Investigating the molecular systematic relationships amongst selected Plesionika (Decapoda: Pandalidae) from the Northeast Atlantic and Mediterranean Sea
Abstract
Despite the high number of species and ecological diversity of pandalid shrimps, there has been no previous attempt to resolve evolutionary relationships of several genera using molecular tools. Although mitochondrial DNA cytochrome oxidase I (COI) is widely used in barcoding studies to delimit species boundaries, additional insights into phylogenetic affinities can be obtained, especially when used in combination with data from additional genes. The knowledge of molecular diversity is essential to understand phylogenetic relationships and will help systematic clarifications. Based on partial fragments of the 16S and COI genes, we have focused specifically on addressing the systematic relationships of the economically and ecologically important shrimp genus Plesionika within a framework of five genera from within the Pandalidae. Our results showed that species within Plesionika are substantially divergent when compared with other genera, exhibiting the highest average nucleotide divergence, with 0.1123 and 0.0846 in COI and 16S genes, respectively. In addition, sequence divergence was found to vary greatly within the genus Plesionika (COI/16S): 0.0247/0.0016 between Plesionika antigai and Plesionika heterocarpus and 0.1616/0.098 between Plesionika heterocarpus and Plesionika edwardsii. We did not find amino acid sequence divergence between P. heterocarpus and P. antigai compared with P. heterocarpus and P. edwardsii (8.10%, K2P distance). Three species of Plesionika (P. antigai, P. heterocarpus and Plesionika scopifera) appear well separated from other Plesionika species in both maximum likelihood and Bayesian analyses. The present study confirms the utility of COI over 16S as a genetic marker to resolve relationships between different species of Plesionika from the Northeast Atlantic and Mediterranean Sea, in addition to species delimitation. The findings highlight the need to further review paraphyly within Plesionika in an attempt to recognize a concordance in the evolutionary history of Plesionika with major ecological and geological events.
Introduction
Most systematic studies of the marine shrimps, Pandalidae, have been based solely on morphology; molecular tools have been rarely applied to solve questions of species status or to determine lower level phylogenetic relationships. Although previous studies exist on phylogenetic relationships within major taxa of the infra-order Caridea exist (Shank et al. 1999; Page et al. 2008; Baeza et al. 2009; Bracken et al. 2009), few studies attempted to understand pandalid molecular phylogeny and biogeographical relationships between the different genera. The Pandalidae is among the most species-rich families due to extensive diversification in the genus Plesionika Bate, 1888 with 92 extant described species (De Grave et al. 2009). Among the pandalid shrimps found in the Northeast Atlantic and Mediterranean Sea, the species of the genus Plesionika have a subtropical and tropical distribution, with the Bay of Biscay region as the northernmost limit (Vafidis et al. 2005). Members of Plesionika are small to intermediate sized shrimps (6–29 mm carapace length) with a benthic or nektobenthic occurrence, distributed on the shelf and slope (Puig et al. 2001; Vafidis et al. 2005). A clear segregation of size and depth of Plesionika spp. has been described and congeneric assemblages of Plesionika species exhibit specific bathymetric distributions, each species having a preferred depth range (Puig et al. 2001; Carbonell et al. 2003). Decapod species play an important ecological trophic role in megabenthic ecosystems (Cartes 1998; Cartes et al. 2002; Fanelli & Cartes 2004), both as predators and prey. They feed on mesopelagic, suprabenthic, epibenthic and infaunal organisms, and are considered non-migratory macroplankton feeders (Cartes et al. 2002). Furthermore, they constitute a large part of the diet of demersal fish and cephalopods (Vafidis et al. 2005). In the Mediterranean demersal fisheries, some species form a by-catch of the trawl fishery, reaching prices similar to those of target species (Vafidis et al. 2005).
The evolutionary history of Plesionika is complex and, despite revisions (Chan & Yu 1991, 1998), its phylogeny, phylogeography and ontogeny remain ambiguous, especially for the species found in the Atlantic Ocean and Mediterranean Sea. Recently, the analysis of cytochrome oxidase I (COI) barcode diversity among 68 families of decapods (Matzen da Silva et al. 2011) revealed high molecular diversity within Plesionika and non-monophyly among species. There has been no previous attempt to resolve evolutionary relationships among any Plesionika species using molecular systematic approaches. Therefore, here we have investigated the molecular systematic relationships of the genus Plesionika in relation to five sister genera from within the Pandadidae.
Systematic and phylogenetic studies of decapods have recently focused on mitochondrial gene sequences; slowly evolving sequences of the ribosomal subunit 16S rRNA gene (16S) have been extremely informative in reconstructing deep relationships (Schubart et al. 2000), whereas the popular DNA barcoding mtDNA marker, COI, is generally used to study shallow to moderately deep relationships among crustaceans (Groeneveld et al. 2007; Mathews & Anker 2009; Schubart et al. 2005; Shih et al. 2007; von Rintelen et al. 2007). To increase the robustness of the phylogenetic analysis of Plesionika we combined partial fragments of the 16S and COI data with the few available molecular data in GenBank among six genera (Plesionika, Heterocarpus, Chlorotocus, Stylopandalus, Pandalus and Pandalopsis) of the family Pandalidae (Table 1), plus an outgroup Macropodia (Brachyura: Inachidae). There are eight species of the genus Plesionika in European waters, from the Mediterranean and Northeast Atlantic waters, which can be easily distinguished based on rostrum length and number of teeth, pleon features, and traits of the second pereiopod (e.g. Zariquiey-Alvarez 1968; Lagardère 1971). We analysed six of the eight reported European Plesionika species: Plesionika acanthonotus (S. I. Smith, 1882), Plesionika antigai Zariquiey (Alvarez, 1955), Plesionika edwardsii (Brandt, 1851), Plesionika heterocarpus (A. Costa, 1871), Plesionika martia (A. Milne-Edwards, 1883) and Plesionika narval (J. C. Fabricius, 1787). This study provides the first DNA sequence data that can be used to accurately identify Plesionika species from the North Atlantic Ocean and Mediterranean Sea. Moreover, as there has been no previous attempts using molecular tools to resolve evolutionary relationships among these species, we analysed whether phylogenetic groupings based on mitochondrial COI and 16S genes, support or refute previously proposed morphological divisions.
| Taxon | Region | GenBank nos. | |
|---|---|---|---|
| COI | 16S | ||
| Chlorotocus crassicornis Costa, A., 1871 | Mediterranean Sea (Italy) | JN412722 a | JN412679 b |
| Dichelopandalus bonnieri Caullery, 1896 | Northeast Atlantic (Scotland) | JN412723a | – |
| Pandalopsis dispar Rathbun, 1902 | Northwest Pacific (Canada) | DQ882107 | – |
| Pandalus borealis Krøyer, 1838 | Northeast Atlantic (Sweden) | FJ403244 | FJ403244 |
| Pandalus danae Stimpson, 1857 | Northeast Pacific (Canada) | DQ882112 | – |
| Pandalus goniurus Stimpson, 1860 | Northeast Pacific (Canada) | DQ882113 | – |
| Pandalus hypsinotus Brandt, 1851 | Northeast Pacific (Canada) | DQ882116 | – |
| Pandalus jordani Rathbun, 1902 | Northeast Pacific (Canada) | DQ882118 | – |
| Pandalus montagui Leach, 1814 in Leach, 1813–1814 | Northwest Atlantic (Canada) | FJ581851 | EU868698 |
| Pandalus platyceros Brandt, 1851 | Northeast Pacific (Canada) | DQ882125 | – |
| Pandalus stenolepsis Rathbun, 1902 | Northeast Pacific (Canada) | DQ882128 | – |
| Heterocarpus amacula Crosnier, 1988 | Southeast Pacific (French Polynesian) | AY612856 | AY612870 |
| Heterocarpus calmani Crosnier, 1988 | Indian Ocean (Madagascar) | AY612857 | AY612871 |
| Heterocarpus ensifer A. Milne-Edwards, 1881 | Northwest Pacific (Palau) | AY612859 | AY612873 |
| Heterocarpus gibbosus Bate, 1888 | Northwest Pacific (Taiwan) | AY612861 | AY612876 |
| Heterocarpus hayashii Crosnier, 1988 | Northwest Pacific (Taiwan) | AY612863 | AY612877 |
| Heterocarpus intermedius Crosnier, 1999 | Southwest Pacific (Fiji) | AY612864 | AY612878 |
| Heterocarpus laevigatus Bate, 1888 | Northwest Pacific (Taiwan) | AY612865 | AY612879 |
| Heterocarpus parvispina de Man, 1917 | Northwest Pacific (Taiwan) | AY612866 | AY612880 |
| Heterocarpus sibogae de Man, 1917 | Northwest Pacific (Taiwan) | AY612867 | AY612881 |
| Heterocarpus woodmasoni Alcock, 1901 | Southwest Pacific (Solomon Island) | AY612868 | AY612882 |
| Plesionika antigai Zariquiey Alvarez, 1955 | Northeast Atlantic (Portugal) | JN412724 a | JN412682 b |
| Plesionika acanthonotus (Smith, 1882) | Northeast Atlantic (Portugal) | JN412725a | JN412680 b |
| Plesionika edwardsii (Brandt, 1851) | Mediterranean Sea (Italy) | JN412726a | JN412683 b |
| Plesionika heterocarpus (Costa, A., 1871) | Northeast Atlantic (Portugal) | JN412727 a | JN412685 b |
| Plesionika martia (A. Milne-Edwards, 1883) | Northeast Atlantic (Portugal) | JN412728 a | JN412685 b |
| Plesionika narval (Fabricius, J.C., 1787) | Northeast Atlantic (Portugal) | JN412729 a | JN412689 b |
| Plesionika scopifera Chan, 2004 | Could not be determined | HQ241552 | HQ241519 |
| Stylopandalus richardii Richard, 1905 | Northeast Atlantic (Portugal) | JN412730 a | – |
| Macropodia longipes*A. Milne-Edwards & Bouvier, 1899) | Northeast Atlantic (Portugal) | JN107573 a | JN412692 b |
- *Outgroup taxa.
- a Matzen da Silva et al. (2011).
- b This study.
- c COI, cytochrome oxidase I.
Material and Methods
Sample collection
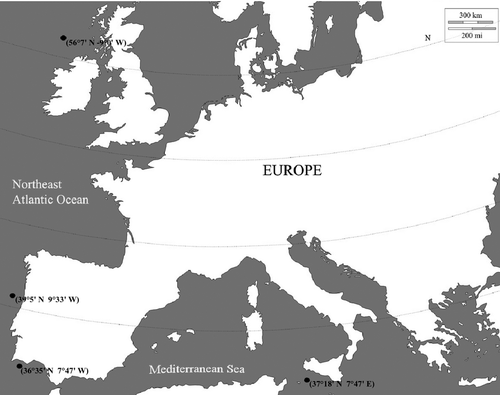
Decapods were collected from the Portuguese west coast, Mediterranean Sea and east coast of Scotland between 2006 and 2008 (Fig. 1). From the Portuguese coast, specimens were harvested as by-catch from rough ground bottom trawls by National Institute of Biological Resources of Portugal (INRB-IPIMAR) research vessels. Specimens were also collected opportunistically from survey work associated with fisheries from Sicily and from Aberdeen Fisheries Research Services from Scotland. Decapods were stored in a freezer at −20 °C and later identified. Morphological identification was assigned to the lowest possible taxonomic category, which, in the majority of cases, was to species level, using Zariquiey-Alvarez (1968), Lagardère (1971) and Crosnier & Forest (1973). A sample of muscle was collected after taxonomic identification and preserved in 95% ethanol and stored at –20 °C in the Natural and History Museum of Portugal (voucher numbers MB89000100 (C. crassicornis); MB89000110 (Dichelopandalus bonnieri); MB89000439 (Plesionika antigai); MB89000745 (Plesionika acanthonotus); Dichelopandalus (Plesionika edwardsii); MB89000291 (Plesionika heterocarpus); MB89000766 (Plesionika narval); MB89000383 (Stylopandalus richardii); MB89000325 (Macropodia Longipes).
DNA isolation, amplification and sequencing
Total genomic DNA was extracted from small amounts of tissue (1 mm3 muscle tissue) using the Chelex dry release method (Hajibabaei et al. 2005). A 10:1 mixture of Chelex buffer with Proteinase K (Sigma®, Haverhill, United Kingdom) was prepared and 110 μl of the mixture was placed in each 96-well plate. A small sample of tissue from each specimen was put into the extraction mixture. Extraction plates were then incubated at 55 °C for 12 h and subsequently heated to 95 °C for 20 min. Extraction plates were centrifuged at 126.4 g (∼1000 rpm in Fisher Centrific, radius 113) for 5 min immediately before setting up the polymerase chain reaction (PCR). The COI gene was amplified with alternative sets of primers depending on PCR reaction success following the protocol by Costa et al. (2007). Forward primer sequences were LCOI490 (5′-GGT CAA CAA ATC ATA AAG ATA TTG G-3′) (Folmer et al. 1994), CrustF1 (5′-TTT TCT ACA AAT CAT AAA GAC ATT GG-3′) (Costa et al. 2007), and the reverse primer HCO2198 (5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′) (Folmer et al. 1994). All PCRs were performed in a 25-μl volume containing 1 × PCR buffer, 3 mm MgCl2, 0.1–0.2 mm dNTP, 1 U TAQ polymerase, 5–10 pmol of each primer, and 2–10 ng of DNA template. The thermal cycling conditions consisted of 94 °C for 60 s; 35–40 cycles of 94 °C for 30 s, 48–56 °C for 90 s, and 72 °C for 60 s; followed by a final extension of 72 °C for 5 min. Alternative thermal cycling conditions consisted of 94 °C for 60 s; five cycles of 94 °C for 30 s, 45 °C for 90 s, and 72 °C for 60 s; 35 cycles of 94 °C for 30 s, 50–56 °C for 90 s, and 72 °C for 60 s, followed by a final extension of 72 °C for 5 min. Amplification of 16S was performed in a 25-μl volume containing 1 × PCR buffer, 1.5–3 mm MgCl2, 0.05–0.1 mm dNTP, 1 U TAQ polymerase, 5–10 pmol of each primer, and 2–10 ng of DNA template. Forward primer was 16SL2 (5′-TGC CTG TTT ATC AAA AAC AT-3′) (Mathews et al. 2002) and the reverse 16S 1472 (5′-AGA TAG AAA CCA ACC TGG-3′) (Schubart et al. 2000). The thermal cycling conditions consisted of 94 °C for 10 min; 35 cycles of 98 °C for 60 s, 48 °C for 120 s, and 72 °C for 120 s, followed by a final extension of 72 °C for 2 min. Following amplification, PCR products were cleaned by incubation with 10 U Exonuclease I (New England Biolabs®, Hitchin, United Kingdom) and 1 U Shrimp Alkaline Phosphate (Promega®, Fitchburg, United Kingdom) at 37 °C for 1 h, followed by heating at 80 °C for 5 min. Samples were sequenced by Macrogen Inc. (Macrogen, Seoul, South Korea) using an Applied Biosystems® 3730 sequencer (Applied Biosystems, Seoul, South Korea).
Sequence analyses
Cytochrome oxidase I and 16S sequences were manually checked for ambiguous base calls and assembled in CODONCODE ALIGNER version 1.3.0 (CodonCode Corporation, Dedham, Massachusetts, United States), and aligned using CLUSTALX in MEGA 4 (Tamura et al. 2007). The amino acid translation of COI was examined to ensure that no gaps or stop codons were present. BLAST searches were performed for all sequences using GenBank's online nucleotide database using the megablast algorithm (Zhang et al. 2000). Nucleotide composition and substitution frequencies were calculated in PAUP v3.1 (Swofford 1993). Pairwise distances were calculated in PAUP v3.1 with the best-fit model of nucleotide substitution, GTR + G + I and TrN + G for COI and 16S, respectively (Table 2), indicated by the Akaike information criterion (AIC) (Akaike 1973) using JMODELTEST (Posada 2008). To evaluate the range of intrageneric sequence identity found among Plesionika species, we compared pairwise distances of COI and 16S, incorporating Kimura 2-parameter (K2P) distances to facilitate comparisons with existing publications. The amount of phylogenetic signal versus noise in the concatenated alignment (ingroup only) was assessed with DAMBE (Xia & Xie 2001). The program DAMBE was used to examine saturation of the mitochondrial COI and 16S sequences and to calculate transition (ts) versus transversion (tv) nucleotide substitutions. The Iss values provide a measure of substitution saturation in molecular phylogenetic datasets, and were calculated for the whole dataset and for each of the codon position separately. Iss is derived from the amount of entropy in the data and needs to be compared to critical values, below which simulation studies showed decreased accuracy (Xia et al. 2003). The DAMBE software was used to calculate Iss values and compare them against critical Iss.c values for symmetric and asymmetric topologies (Xia et al. 2003) for both datasets, respectively.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Macropodia longipes | 0.214 | 0.247 | 0.226 | 0.239 | 0.226 | 0.231 | 0.221 | 0.205 | 0.231 | 0.234 | 0.213 | 0.197 | 0.231 | 0.224 | 0.261 | 0.255 | 0.229 | 0.240 | 0.306 | 0.244 | |
| 2 Chlorotocus crassicornis | 0.242 | 0.028 | 0.044 | 0.030 | 0.030 | 0.029 | 0.047 | 0.033 | 0.029 | 0.029 | 0.040 | 0.057 | 0.063 | 0.044 | 0.099 | 0.053 | 0.099 | 0.061 | 0.061 | 0.109 | |
| 3 Heterocarpus amacula | 0.111 | 0.089 | 0.028 | 0.008 | 0.020 | 0.008 | 0.031 | 0.017 | 0.008 | 0.008 | 0.028 | 0.043 | 0.042 | 0.038 | 0.082 | 0.046 | 0.077 | 0.035 | 0.039 | 0.085 | |
| 4 Heterocarpus calmani | 0.177 | 0.125 | 0.080 | 0.023 | 0.022 | 0.024 | 0.003 | 0.025 | 0.024 | 0.023 | 0.002 | 0.058 | 0.064 | 0.042 | 0.106 | 0.054 | 0.102 | 0.048 | 0.058 | 0.099 | |
| 5 Heterocarpus ensifer | 0.111 | 0.087 | 0.002 | 0.080 | 0.016 | 0.000 | 0.025 | 0.015 | 0.000 | 0.000 | 0.025 | 0.044 | 0.041 | 0.035 | 0.097 | 0.042 | 0.097 | 0.039 | 0.043 | 0.092 | |
| 6 Heterocarpus gibbosus | 0 199 | 0.142 | 0.080 | 0.060 | 0.082 | 0.017 | 0.025 | 0.015 | 0.017 | 0.016 | 0.023 | 0.046 | 0.048 | 0.039 | 0.098 | 0.052 | 0.094 | 0.038 | 0.053 | 0.099 | |
| 7 Heterocarpus hayashii | 0.111 | 0.088 | 0.000 | 0.079 | 0.002 | 0.080 | 0.026 | 0.016 | 0.000 | 0.000 | 0.026 | 0.043 | 0.041 | 0.035 | 0.095 | 0.041 | 0.095 | 0.039 | 0.042 | 0.088 | |
| 8 Heterocarpus intermedius | 0.202 | 0.133 | 0.104 | 0.013 | 0.102 | 0.070 | 0.104 | 0.027 | 0.026 | 0.025 | 0.004 | 0.057 | 0.063 | 0.037 | 0.115 | 0.052 | 0.111 | 0.044 | 0.054 | 0.106 | |
| 9 Heterocarpus laevigatus | 0.149 | 0.108 | 0.086 | 0.065 | 0.086 | 0.061 | 0.085 | 0.075 | 0.016 | 0.015 | 0.026 | 0.056 | 0.053 | 0.033 | 0.092 | 0.045 | 0.088 | 0.033 | 0.044 | 0.091 | |
| 10 Heterocarpusparvispina | 0.110 | 0.087 | 0.003 | 0.076 | 0.003 | 0.079 | 0.003 | 0.104 | 0.082 | 0.000 | 0.026 | 0.043 | 0.041 | 0.035 | 0.095 | 0.041 | 0.095 | 0.039 | 0.042 | 0.088 | |
| 11 Heterocarpus sibogae | 0.109 | 0.088 | 0.001 | 0.076 | 0.002 | 0.081 | 0.001 | 0.102 | 0.083 | 0.002 | 0.025 | 0.044 | 0.042 | 0.036 | 0.094 | 0.043 | 0.094 | 0.039 | 0.043 | 0.089 | |
| 12 Heterocarpus woodmasoni | 0.235 | 0.127 | 0.109 | 0.011 | 0.109 | 0.066 | 0.108 | 0.018 | 0.062 | 0.106 | 0.107 | 0.058 | 0.067 | 0.041 | 0.102 | 0.052 | 0.098 | 0.050 | 0.059 | 0.098 | |
| 13 Pandalus borealis | 0.147 | 0.161 | 0.085 | 0.120 | 0.086 | 0.123 | 0.084 | 0.131 | 0.152 | 0.084 | 0.082 | 0.1 42 | 0.005 | 0.039 | 0.097 | 0.064 | 0.095 | 0.056 | 0.068 | 0.088 | |
| 14 Pandalus montagui | 0.154 | 0.153 | 0.106 | 0.137 | 0.110 | 0.150 | 0.105 | 0.151 | 0.140 | 0.106 | 0.103 | 0.1 29 | 0.022 | 0.041 | 0.108 | 0.063 | 0.109 | 0.051 | 0.066 | 0.103 | |
| 15 Plesionika acanthonotus | 0.166 | 0.135 | 0.082 | 0.110 | 0.083 | 0.108 | 0.083 | 0.124 | 0.119 | 0.083 | 0.082 | 0.1 53 | 0.145 | 0.183 | 0.091 | 0.031 | 0.091 | 0.027 | 0.046 | 0.089 | |
| 16 Plesionika antigai | 0.140 | 0.160 | 0.129 | 0.111 | 0.129 | 0.154 | 0.128 | 0.128 | 0.121 | 0.125 | 0.127 | 0.1 18 | 0.089 | 0.114 | 0.142 | 0.106 | 0.002 | 0.108 | 0.107 | 0.012 | |
| 17 Plesionika edwardsii | 0.163 | 0.168 | 0.121 | 0.122 | 0.119 | 0.088 | 0.120 | 0.129 | 0.115 | 0.120 | 0.119 | 0.1 40 | 0.137 | 0.165 | 0.060 | 0.137 | 0.098 | 0.046 | 0.065 | 0.112 | |
| 18 Plesionika heterocarpus | 0.125 | 0.143 | 0.108 | 0.115 | 0.104 | 0.138 | 0.107 | 0.129 | 0.124 | 0.109 | 0.104 | 0.1 21 | 0.115 | 0.127 | 0.139 | 0.025 | 0.162 | 0.099 | 0.101 | 0.011 | |
| 19 Plesionika martia | 0.180 | 0.178 | 0.176 | 0.100 | 0.173 | 0.114 | 0.175 | 0.102 | 0.083 | 0.177 | 0.174 | 0.096 | 0.103 | 0.120 | 0.141 | 0.130 | 0.130 | 0.140 | 0.050 | 0.105 | |
| 20 Plesionika narval | 0.206 | 0.167 | 0.099 | 0.108 | 0.100 | 0.139 | 0.099 | 0.115 | 0.124 | 0.102 | 0.098 | 0.1 26 | 0.133 | 0.148 | 0.146 | 0.126 | 0.124 | 0.081 | 0.132 | 0.095 | |
| 21 Plesionika scopifera | 0.138 | 0.150 | 0.107 | 0.079 | 0.110 | 0.120 | 0.107 | 0.104 | 0.107 | 0.101 | 0.103 | 0.093 | 0.117 | 0.112 | 0.114 | 0.042 | 0.129 | 0.028 | 0.121 | 0.108 |
COI phylogenetic analyses
To provide a comprehensive sister-species coverage and survey of between species variation of pandalid shrimps, sequences for target species from GenBank were merged with our data (in Table 1). To reconstruct phylogenetic relationships within each genus, the corresponding mitochondrial sequence data were analysed for 30 specimens using maximum likelihood (ML) and Bayesian inference (BI) methods. Due to the absence of information about the phylogenetic relationship between pandalid genera and molecular data, we chose a decapod crab specimen, Macropodia longipes (A. Milne-Edwards & Bouvier, 1899) (Brachyura: Inachidae) to serve as a phylogenetically distinct outgroup and JMODELTEST (Posada 2008) identified the model TVM + I + G (Posada 2003) as best indicated by AIC. However, as not all potential models are implemented in the current version of RaxML, the GTR + I + G model was selected as the closest matching alternative. Ten independent ML analyses were conducted using GTR + I + G with invariant sites (I) and gamma distributed rates (G) (Yang 1994) as the model (using the GTR + CAT setting) with four categories of rate variation (500 bootstrap replicates were undertaken for estimation of node support) of COI data. To find the ML tree, 10 independent runs of RAXML 7.0.4 (Stamatakis 2008) were conducted.
As variation among substitution rates among the four nucleotides and among different nucleotide sites in mitochondrial protein-coding genes has been reported (Kumar 1996; Yang & Yoder 1999; Yang et al. 2000), a COI phylogenetic tree was constructed with MRBAYES 3 (Huelsenbech & Ronquist 2001), with the best-fit model of each codon position indicated by the likelihood ratio test of JMODELTEST (Posada 2008): the models TIM3ef + I (Posada 2003), F81 + I (Felsenstein 1981), and TIM3 + G (Posada & Buckley 2004) were used for the 1st, 2nd and 3rd codon position of COI, respectively (see species analysed in Table 1).
Combined COI and 16S data phylogenetic analysis
A combined analysis was conducted with concatenated sequences from the two genes with 21 specimens (Table 1). The best indicated models by AIC (Akaike 1973) were TIM3ef + I + G (Posada 2003), TIM 3 + G (Posada 2003) and TIM 1 + G (Posada 2003) for the 1st, 2nd and 3rd codon position of COI, respectively.
The TrN + G (Tamura & Nei 1993) model was used for the 16S sequences. ML analysis was conducted using the closest matching GTR + I + G model for each partition data, as the AIC selected models are not implemented in the current version of RaxML. In the BI analyses, the two gene regions were partitioned according to the previously determined model parameters. Minor gaps in 16S sequences were treated as fifth character-state in subsequent BI phylogenetic analyses. The BI analysis employed two independent chains, which were conducted for 5 × 106 generations under the substitution model for each gene. After discarding the first 1 million generations (20%) cycles as burn-in, we monitored the fluctuating value of the likelihood graphically with TRACER v1.4 (Drummond & Rambaut 2007) to check that the generations had been reached stationary values. The consensus tree was selected and visualized using FIGTREE V.1.0 (http://tree.bio.ed.ac.uk/software/figtree/).
Results
BLAST search
A BLAST search of the nucleotide and protein sequences revealed that four sequences from GenBank of Pandalidae family require special attention. The sequences of Plesionika ensis (AY612869; AY612883) show high similarity with Heterocarpus spp.; Pandalopsis coccinata (Urita, 1941) (AB290213) apparently does not belong to the Caridea suborder, and Pandalus eous (Makarov, 1935) (AB211294) is not even a Decapod. Thus, these sequences were not included in our analyses.
Diversity
Gene sequences of approximately 600 base pairs (bp) of COI and 500 bp of 16S were amplified successfully from all specimens and sequences of both genes were deposited in GenBank (Table 1). Following the addition of GenBank sequences, our resulting dataset comprised 540 bp of COI and 463 bp of 16S.
The COI alignment contained 239 variable characters, of which 220 were parsimony informative. There was also evidence for base composition bias in the sequences, notably a pronounced underrepresentation of guanine at the third codon positions (mean frequency of nucleotides of 30 Pandalidae species: 27.26% A; 22.08% C; 18.41% G; 32.24% T), a phenomenon commonly observed in metazoan mitochondrial sequences (Wolstenholme 1992). The pattern of nucleotide substitutions (excluding the outgroup) produced an average ts:tv ratio of 1.2, indicating a clear transition over transversion bias. The number of variable sites was much greater at the third (179/180) than at the first (53/180) and second (7/180) positions. Previously, it has been shown that when third codon positions are analysed separately, saturation can prevent reliable phylogenetic inference (Tong et al. 2000). Results from the substitution saturation analysis showed an Iss < Iss.c (P < 0.001, in all three datasets), indicating that the sequences are highly useful in phylogenetic reconstruction (Xia et al. 2003).
The 16S alignment showed that the pandalid sequences were highly conserved for the group of 21 species of this family in some regions with 199/463 variable characters, of which 157/463 were parsimony informative. The 16S sequences are AT-rich (0.67), indicating a moderate compositional bias. The pattern of nucleotide substitution was also biased in favour of transitions over transversions, yielding an average ts:tv ratio of 1.2 among all pairwise comparisons.
The mean divergence distance (ML distance with substitution model GTR + I + G) within 30 species was 0.1164. The Plesionika genus (represented here by eight species) diverges substantially from the other genera and showed the highest average nucleotide divergence of 0.1123 (range 0.0247–0.1616) and 0.0846 (range 0.0016–0.1116) within COI and 16S among Pandalidae genera. For example, Heterocarpus, the most representative genus in our dataset (with 11 species), exhibited a nucleotide divergence of 0.0620 (range 0.002–0.1023) and 0.0167 (range 0.000–0.0308), respectively. On the other hand, sequence divergence was found to vary greatly within Plesionika (COI/16S): 0.00247/0.0016 between P. antigai and P. heterocarpus and 0.1616/0.0983 between P. heterocarpus and P. edwardsii (Table 2). In addition, using the genetic code for Drosophila mtDNA, the lowest and the highest distance in the amino acid sequence divergence (Poisson correction) observed was between P. heterocarpus and P. antigai (0.000) and between P. heterocarpus and P. edwardsii (0.0810), respectively. No amino acid divergence was observed between Heterocarpus amacula (Crosnier, 1988), Heterocarpus hayashii (Crosnier, 1988), Heterocarpus sibogae (De Man, 1917), Heterocarpus parvispina (De Man, 1917), and Heterocarpus ensifer (A. Milne-Edwards, 1881) species.
Phylogeny
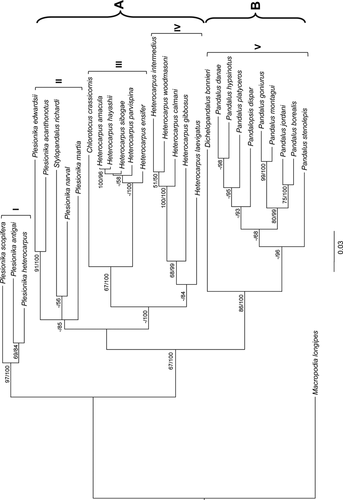
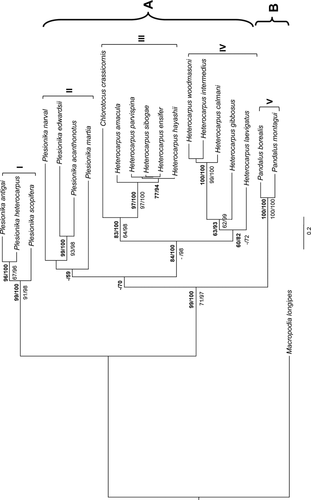
Phylogenetic analyses using ML and BI analysis based on 21 species of the COI dataset found the same grouping patterns in similar tree topologies as the combined data set (Figs 2 and 3). The 16S data were unable to resolve relationships among the Plesionika species with any significant support. Combining data increased separation between Plesionika antigai, Plesionika heterocarpus and Plesionika scopifera from the rest of the Plesionika species, which are separated into two distinct clades. This also improved bootstrap and probability support values between the two distinct clades of the Heterocarpus genus (Fig. 3). Both BI trees (see Figs 2 and 3) support relationships between two groups: group A, which possibly represents species preferring subtropical and temperate zones, and group B, inhabiting temperate and frigid zones. However, we cannot exclude the possibility of a taxonomic against ecological split, as group B comprise only Pandalus in both cases. When amino acid divergence was compared within the COI dataset, group A showed a mean amino acid divergence of 3.5 versus 0.9% for group B. The number of variable and parsimony informative sites was much greater for the sequences in group A (27/180; 13/180) than those in group B (15/180; 3/180). Plesionika antigai and P. heterocarpus showed an absence of amino acid divergence in contrast with the remaining Plesionika species with an average of amino acid divergence of 3.40%. The amino acid distance between the two Plesionika groups was 7.20%. The paraphyly of the genus Plesionika was supported by high bootstrap support and Bayesian posterior probabilities (ML/BI), notably 99% in ML and 100% in BI analyses (Fig. 3). The two distinct Heterocarpus groups are represented by clades III and IV. Clade III showed an absence of amino acid divergence, in contrast to samples from the clade IV, which had an average amino acid K2P distance of 2.00%.
Discussion
Species diversity
Plesionika is distributed globally, consisting mainly of morphologically similar deep-water shrimps (Ohtomi 1997; Vafidis et al. 2005). However, the rate of evolution of morphological features is known to differ from the rate of molecular evolution (Tong et al. 2000). Genetic divergence of COI (K2P distance) within Plesionika species (mean 25.08%) was slightly higher than the mean congeneric divergences in two other pandalid shrimps, Heterocarpus (15.79%) and Pandalus (15.80%), one alvinocaridid shrimp Alvonicaris (16.60%) (Shank et al. 1999), two hyppolitid shrimps Spirontocaris (15.40%) and Eualus (15.20%), one crangonid shrimp Crangon (18.91%) (Costa et al. 2007), and three palaemonid shrimps Macrobrachium (20.30%), Palaemon (18.11%) and Periclimenes (20.19%) (Matzen da Silva et al. 2011). Thus, it may be suggested from the present study that the relationship between molecular and morphological evolution exhibits high variance in Plesionika shrimps. Similar features have also been reported in other crustaceans, e.g. Amphipoda (Macdonald et al. 2005) and Copepoda (Rocha-Olivares et al. 2001) and the high variability observed within genera can raise two major questions: (i) whether molecules and morphology yield strongly supported, conflicting hypotheses of relationships or (ii) are the observations attributable to undersampling of phylogenetic characters in one or both types of data (morphological versus molecular).
The mean genetic divergence of 16S (K2P distance) within Plesionika species was 22.20%. Similar studies for penaeid shrimp species have reported an average (K2P) of 6.4% and 11% nucleotide divergence in partial 16S sequences among seven Metapenaeopsis and 13 species of Penaeus genus, respectively (Garcia-Machado et al. 1993; Tong et al. 2000).
The present data show 89.50% and 95.66% similarity at the amino acid sequences of COI among Plesionika spp. and Heterocarpus spp., respectively. Very similar levels of similarity were observed among Metapenaeopsis of 98% (Tong et al. 2000). Despite considerable conservation at amino acid sequences, large differences at silent sites of COI were observed in both Plesionika (61.4%) and Heterocarpus (44.2%) genera.
The range of COI and 16S divergence was found to vary greatly within Plesionika (Table 2). The closest observed distance between Plesionika antigai and Plesionika heterocarpus supports the morphological similarity of these two species (Zariquiey-Alvarez 1968, Lagardère 1971, Crosnier & Forest 1973). Studies with different molecular markers in decapods revealed considerable allozyme differentiation among morphologically similar species (Mulley & Latter 1980; Tam & Chu 1993; Stewart et al. 2003; Zaslavskaya et al. 2007), suggesting that there is no conflict between molecular and morphological phylogenetic hypotheses among Plesionika species.
Molecular phylogeny
Results from the substitution saturation analysis of our COI and 16S dataset showed that sequences are highly informative for phylogenetic reconstruction (Xia et al. 2003). Comparing both markers, COI is shown to be more useful in phylogenetic reconstruction of Plesionika compared with 16S. A sensible approach for tackling this problem was to use an appropriate nucleotide substitution model of evolution that incorporates multiple mutations at the same site for each gene, to correct the observed distance for the multiple hits. The COI and the combined mitochondrial dataset phylogenetic analysis identified several well-supported groupings within the Pandalidae species.
Molecular systematics of Plesionika and ecological/life history implications
Cytochrome oxidase I sequence divergence between species in clades I and II (13.07%; Fig. 3) and within species (3.16% and 11.24%, respectively) indicates that although the Plesionika genus is genetically distinct, it is not monophyletic as currently defined, highlighting some discrepancies between the current taxonomy and systematics.
Recently, Komai et al. (2010) reported a pattern of molecular variation between two bathyal hydrothermal Palaemonidae shrimps, Periclimenes, Periclimenes thermohydrophilus and Periclimenes cannaphilus (interspecific variability 11.3–19.9%), reflecting their isolated geographical distribution without partial overlap, which suggest a parapatric speciation event. The two Periclimenes species appear restricted to the tube worm habitat at rather shallow hydrothermal vents (shallower than 500 m), and therefore larval dispersal and opportunity for settlement can be rather limited (Komai et al. 2010). The observed phylogenetic patterns of Plesionika do not reflect isolated geographical distributions of the two clades. It was recently reported that members of the Plesionika genus have a preference for divided bathymetric space depending on the season, sex and life history of the species (Puig et al. 2001; Carbonell et al. 2003). Plesionika heterocarpus and Plesionika antigai co-habit comparatively shallower depths (clade I), being found in different proportions between 380 and 500 m in the Mediterranean Sea (Campisi et al. 1998), whereas Plesionika acanthonotus occupies the deepest bathymetric distribution (clade II) (Puig et al. 2001; Carbonell et al. 2003). Here, the shallow water species P. antigai, P. heterocarpus and Plesionika scopifera are paraphyletic to the deeper water species (depth >400 m) found in clade II. Similar observations have been reported recently for COI data from the annelid genus Osedax study (Vrijenhoek et al. 2009), and for 16S data derived from deep-sea amphipods Eurythenes gryllus, indicating monophyly among sites within the same depth zone at the scale of ocean basins (France & Kocher 1996). Similarly, using COI and 16S data of penaeid Metapenaeopsis spp., it was found by Tong et al. (2000) that species with deeper water preferences clustered together in phylogenetic analyses.
Plesionika are benthic species of moderate locomotory ability. To date, there have been no descriptions of adults showing diel water column migrational behaviour (Cartes 1993). Because so many benthic marine animals move little in adulthood, movements by larvae have been expected to be responsible for most dispersal and gene flow between populations and can reveal historical and contemporary patterns of connectivity (Hellberg 2009), including the identification of phylogeographic breaks. Such putative boundaries are usually assumed to be the result of long-term barriers to gene flow. However, it was shown recently that phylogeographic breaks can be related to the decrease in average dispersal distance and population size in the absence of barriers to gene flow (Irwin 2002). To date, only five Plesionika species (P. heterocarpus, Plesionika edwardsii, Plesionika gigliolii, Plesionika martia and P. acanthonotus), from 92 extant species, have yielded insights into the relationship between population structure of adults and juveniles in relation to life history and oceanography (Puig et al. 2001). Related taxa are often located within the same broad geographical or habitat types. The depth of maximum abundance of P. heterocarpus, P. edwardsii and P. martia juveniles exhibited a strong bathymetric preference (100, 400 and 600 m depth, respectively), in contrast to P. acanthonotus, which showed an absence of any spatial trend for juveniles (700–1000 m). Puig et al. (2001) suggested a trophic linkage between availability of food and the population structure of the Plesionika spp. (larvae, juvenile and adult stage). The original paradigm of high connectivity in marine taxa of infinite population size is increasingly being contested: numerous studies (reviewed in Hauser and Carvalho, 2008) show more restricted dispersal and gene flow, as well as high levels of self-recruitment (Sponaugle et al., 2002). Also, species with life history and oceanographical similarities are clustered together here. Data on Plesionika indicate an increasing seasonality in reproductive periods from the shallowest species P. heterocarpus and P. antigai (clade I), with ovigerous females present throughout the year (Campisi et al. 1998), to the deepest species (clade II) P. acanthonotus, with ovigerous females present only in late spring and early summer months (Company & Sardà 1998). To date, morphological descriptions of larvae are available only for the first larvae stage (zoea) of P. acanthonotus species (Bourdillon-Casanova, 1960), all larvae stages of P. narval (Lebour, 1940; Landeira et al., 2009a), and the first seven larval stages (zoea) of P. edwardsii (Landeira et al., 2009b). No information is available on the distribution and trophic behaviour of these stages. Variation in larval developmental modes, and the expected variation in gene flow between populations that arise from the dispersal potential of different developmental modes, has important consequences over longer ecological and evolutionary timescales (Hart & Marki, 2010). Accordingly, there is still research to be done on the description of larvae before we can assign precise identification to extant cohorts in space and time in the water column. These observations suggest that ecological and physical conditions are important isolating mechanisms that may lead to speciation in Plesionika shrimps, but the addition of comparative sequence data from other shallow-dwelling taxa may confirm or refute this hypothesis in a more robust manner. Otherwise, no clear evolutionary pattern of depth utilization is apparent among the major Plesionika clades. However, the unique phylogenetic distance observed in our data does provide an extraordinary opportunity to study population evolution and larval distribution of Plesionika spp. and its phylogenetic relationships with other genera within the family Pandalidae.
Molecular systematics of selected Pandalidae species and ecological implications
The assignment of all deep-sea Pandalidae species in the same clade V was well supported (Figs 2 and 3, respectively). This clade was further split into two main groups that we further recognized as deep-sea species with a preference for subtropical and temperate waters (A) or temperate and frigid waters (B). Group B showed high support values for both ML and BI, supporting the idea that the genus Dichelopandalus is closely related to the Pandalus genus (Chan & Yu 1991; Komai 1999). Dichelopandalus bonnieri (Caullery, 1896) has the most southern limit distribution of the temperate and frigid water species. Whereas Pandalus species have their southern limit in the English Channel, D. bonnieri seems to have its limit of southern distribution on the Northwest Portuguese coast based on reported larval occurrence (Calado & Narciso 2002; Santos et al. 2008). The pandalid shrimp genus Pandalopsis (Fig. 2) is readily distinguished from other pandalid genera by its broad ischial laminar expansion of the first pereiopod; however, a comprehensive revisionary study of the genus has not been published (Komai 1994). Moreover, both ML and BI analyses uncovered identical relationships regarding the molecular systematic biogeography, life history and ecological implications of selected species of pandalid shrimps.
Conclusions
The present study confirms the utility of COI over 16S as genetic markers to further resolve relationships among species of Plesionika from the Northeast Atlantic and Mediterranean Sea. Large genetic distances between morphologically similar taxa may either be due to elevated mutation rates or may reflect divergent lineages. We suggest that the latter could be true for Plesionika and that the morphological similarity between species is masking a long history of evolution maintained by stabilizing selection, which has led to phenotypic stasis. Among the Pandalidae, Plesionika is one of the most speciose genera, and it would appear warranted to obtain further systematic clarification within this group, based on future and robust phylogenetic frameworks. Given the high degree of morphological convergence among allopatric species of Plesionika in the Atlantic and Pacific Oceans and the Mediterranean Sea, we believe that molecular data (with more extensive sequencing of various genetic loci) would enhance our understanding of phylogenetic relationships, compared with using morphology alone. Our findings underscore the need for comprehensive studies of the molecular systematic nature of paraphyly within Plesionika and Stylopandalus throughout the Northeast Atlantic and the Mediterranean Sea, in an attempt to recognize a concordance in the evolutionary history of Plesionika with major ecological and geological events.
Acknowledgements
The ‘Fundação para a Ciência e Tecnologia’ (Portugal) provided a doctoral fellowship (SFRH/BD/25568/2006) to Joana Matzen da Silva. This research was partially supported by LusoMarBol FCT research grant PTDC/MAR/69892/2006. Thanks are due to many friends, colleagues and respective institutions (Dr Sarah Helyar from Bangor University; Jim Drewery from Aberdeen Fisheries Research Services from Scotia; Dr Marco Arculeo from the University of Palermo; Cristina Silva and all the technicians and crew members of the R/V Noruega for the samples taken on the Crustacean cruises (project PNAB-NP/EU-DCF); Dr Markos Alexandrou, and Axel Barlow from Bangor University; Dr Ana Elisabete Pires and Dr José António Matos from INRB (Instituto Nacional de Recursos Biológicos) who collaborated throughout this study by making available essential specimens and literature, and sharing interesting scientific discussions. We thank Dr Judite Alves and Alexandra Cartaxana for archiving and being responsible for the Crustacean collection in the Natural and History Museum of Portugal.




