain after the second injection of ImprovacTM during the 4 weeks before slaughter. Daily, immunized pigs grew approximately 150 and 170 g more than the entire male and surgically castrated pigs respectively. Daily weight gain and feed conversion ratio for the whole growing/finishing period did not differ significantly between the groups. However, entire male pigs had slightly lower feed conversion ratio than surgically castrated pigs (p = 0.058). In the period after the second injection of ImprovacTM in the 4 weeks before slaughter, daily feed intake was 0.3 kg higher for immunized pigs than for entire male pigs, but 0.2 kg lower than for castrated pigs.
| Entire male pigs (n = 48) | Surgically castrated male pigs (n = 23) | p-value, treatment | |
|---|---|---|---|
| Birth weight per piglet, kg | 1.73 ± 0.06 | 1.74 ± 0.08 | 0.956 |
| Daily weight gain, g | |||
| Birth to weaning | 265 ± 0.01 | 235 ± 0.01 | 0.050 |
| Weaning to 9 week | 448 ± 0.02 | 447 ± 0.03 | 0.950 |
| Birth to start of experiment | 353 ± 0.01 | 339 ± 0.01 | 0.371 |
- Data are presented as least squared means and standard error.
| Entire male pigs (n = 48) | Surgically castrated male pigs (n = 23) | Immunized male pigs (n = 47) | p-value, treatment | |
|---|---|---|---|---|
| Initial weight, kg | 27.9 ± 1.94 | 27.1 ± 2.07 | 28.1 ± 1.94 | 0.671 |
| Final weight, kg | 123.4 ± 1.83 | 123.6 ± 2.43 | 126.1 ± 1.81 | 0.450 |
| Age at slaughter, days | 168 ± 1.1 | 167 ± 1.5 | 168 ± 1.1 | 0.889 |
| Daily weight gain, g | ||||
| Start to slaughter | 971 ± 20.0 | 997 ± 25.1 | 1007 ± 19.7 | 0.251 |
| Start to second injection | 915 ± 21.8 | 951 ± 27.4 | 909 ± 21.5 | 0.352 |
| Second injection to slaughter | 1107a ± 92.7 | 1090a ± 96.7 | 1257b ± 92.5 | 0.005 |
| Feed consumption, kg | 280 ± 15.9 | 318 ± 19.4 | 294 ± 15.9 | 0.130 |
| Feed conversion ratio, kg/kg | 2.90 ± 0.136 | 3.20 ± 0.165 | 3.05 ± 0.136 | 0.137 |
| Carcass weight, kg | 91.4 ± 1.28 | 92.7 ± 1.70 | 92.5 ± 1.26 | 0.746 |
| Dressing percentage | 74.7a ± 0.30 | 75.3a ± 0.38 | 73.5b ± 0.29 | 0.005 |
| Lean meat content, % | ||||
| Commercial grading | 57.8a ± 0.59 | 54.9b ± 0.67 | 56.1b ± 0.57 | 0.006 |
| Estimated | 60.2a ± 0.44 | 56.5b ± 0.51 | 58.5c ± 0.41 | 0.001 |
| pHLD | 5.45 ± 0.022 | 5.45 ± 0.022 | 5.44 ± 0.022 | 0.758 |
| pHBF | 5.53 ± 0.022 | 5.49 ± 0.023 | 5.52 ± 0.022 | 0.423 |
| FOPLD | 35.2 ± 2.15 | 34.8 ± 2.15 | 34.7 ± 2.14 | 0.983 |
| FOPBF | 38.5 ± 0.85 | 38.1 ± 0.88 | 39.6 ± 0.85 | 0.470 |
- Data are presented as least squared means and standard error. Means with different superscripts within the rows differ at p < 0.05.
Estimated lean meat content differed significantly between the groups (Table 4). Immunized pigs had higher estimated lean meat content than surgically castrated pigs, but lower than entire male pigs. When graded commercially using the Hennessy Grading Probe, lean meat content did not differ between immunized and castrated pigs, but did differ between immunized and entire male pigs. The amount of interstitial abdominal fat (only recorded in the second trial) was significantly higher in immunized pigs compared with entire male pigs (1.28 vs 0.98 kg; p = 0.022). Dressing percentage was significantly lower in immunized male pigs than in surgically castrated and entire male pigs. Internal reflectance values (FOP) and pH did not differ between groups (Table 4).
Entire male pigs had more skin damage recorded at slaughter than surgically castrated pigs (1.48 vs 0.39 points; p = 0.054), however, overall skin damage was low in spite of mixing with unfamiliar pigs on the transport and at the abattoir (only recorded in the first trial). The frequency of skin damage did not differ between immunized and entire male pigs (1.08 vs 1.48 points; p = 0.378) or between immunized and surgically castrated pigs (1.08 vs 0.39 points; p = 0.158).
Discussion
Immunization against reproductive hormones has been used in some livestock species including pigs to control their reproduction function and meat quality. In the present study, immunization against GnRH with commercially available ImprovacTM was for the first time performed in Sweden, demonstrating efficacy of this method to suppress testicular development and secretory activity in a sample of the Swedish pig population. As expected, the GnRH antibody titres were higher in immunized pigs after the first vaccination and were greatly increased after the second vaccination.
The sizes of reproductive organs at slaughter (testes weight and bulbourethral gland length) were significantly reduced in the pigs immunized against GnRH in our study and other studies (Dunshea et al. 2001; Zeng et al. 2002b). However, the size of reproductive organs may not be a reliable indicator of a response to vaccination because some entire males pigs at slaughter could also have short bulbourethral glands and low testes weight.
To evaluate the effect of immunization on testicular secretory activity, measurement of plasma testosterone, the principal androgen produced by the testes, is often used. As expected, the pigs immunized against GnRH had undetectable levels of testosterone in plasma in our study and other studies (Bonneau et al. 1994; Zeng et al. 2002a,b). Testosterone levels in surgically castrated pigs were also undetectable. As entire male pigs produce oestrogens in large amounts and their physiological levels increase at puberty (Claus and Hoffmann 1980), concentrations of these hormones could also be used to evaluate the secretory activity of the testes in male pigs. Our study showed that immunization against GnRH reduced E1S levels in plasma to non-detectable levels the day before slaughter, confirming that testicular function was markedly suppressed. Similarly, E1S levels were non-detectable in surgically castrated pigs.
In our study, both removal of testicular function by surgical castration or immunization against GnRH were associated with a marked decrease in androstenone production, as indicated by undetectable concentrations of androstenone in adipose tissue. In contrast, as many as 50 and 82% of the entire male pigs in our study had androstenone levels above 1 ppm. Previous studies have shown that androstenone (Doran et al. 2002b; Tambyrajah et al. 2004) or other steroids produced by the testes, such as oestrogens, (Zamaratskaia et al. 2005a,b) might be involved in the regulation of skatole levels. Despite the efforts of several research groups it is still unclear how testicular steroids affect skatole. Claus et al. (1994) suggested that testicular steroids are involved in skatole production by affecting the intestinal cell apoptosis, whereas Babol et al. (1999) proposed the involvement of testicular steroids in skatole metabolism in the liver. The facts that androstenone inhibited skatole-induced expression of CYP2E1 (Doran et al. 2002b), the main enzyme in skatole metabolism, and that some steroids may modify CYP2E1 activity in vitro (Zamaratskaia et al. 2007), support the latter hypothesis. High skatole levels are usually associated with high levels of steroids. Generally, neither females nor surgically castrated pigs accumulate high skatole levels in adipose tissue. This might be due to sex-related differences in the activities of enzymes involved in skatole metabolism in the liver (Whittington et al. 2004; Zamaratskaia et al. 2006). Overall, skatole levels in fat were lower in immunized and surgically castrated pigs compared with those in entire male pigs confirming the importance of testicular steroids in the regulation of skatole levels.
Skatole levels in plasma were significantly lower in immunized pigs than in entire male pigs in the second trial, but not in the first due to overall low skatole levels. In contrast, indole levels in plasma were significantly lower in immunized pigs from both trials. These lower levels were not due to reduced indole levels after the second immunization because indole levels at second and third sampling were similar. At the third sampling, indole levels in entire male pigs increased, probably as a result of increased age and LW (Babol et al. 2004), whereas no further age-related increase was observed in immunized pigs. Presuming that testicular steroids affect indole metabolism (Chen et al. 2006) it can be speculated that the absence of age-related increase in indole levels in plasma and the lower indole levels in fat were due to suppressed steroid production. Interestingly, increased steroid levels induced by the use of human chorionic gonadotropin in entire male pigs were accompanied by increased indole levels (Chen et al. 2006). Thus, the amount of testicular steroids appears to be important in the regulation of either metabolism or production of indole.
The lower growth rate during the suckling period for surgically castrated piglets in our study indicates that castration causes stress to the piglets. McGlone et al. (1993) reported that castration caused reduced suckling and standing times, but increased lying time, compared with that of entire piglets. In addition, castration without anaesthesia significantly changed physiological reactions, such as endocrine status and activation of adrenal and sympathetic axes (Prunier et al. 2006).
For the whole growing/finishing period, the growth rate of immunized pigs was similar to both surgically castrated and entire male pigs. After the second injection, the immunized pigs had higher daily weight gain than entire male pigs and castrated pigs, probably due to higher feed consumption. Similar results were found by Dunshea et al. (2001) and Cronin et al. (2003), which they explained in terms of a reduction in aggression and mounting events. Moore et al. (2006) also reported that ImprovacTM improved daily weight gain and increased feed intake compared with entire male pigs. Reduced oestrogen levels in immunized pigs might partly be responsible for the increased feed intake, as oestrogens are known to have a direct negative effect on feed intake (Bonavera et al. 1994). No effect on the overall growth rate between immunized and surgically castrated pigs was found by Jaros et al. (2005).
The estimated lean meat content was improved in immunized pigs in comparison with the castrated pigs, but was lower than in the entire male pigs. Accordingly, Dunshea et al. (2001) reported that back fat thickness of immunized pigs was in-between that of entire male pigs and castrated pigs. Hennessy et al. (2006) also found higher lean meat content and a lower back fat thickness for immunized pigs compared with surgically castrated pigs. In our study a lower dressing percentage in immunized pigs in comparison with entire male pigs was found, but with no difference in carcass weight, which is unexpected because the genital organs are much smaller after immunization. Dunshea et al. (2001) also reported a lower dressing percentage in immunized male pigs than in control pigs at a LW at slaughter similar to the pigs in our study, which they interpreted as depending on the greater feed intake and gut fill in the former group. However, the higher feed consumption during the 4 weeks before slaughter, and the consequently higher gut fillings, for the immunized pigs compared with the entire male pigs in our study was not sufficient to totally explain the lower dressing percentage. A part of the difference can be explained by the increased amount of abdominal fat, which is of limited economical value. Because pH and internal reflectance values did not differ in either LD or BF among the groups, meat quality appears not to be affected by vaccination with ImprovacTM.
While ImprovacTM is definitely one of the most effective ways to prevent boar taint and aggressive behaviours in entire male pigs, more investigations are needed. The public should be fully informed about the concept of immunocastration before the introduction of this technique into pig husbandry. In a questionnaire about several welfare aspects involved in pig production in Sweden such as raising system, castration, tail docking and fixation of sows, Swedish consumers were asked how much they wanted to pay for pork loin (Lagerkvist et al. 2006). The consumers were willing to pay 21% more for pork from immunized pigs and 21% less for pork from uncastrated male pigs compared with the present situation with castrated male pigs. By comparison, they were willing to pay 46% more when the pigs have a thick layer of straw and 64% more when the pigs are reared outdoors (Lagerkvist et al. 2006). Two other important issues are the accessibility for consumers to a comprehensive description of the safety of the products from immunized pigs and the cost of vaccine.
In summary, our data support earlier findings that immunization against GnRH suppresses reproductive function, as demonstrated by reduction in the size of reproductive organs and levels of the testicular steroids E1S and testosterone, and that it reduces androstenone and skatole levels in male pigs. None of the immunized pigs expressed androstenone or skatole levels in fat above the commonly accepted threshold for eating quality. Our results indicate that entire male pigs had better feed efficiency than castrated pigs and yield a more valuable carcass due to higher lean meat content. This disadvantage of castrated pigs is compensated for by the absence of boar taint, as assessed by the levels of androstenone and skatole. Immunocastration offers an advantage over surgical castration through improved animal welfare and better carcass quality.
Acknowledgements
This work was supported by grants from Swedish Animal Welfare Agency. Pfizer is gratefully acknowledged for providing additional financial support and ImprovacTM. The authors want to thank Mr Michael Pearce for valuable comments on the manuscript. We also thank the staff at Funbo-Lövsta Research Station for taking excellent care of the animals and for collecting data, and Dr Ingemar Hansson and Ms Ulla Schmidt for all assistance at the slaughterhouse.




