Artificial Insemination of Gilts with 1.5 Billion Sperms Stored in Different Periods Associated with Different Pre-ovulatory Intervals
Contents
This study evaluated the reproductive performance of gilts inseminated at three intervals before ovulation (0–12, 13–23, 24–30 h) with sperm doses (SD) stored for 0–48 and 96–120 h. A total of 218 PIC Camborough 22® gilts were inseminated once with SD of 1.5 × 109 sperms. Pregnant gilts (n = 166) were slaughtered 30.8 ± 3.7 days after artificial insemination. The number of corpora lutea (CL) and total embryos (TE) was counted. Pregnancy rates (PR) were analysed by chi-square test. TE and embryonic survival (ES), obtained as the ratio between viable embryos and CL, were analysed by GLM procedure (SAS) and mean values were compared by Tukey's test. Pregnancy rate was similar among artificial insemination–ovulation (AIOV) intervals when semen was stored for 0–48 h. However, the lowest PR was observed in the 24–30 h AIOV interval with storage time (ST) of 96–120 h (p < 0.05). There was a significant effect of the interaction between ST and AIOV (p < 0.05) on TE and ES variables. Total embryos and ES did not differ (p > 0.05) among AIOV intervals in ST of 0–48 h. However, gilts inseminated at 24–30 h AIOV interval with ST of 96–120 h showed a reduction of 6.7 embryos (p < 0.05) compared with gilts in the same interval inseminated with semen stored for 0–48 h. ES for the 24–30 h AIOV interval and ST of 96–120 h was lower than that observed in the other groups (p < 0.05).
Introduction
Real-time transcutaneous ultrasonography has allowed a noninvasive study of follicular dynamics and the moment of ovulation (Weitze et al. 1989). Using ultrasonography, it has been possible to evaluate the influence of artificial insemination (AI) intervals on fertility (Soede et al. 1995; Nissen et al. 1997). The optimal fertility results occurred when AI was performed until 24 h before ovulation in sows (Soede et al. 1995; Nissen et al. 1997) and gilts (Waberski et al. 1994). In AI performed at intervals higher than 24 h before ovulation, a reduction in the fertilization rate was observed (Soede et al. 1995; Kemp and Soede 1997). Nevertheless, in another study, it has been demonstrated that successful fertilization occurred in approximately 50% of gilts inseminated at an interval higher than 24 h (Soede et al. 1995). Other authors also observed that 26.7% of gilts showed embryonic survival rate higher than 78% when AI was performed at an interval higher than 24 h before ovulation (Uemoto 1999). Probably, this phenomenon could be associated with sperm quality (Popwell and Flowers 2004) or inherent female aspects (Rousseau and Ménézo 1993). Studying different insemination–ovulation intervals in gilts, a group of authors observed a negative relationship between in vivo ageing semen and fertilization rate (Waberski et al. 1994).
Information about the optimal insemination–ovulation interval, mainly concerning the pre-ovulatory period, and its interaction with semen storage time in vitro are scarce for gilts. The objective of this study was to evaluate the reproductive performance of gilts submitted to AI in different artificial insemination–ovulation (AIOV) intervals using liquid boar semen stored up to 120 h.
Materials and Methods
Animals and housing
Camborough 22® gilts (n = 218), 179 ± 12 days of age and 110 ± 10 kg weight were used in the experiments. The gilts were housed in groups of eight animals per pen (1.8 m2/gilt).
Oestrus detection
Oestrus detection started in the first post-housing day and was performed at 12-h intervals (07:30 and 19:30 hours) using a mature boar. Oestrus onset was defined as the first time a gilt showed a standing response to back pressure in the presence of a mature boar, minus 6 h. The end of oestrus was defined as the last time a gilt showed a standing response, plus 6 h.
Time of ovulation
Transcutaneous ultrasonography of ovaries (Weitze et al. 1989) was performed at 12-h intervals (08:00 and 20:00 hours) beginning 12 h after oestrus onset. The presence of follicles and the determination of time of ovulation was performed with a 5-MHz sector scanner (Aloka Co., Ltd., Mure, Mitaka-shi, Tokyo, Japan). The time of ovulation was defined as the interval between the onset of oestrus and the first time when no follicles were observed, minus 6 h. The ovulation was confirmed by one additional scanning 12 h later.
Inseminating doses
Semen was collected twice weekly from three mature boars (Agroceres PIC®). The semen was macroscopically (colour, odour, aspect and volume) and microscopically (motility, sperm concentration and morphology) evaluated. Only ejaculates that presented at least 75% spermatic motility were processed. Spermatic concentration (sperm/ml) was evaluated by Neubauer Improved® chamber counting (Karl Hecht GmbH & Co., Sondheim/Rhön, Germany). A re-count of sperm number in the semen doses (SD) was performed after dilution. The same ejaculate was diluted in three different extenders, one of short-term and the other two of long-term storage, and doses of 1.5 billion sperms were produced with a total of 90 ml volume. SDs were stored at 15–18°C and sperm motility was evaluated every 24 h until 120 h.
Artificial insemination
Gilts that showed oestrus, and were at least 220 days old and weighed more than 120 kg, were randomly selected to be inseminated once between 18 and 30 h after oestrus onset in order to have different insemination–ovulation intervals. AI were performed with a Melrose® catheter (Minitüb GmbH & Co., Tiefenbach, Germany) and SD of 1.5 × 109 sperms in the presence of a mature boar.
Pregnancy detection
Pregnancy detection was performed through transcutaneous ultrasonography 21–23 days after AI. The gilts were slaughtered at 30.8 ± 3.7 days of pregnancy and their genital tracts were removed. The corpora lutea (CL) and embryos were counted.
Experimental design and statistical analyses
The semen was processed on a split-sample basis taking into account the distribution in three extenders and in two storage times for each extender. One SD was used between 0 and 48 h and the other between 96 and 120 h of storage time. The gilts of the first trio received only one SD stored up to 48 h (10 ± 14 h). The second trio received a SD of the same ejaculate, but stored from 96 to 120 h (101 ± 8 h). Females were separated according to three AIOV intervals: 0–12, 13–23 or 24–30 h before ovulation. Those with an AIOV interval higher than 30 h were not included in the analysis. Pregnancy rate (PR) was analysed by the chi-square test. The total number of embryos (TE) and embryonic survival (ES) were analysed using the GLM procedure (SAS Institute 1998). In the model, the effects of extenders, semen storage time (ST), AIOV intervals, boars and of their interaction were considered. Furthermore, the number of CL was maintained as a co-variable in the model used to analyse the number of embryos. The mean values were calculated by the LSMEANS procedure (SAS Institute 1998) and compared by the Tukey's test.
Results
The duration of oestrus of all gilts was 52.3 ± 13.3 h (24–84) and the interval between oestrus onset and moment of ovulation was 33.9 ± 8.4 h (24–48), on average. The average spermatic motility was 90.2 ± 5.6% (75–95) and 81.3 ± 5.8% (75–95) in 0–48 and 96–120 h of ST respectively.
There was no effect of extenders on PR, TE and ES (p > 0.05). In all AIOV intervals, PR for ST of 0–48 h were higher (p < 0.05) than those for ST of 96–120 h (Table 1). When semen was stored for 0–48 h, PR was similar for all AIOVs. However, in ST of 96–120 h, the AIOV interval of 24–30 h resulted in the lowest PR (p < 0.05).
| Storage time (h) | AIOV (h) | PR (n/n) | n | CL* | TE* | ES (%)* |
|---|---|---|---|---|---|---|
| 0–48 | 0–12 | 93.4 (57/61)a | 55 | 17.6 ± 2.6 | 14.1 ± 3.2a | 78.6 ± 17.5a |
| 13–23 | 91.9 (34/37)a | 33 | 18.0 ± 3.4 | 14.2 ± 4.8a | 75.1 ± 20.9a | |
| 24–30 | 85.7 (18/21)a | 18 | 17.1 ± 2.5 | 13.2 ± 3.9a | 71.9 ± 21.0a | |
| 96–120 | 0–12 | 78.3 (47/60)b | 43 | 17.5 ± 2.6 | 13.2 ± 4.4a | 73.3 ± 23.7a |
| 13–23 | 73.1 (19/26)b | 13 | 16.1 ± 1.7 | 14.3 ± 4.7a | 76.1 ± 26.0a | |
| 24–30 | 30.8 (4/13)c | 4 | 15.7 ± 2.5 | 6.5 ± 2.1b | 31.9 ± 13.2b |
- *LS mean ± standard deviation.
- Values in the same column with different superscript letters are statistically different (p < 0.05).
Interaction was observed between ST and AIOV intervals (p < 0.05) for TE and ES variables. Total embryos did not differ (p > 0.05) among AIOV intervals when SDs were stored up to 48 h (Table 1). However, in ST of 96–120 h and AIOV interval 24–30 h, a reduction of 6.7 embryos (p < 0.05) was observed in comparison with the same interval with ST of 0–48 h (Table 1). ES of the 24–30 h AIOV interval and ST of 96–120 h was lower (p < 0.05) than that observed in the other groups (Table 1).
Discussion
In this experiment, gilts were inseminated once with a 1.5 × 109 sperm dose. The moment of ovulation was determined by transcutaneous ultrasonography. On average, ovulation took place at 64.7% of the oestrus period. This results are in accordance with Bracken et al. (2003), who observed the moment of ovulation after 60% of oestrus period in gilts.
Pregnancy rates were not affected by AIOV interval when semen was stored up to 48 h. Likewise, Waberski et al. (1994) did not observe differences on fertilization rates in gilts inseminated at intervals of <12 h (82.5%), 12–24 h (79.9%) and >24 h (74.2%) before ovulation using SD stored up to 48 h. However, Uemoto (1999), working with different pre-ovulatory intervals, observed that PR on day 24 after AI was affected by AIOV interval, even with semen stored up to 40 h. In that case, the PR significantly decreased as the AIOV interval increased (100, 89 and 73% for 0 to <16, 16 to <32 and >32 h respectively).
The sperm cells ageing in vitro for 96–120 h associated with semen ageing in vivo in the AIOV >23 h resulted in a reduction of PR (Table 1). According to Waberski et al. (1994), the interval between insemination and ovulation has to be considered as a major factor of variation in fertility results not related to semen quality. The same authors observed that when semen was stored by 87–118 h, the fertilization rate significantly decreased with the increase in the AIOV interval. The authors observed fertilization rates of 73.0% (<12 h), 50.0% (>12 to 24 h) and 16.3% (>24 h).
Embryonic survival at 30.8 ± 3.7 days after AI ranged from 71.9 to 78.6% in ST of 0–48 h, confirming embryonic losses of 20–40% until 35 days of pregnancy (Pope and First 1985). The low number of embryos in females inseminated at 24–30 h AIOV interval with semen stored for 96–120 h (Table 1) shows the effect of both in vitro and in vivo ageing of semen cells. This occurred probably because of the insufficient number of capacitated sperm cells in the oviducts. This hypothesis was suggested by Soede et al. (1995) who obtained a low number of accessory sperm cells in the embryos of sows with AIOV intervals ≥24 h. Waberski et al. (1994) also observed a reduction in accessory sperm cells associated with sperm ageing. This fact could be indicative of a sperm transport reduction in oviduct because of sperm cell ageing. According to Steverink et al. (1997), there was an inverse relationship between AIOV interval and fertilization rate when semen was stored up to 36 h. In the present experiment, however, this relationship was not observed because the AIOV interval did not affect the PR and TE when semen was stored up to 48 h (p > 0.05).
It is worth noting that even when insemination was performed at a 24–30-h AIOV interval and semen stored up to 48 h, 38.9% (7/18) of gilts showed an ES rate higher than 80% (Fig. 1). In others studies, it has been demonstrated that when females were inseminated at a >24-h AIOV interval, some of them showed a ES rate higher than 78% (Uemoto 1999) and fertilization rate above 90% (Soede et al. 1995; Bracken et al. 2003). This fact could be related to a longer life of sperm cells in the female genital tract. Xu et al. (1998) describe a possible division of sperm population in sub-populations that could capacitate slower or faster. Based on in vitro results, these authors suggest that a sub-population that responds slowly would have capability to fertilize the oocytes in vivo for a longer period in the female genital tract. In this way, these sub-populations could be responsible for good results obtained in some females with AIOV intervals higher than 23 h. In contrast, in ST of 96–120 h, other factors related to sperm cell ageing could be involved. Flowers (1997) suggests that spermatozoa of dominant males could have a longer life in the female genital tract and this effect is related, in part, to the composition of seminal plasma. Popwell and Flowers (2004) showed differences among boars in the farrowing rate and litter size even when spermatic motility and morphology were similar among them. For Harkema et al. (2004), the boar has a significant effect on the number of females with a 100% fertilization rate. However, the boar effect on the pregnancy rate and total embryos was not observed in the present study (p > 0.05). Furthermore, according to Rousseau and Ménézo (1993), inherent female aspects could be related to a better fertilization rate. The authors suggest that some females could have better conditions for embryonic survival in their genital tract. These conditions could be associated with the composition of uterine secretion, which would be favourable to maintain sperm viability and fertilizing capacity. However, the mechanism by which some females present good results of fertilization and number of embryos, even without an optimal AIOV interval, is unclear and more studies are needed.
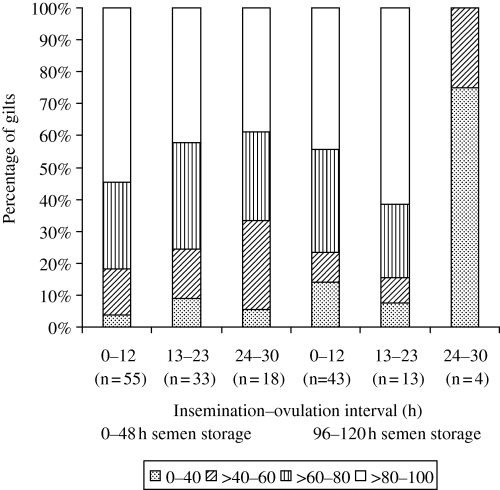
Embryonic survival distribution in gilts inseminated at different pre-ovulatory intervals with semen dose of 1.5 billion sperms stored up to 120 h
Based on the present results, it is possible to conclude that pregnancy rate in gilts inseminated once with 1.5 billion sperms is negatively influenced by in vivo and in vitro spermatozoa ageing. Embryonic survival and number of embryos are reduced by a longer in vitro semen storage (96–120 h) associated with a higher insemination–ovulation interval (24–30 h).




