Effects of Oxygen Exposure and Gentamicin on Stallion Semen Stored at 5 and 15°C
Contents
This study was undertaken to investigate the effects of storage of stallion semen in a defined milk protein extender at 5 and 15°C under either anaerobic or aerobic conditions, with or without addition of the antibiotic gentamicin. Semen samples were collected from eight fertile stallions and stored for 96 h (day 0–4) and assessed daily for motility, velocity and membrane integrity (viability) using a CASA system. Samples for bacteriology assessment were taken on day 2 of storage. No significant (p > 0.05) differences in motility, velocity or viability were observed between treatments on days 0–2. On days 3 and 4, semen stored without gentamicin at 5°C had a significantly (p < 0.05) better semen quality compared with storage at 15°C without gentamicin, irrespective of oxygen exposure. On days 3 and 4, motility and velocity were greater in samples stored at 15°C with gentamicin, compared with the corresponding treatments without antibiotic (p < 0.05). This effect was also evident for viability on day 4. The decline in semen quality observed at 15°C most likely resulted from the effect of bacterial growth. Bacterial growth was the greatest in samples stored at 15°C without gentamicin, under both anaerobic and aerobic conditions (p < 0.05). Bacterial growth was inhibited by adding of gentamicin at 15°C, which accordingly reduced the decline in semen quality. Addition of antibiotic to samples stored at 5°C had no significant effect on any parameter analysed. In conclusion, storage at 15°C can be achieved by using an extender containing the antibiotic gentamicin. Storage at 5°C tended to maintain better semen quality irrespective of oxygen exposure, and did not necessitate an antibiotic treatment.
Introduction
The use of cooled semen for AI has become an integral part of many equine breeding programmes (Aurich and Aurich 2006). When transport from the collection centre to the site of insemination occurs within 24 h, excellent fertility results can be achieved with cooled transported semen. If an acceptable fertilizing capacity of cooled stallion semen could be maintained for several days, this would allow for easier timing of insemination and transport of semen. Several attempts have been made to improve semen extenders and storage conditions in order to prolong the time semen can be maintained viable during storage (e.g. Aurich et al. 1997; Batellier et al. 1997, 2001; Varner et al. 1998; Pagl et al. 2006).
Most extenders for storage of equine semen are based on either skim milk or egg yolk and are routinely used at a temperature between 4 and 6°C (reviewed by Aurich 2005). In some extenders, biological products such as skim milk have been replaced by defined proteins to improve standardization and reduce the risk of microbiological contamination and such extenders were superior to traditional skim milk extenders (Batellier et al. 1998, 2001; Pagl et al. 2006). An extender containing phosphocaseinates maintained fertility of equine semen for up to 72 h when stored at 15°C under aerobic conditions and a per cycle pregnancy rate of 48% could be achieved. After storage for 24 h at 15°C, per cycle pregnancy rate was 57% compared to 40% with skim milk-based semen extenders (Batellier et al. 1998, 2001).
The penis of the stallion harbours a natural microbial population, which can contaminate semen during ejaculation (Varner et al. 1998). Traditionally, antibiotics have been added to semen extenders to inhibit bacterial growth in stored semen and protect the mare from post-coital endometritis (Jasko et al. 1993). However, there is evidence that not only bacteria but also certain antibiotics may have detrimental effects on spermatozoa. Depending on the extender used, gentamicin and polymixin B sulphate at a concentration of 1 g/l or higher concentrations decrease semen motility during cooled storage (Jasko et al. 1993; Aurich and Spergser 2006).
The aim of this study was to analyse the effects of storage temperature, oxygen exposure and addition of gentamicin on motility parameters and membrane integrity of stallion spermatozoa during liquid storage in an extender containing defined milk proteins. In addition, bacterial growth was investigated.
Materials and Methods
Animals and semen collection
A total of eight fertile stallions of different breeds (three Hannoverian sport horses, four Shetland ponies, one Welsh pony) were available for semen collections. The stallions were aged between 4 and 14 years. The Hannoverians were fed concentrates three times per day and all stallions were fed hay twice daily. Mineral supplements and water were available ad libitum.
In all two ejaculates were assessed from each stallion over a period of 2 months (June–July). Ejaculates were collected at intervals of 1–2 weeks. For spermatology parameters, the mean from the two ejaculates was then used for further statistical analysis. Semen collection and analysis were performed with standard techniques as described (Aurich et al. 1997). Semen sample was collected using an artificial vagina (Hannover Model; Minitüb, Tiefenbach, Germany) on a dummy or on an ovariectomized mare. Semen collections were always performed between 10:00 and 11:30 a.m. After collection, the gel fraction was removed and the remainder of the ejaculate was filtered through sterile gauze, the volume of gel was then recorded. Volume, colour, consistency and pH were also recorded from the gel free fraction. Sperm concentration was assessed photometrically (SpermaCue; Minitüb) and the total sperm count was calculated. The analysis of semen motility is given in a separate section here.
Semen processing and storage
After removal of the gel fraction, the remainder of the ejaculate was divided into eight aliquots and diluted to a concentration of 25 million sperm/ml in EquiPro extender (Minitüb; Pagl et al. 2006) with or without the antibiotic gentamicin. Aliquots 1–4 did not contain gentamicin while aliquots 5–8 were diluted in EquiPro plus gentamicin at a concentration of 250 mg/l. Aliquots were stored either anaerobically by filling a 10-ml syringe with 5 ml diluted semen and no air, or aerobically by filling a 10-ml syringe with half air and half diluted semen as described (Batellier et al. 1997). All syringes were sealed with a cap and stored horizontally for 96 h at 5 or 15°C. Syringes were gently rotated every 24 h. The storage conditions and the abbreviations used for different semen treatments are summarised in Table 1. On day 2 of storage, samples for bacteriology assessment were also taken.
| Aliquot No. | Additions to extender | Oxygen exposure | Temperature (°C) | Abbreviation |
|---|---|---|---|---|
| 1 | – | Anaerobic | 5 | 5 An |
| 2 | – | Aerobic | 5 | 5 Aer |
| 3 | – | Anaerobic | 15 | 15 An |
| 4 | – | Aerobic | 15 | 15 Aer |
| 5 | Gentamicin | Anaerobic | 5 | 5 AB An |
| 6 | Gentamicin | Aerobic | 5 | 5 AB Aer |
| 7 | Gentamicin | Anaerobic | 15 | 15 AB An |
| 8 | Gentamicin | Aerobic | 15 | 15 AB Aer |
Semen analysis
Total motility, velocity and membrane integrity of spermatozoa were analysed using a computer-assisted sperm analysis system (SpermVision; Minitüb) as described (Aurich and Spergser 2006; Pagl et al. 2006).
A 6-μl drop of diluted semen was placed on a standard microscope slide and seven fields per sample were evaluated on each day. Assessment on day 0 occurred immediately after dilution and prior to cooling. On days 1–4, 200 μl of diluted semen was removed from each syringe and warmed in a water bath (37°C) for 5 min before analysis. Motility analysis utilised a phase contrast microscope (Optiphot-2; Nikon, Vienna, Austria, magnification ×200), with a warmed stage (35°C) and a high-speed digital camera that identifies 60 frames per second. Total motility, average path velocity (VAP, μm/s), curved line velocity (VCL, μm/s) and straight line velocity (VSL, μm/s) were recorded.
Membrane integrity (viability) of spermatozoa was assessed using a phase contrast fluorescent microscope (Olympus AX70, Olympus, Vienna, Austria; U-MWB filter block, BP420-480 excitation filter, BA515 suppressor filter, dichromatic mirror: DM500, magnification ×400) following procedures established in our laboratory (Schäfer-Somi and Aurich 2005, in press). Samples were stained by incubating 1 ml diluted semen with 2.6 μl fluorescent dye SYBR-14/propidium iodide nucleic acid (PI) for 7 min at room temperature. The stain was prepared by mixing 100 μl of SYBR-14 solution (Minitüb), 2400 μl of PI solution (Minitüb) and 2500 μl of Androhep extender (Minitüb). Using this stain, sperm with intact membranes stained green, and sperm with damaged membrane stained red. Results are given as percentage of live, i.e. membrane-intact, spermatozoa.
Samples for bacteriology assessment were taken with a sterile swab and were analysed after culture on blood agar using routine techniques (Aurich et al. 2003). The bacteria species were recorded qualitatively and bacterial growth was analysed semi-quantitatively by counting the number of colonies per plate. Bacterial growth was scored as absent (0), low (1), intermediate (2) and high (3).
Statistical analysis
All statistical comparisons were made with the spss statistical package (SPSS, Chicago, IL, USA). Data were normally distributed and thus analysis of variance (anova) and subsequent Tukey's Test were used to compare the overall effects of treatments for each day. Only comparisons of treatments which differed in only one parameter (e.g. anaerobic vs aerobic, 5°C vs 15°C) are given in the results. All values are expressed as means ± standard error of the mean (SEM) and a p-value <0.05 was considered significant.
Results
Motility
Total motility did not differ between treatments immediately after dilution with semen extender (day 0) or on days 1 and 2 of storage. On days 3 and 4, in all aliquots without gentamicin, motility was significantly (p < 0.05) lower at a storage temperature of 15°C compared with that at 5°C (e.g. day 3: 5°C An 63 ± 3% vs 15°C An 8 ± 4%; 5°C Aer 64 ± 3% vs 15°C Aer 9 ± 3%, p < 0.05; see Fig. 1A). When the extender contained gentamicin, differences in motility between storage temperatures were reduced but existed only on day 4 between semen stored anaerobically at 5°C (59 ± 7%) vs 15°C (20 ± 4%, p < 0.05; see Fig. 1B).
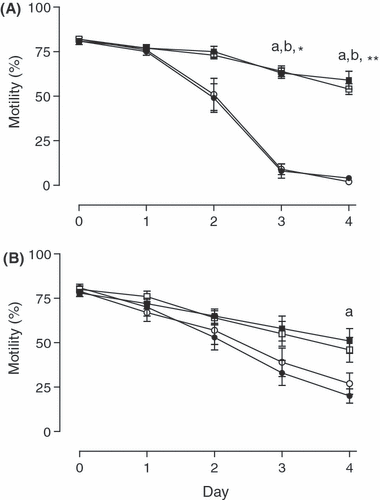
Total motility of spermatozoa stored either anaerobically at 5 () or 15°C () and aerobically at 5 () or 15°C (), without (A) and with (B) addition of gentamicin (250 mg/l). Significant differences: a, anaerobic storage at 5 vs 15°C; b, aerobic storage at 5 vs 15°C; *anaerobic and aerobic storage at 15°C with vs without antibiotic; **aerobic storage at 15°C with vs without antibiotic (p < 0.05)
On day 3, total motility in semen stored either anaerobically or aerobically at 15°C was higher when gentamicin had been added (33 ± 7 and 39 ± 8% respectively) than that in semen without gentamicin (8 ± 4 and 9 ± 3%, respectively, p < 0.05). On day 4, total motility in semen stored aerobically at 15°C was higher when gentamicin had been added (27 ± 6%) than that in semen without gentamicin (2 ± 1%, p < 0.05). Independent of storage temperature and gentamicin semen motility did not differ between anaerobic or aerobic storage at any time.
Velocity parameters
None of the parameters VAP, VCL and VSL differed between treatments on days 0–2 of storage. On days 3 and 4, in semen stored without gentamicin, all three velocity parameters were higher in semen kept at 5°C than in corresponding aliquots at 15°C (p < 0.05; see 2-5). In semen containing gentamicin, no differences between groups were found on day 3, but on day 4 VAP and VSL were significantly higher (p < 0.05) for aerobic storage at 5°C than at 15°C. Day 4, VCL was also higher in samples stored anaerobically at 5°C than in corresponding aliquots at 15°C (p < 0.05). Addition of gentamicin to aliquots stored at 15°C reduced the loss in all three velocity parameters at 15°C on days 3 and 4 for both anaerobic and aerobic storage (p < 0.05; see 2-4).
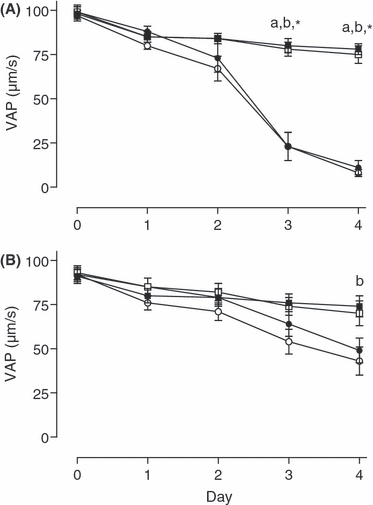
Velocity average path (VAP) of spermatozoa stored either anaerobically at 5 () or 15°C () and aerobically at 5 () or 15°C (), without (A) and with (B) addition of gentamicin (250 mg/l). Significant differences: a, anaerobic storage at 5 vs 15°C; b, aerobic storage at 5 vs 15°C; *anaerobic and aerobic storage at 15°C with vs without antibiotic (p < 0.05)
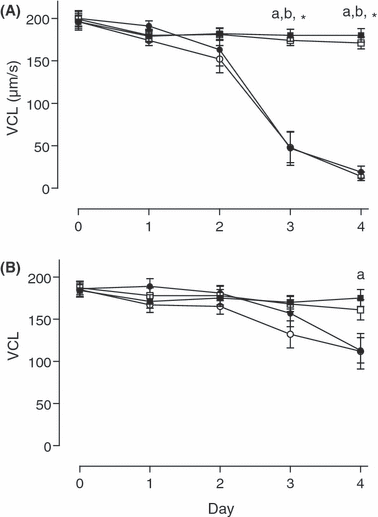
Velocity curved line (VCL) of spermatozoa stored either anaerobically at 5 () or 15°C () and aerobically at 5 () or 15°C (), without (A) and with (B) addition of gentamicin (250 mg/l). Significant differences: a, anaerobic storage at 5 vs 15°C; b, aerobic storage at 5 vs 15°C; *anaerobic and aerobic storage at 15°C with vs without antibiotic (p < 0.05)
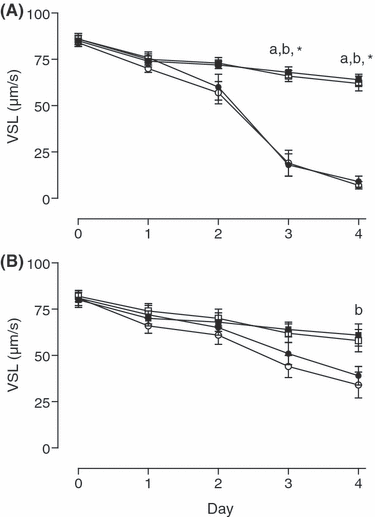
Velocity straight line (VSL) of spermatozoa stored either anaerobically at 5 () or 15°C () and aerobically at 5 () or 15°C (;), without (A) and with (B) addition of gentamicin (250 mg/l). Significant differences: a, anaerobic storage at 5 vs 15°C; b, aerobic storage at 5 vs 15°C; *anaerobic and aerobic storage at 15°C with vs without antibiotic (p < 0.05)
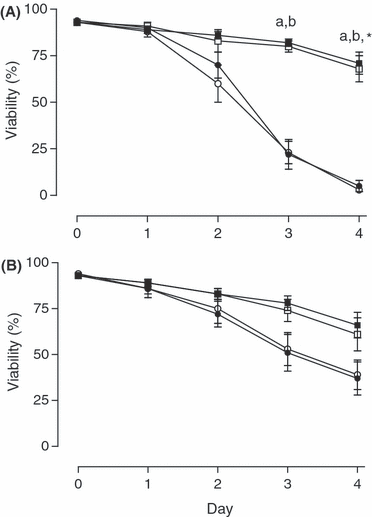
Percentage of membrane-intact (viable) spermatozoa stored either anaerobically at 5 () or 15°C () and aerobically at 5 () or 15°C (), without (A) and with (B) addition of gentamicin (250 mg/l). Significant differences: a, anaerobic storage at 5 vs 15°C; b, aerobic storage at 5 vs 15°C; *aerobic storage at 15°C with vs without antibiotic (p < 0.05)
Membrane integrity
As reflected in motility parameters, the viability of spermatozoa determined as membrane integrity did not differ between treatments on days 0–2. On days 3 and 4 of storage, the percentage of membrane-intact spermatozoa was significantly higher at 5°C than in corresponding aliquots at 15°C for semen without gentamicin (p < 0.05). No significant difference existed between aliquots containing gentamicin on any day. Comparison of treatments with and without gentamicin found that the percentage of membrane-intact spermatozoa on day 4 was higher in semen stored aerobically at 15°C with gentamicin (37 ± 9%) than without gentamicin (3 ± 1%, p < 0.05; see Fig. 5).
Bacteriology
Storage without gentamicin at 15°C under anaerobic and aerobic conditions significantly (p < 0.05) increased bacteria growth compared to all other storage conditions (see Fig. 6). Storage with gentamicin at 5°C under anaerobic conditions contained minimal bacterial growth within 48 h of storage. Low levels of bacterial growth were detected in all other samples. Bacteria species identified included normal mucosal flora, Bacillus spp, Streptococcus spp, Staphylococcus spp, Enterococcus spp and Enterobacteriaceae spp (see Table 2).
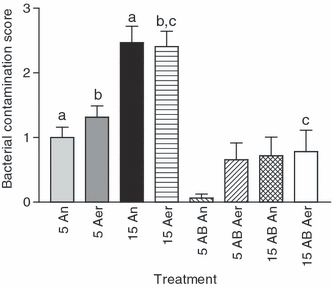
Bacterial contamination score (scale 0–3) of diluted semen on day 2 of storage. Significant differences: a, anaerobic storage at 5 vs 15°C; b, aerobic storage at 5 vs 15°C; c, storage at 15°C with vs without gentamicin (250 mg/l)
| Treatment | Negative | Mucosal flora | Enterobacteriaceae | Enterococcus | Bacillus spp | Escherichia coli | Othersa |
|---|---|---|---|---|---|---|---|
| 5 An | 2 | 12 | – | 2 | 2 | – | 1 |
| 5 Aer | 1 | 15 | – | – | 1 | – | – |
| 15 An | 1 | 2 | 5 | – | 8 | 2 | 2 |
| 15 Aer | – | 1 | 7 | 3 | 6 | 2 | 1 |
| 5 AB An | 15 | 1 | – | – | – | – | – |
| 5 AB Aer | 10 | 6 | – | – | – | – | 1 |
| 15 AB An | 8 | 5 | 1 | – | 3 | – | 1 |
| 15 AB Aer | 11 | 1 | 2 | – | 4 | 1 | – |
- aOthers includes bacteria species Lactococcus, α-haemolysing Streptococcus, Streptococcus equisimilis ssp. zooepidemicus, Alcaligenes faecalis, Staphylococcus intermedius (n = 1–2).
Discussion
This study demonstrates clear effects of temperature on the quality of cooled stored equine semen. With milk-based extenders, a temperature between 4 and 6°C has been recommended as optimal for the cooled storage of stallion semen (Varner et al. 1988, 1989; Moran et al. 1992). However, with an extender containing native phosphocaseinates instead of skim milk, equal fertility results were obtained when semen was stored at 15°C under aerobic conditions and at 4°C under anaerobic conditions (Batellier et al. 2001). Storage at 15°C has thus been recommended as an alternative to storage at 4–6°C (Batellier et al. 1998, 2001). In our study, after 48 h of storage, no significant differences in motility or viability parameters were apparent between treatments, irrespective of temperature, oxygen exposure and gentamicin content. At no time did 15°C storage result in better semen quality than storage at 5°C. In contrast, several parameters indicated a reduced semen quality at 15°C, especially but not exclusively when no antibiotic was added. Our data are thus in contrast to results obtained with an extender containing native phosphocaseinates (Batellier et al. 1998, 2000, 2001), which gave the best semen quality after storage at 15°C. The authors suggested that this is due to a favourable influence of the native micelle structure of the casein observed only at 15°C (Batellier et al. 2000). However, Batellier et al. (1998, 2001) did not compare semen storage temperatures for more than 24 h. Our findings based on motility and viability parameters do suggest that a temperature of 15°C should not be preferrentially used for the defined milk protein extender EquiPro.
When semen was stored at 15°C without gentamicin, a dramatic decrease in all parameters analysed occurred on day 3 of storage compared to storage at 5°C. This coincided with an increase in bacterial growth which became apparent after 2 days of storage. Bacterial samples taken from semen at 48 h detected a variety of bacterial species. The bacteria identified constitute the non-pathogenic, commensal microflora of the stallion's genital tract (Varner et al. 1998). In a recent paper (Aurich and Spergser 2006) it was demonstrated that from a variety of potentially pathogenic bacteria only Streptococcus equisimilis and Pseudomonas aeruginosa negatively affected semen quality during storage at 5°C. At higher temperatures, other commensal bacteria are also clearly detrimental to semen quality.
The decrease in semen quality on days 3 and 4 of storage was less pronounced when gentamicin at a concentration of 250 mg/l had been added to the extender. A dose of 1 g/l is suggested by others (Jasko et al. 1993; Varner et al. 1998) but this concentration of gentamicin has been found to have negative effects on semen quality when used in EquiPro extender (Aurich and Spergser 2006). However, gentamicin at a concentration of 250 mg/l in the present study was effective in reducing the loss in semen quality between days 2 and 3 of storage. Semen extenders for storage at 15°C thus should contain antibiotics to control bacterial growth. In agreement with this recommendation the INRA 96 extender also contains antibiotics (Batellier et al. 1998, 2001).
Despite a decline over time in all parameters determined in our study, there was no major effect of bacterial growth over 4 days when semen was stored at 5°C. Thus with adequate hygienic precautions during semen collection and processing, the use of antibiotics in semen extender can be completely avoided. Antibiotics in semen extender, besides their desired effects on bacteria, may negatively affect spermatozoal function (Varner et al. 1998; Aurich et al. 2003). In addition, the use of broad spectrum antibiotics may increase resistance of bacteria. Antibiotics brought into the genital tract of mares may cause changes in the natural vaginal microflora, which may lead to proliferation of pathogenic organisms. Accordingly, widespread use of antibiotics in semen extenders thus may be questioned. Our present data show that for at least 24 h even storage of semen at 15°C without antibiotic is possible with adaequate hygienic precautions during semen collection and processing.
In conclusion, storage of stallion semen at 15°C resulted in a decrease in semen quality on days 3 and 4 of storage compared to 5°C. This is most likely caused by the effects of bacteria growing at 15°C. Bacterial growth can largely be avoided by adding gentamicin, but at 5°C semen quality can also be maintained for more than 24 h without antibiotics in semen extender.




