Vegetative reproduction by multicellular propagules in Rhodophyta: an overview
Abstract
This paper deals with vegetative reproduction by multicellular propagules in Rhodophyta. An extensive examination of the relevant literature shows that this phenomenon in Rhodophyta is not well known. A propagule is here defined as a vegetative multicellular structure which spontaneously detaches from the parent thallus and gives rise to a new individual. The origin and morphological features of multicellular propagules are examined in the various known propagule-forming species. The importance of multicellular propagules as both overwintering and resting organs and as a taxonomic feature is also shown. Their role in increasing local populations and/or for long-distance dispersal is discussed. The relative abundance of vegetative reproduction by multicellular propagules versus sexual reproduction, as well as the advantages of this additional mode of reproduction, are shown and discussed. The production of multicellular propagules may contribute to the capacity of species to increase populations, to weather unpredictable environmental changes, to survive in conditions that would be lethal for entire thalli, and to reach new habitats. As resting organs, they may also be responsible for long-distance dispersal and may account for the introduction of some alien species. Accordingly, propagule-forming species are probably more competitive than taxa that do not produce such propagules. These considerations should be taken into account in future studies of the biology, ecology and demography of Rhodophyta.
Introduction
Few investigations deal with the vegetative reproduction by multicellular propagules in macroalgae. Research studies to identify both the presence and the modalities of this form of reproduction are rare; moreover, neither the extent of the phenomenon nor the multiplicity of its forms are widely known by the scientific community and most of the data are contained in papers in which the presence of multicellular propagules was observed by chance, usually when new taxa were described. The present paper is part of a series of reviews of vegetative reproduction by multicellular propagules in macroalgae, including red, brown and green macroalgae. The aim of this work is to call researchers’ attention to this kind of reproduction to expose the possible gaps to be filled by future studies, especially considering that the presence of multicellular propagules in algal diaspore banks has been neglected and, consequently, their ecological role in the structure and functioning of macroalgal assemblages has not often been considered (Worm et al. 2001).
In the light of the fragmentary nature of the information in this field, the first part of this review describes the present state of knowledge of vegetative reproduction by means of multicellular propagules in red macroalgae (Rhodophyta). A meticulous search for papers reporting these propagules turned up several publications, showing that an interesting and relevant literature does indeed exist. For nomenclature purposes the taxonomic database AlgaeBase (Guiry & Guiry 2010) was used.
History
Previous reviews of vegetative reproduction have dealt mainly with fragmentation (Chemin 1928; Dixon 1965; Norton & Mathieson 1983). Vegetative fragments can become detached, continue to grow and reattach in a great number of Rhodophyta (Fritsch 1965; Dixon 1973), and spontaneous fragmentation and cutting are routine methods to reproduce macroalgae in aquaculture and laboratory studies (e.g.Simpson et al. 1979; Pérez et al. 1992; Yokoya et al. 2003).
Multicellular outgrowths in red algae have been described by naturalists for centuries. On some occasions, researchers correctly hypothesized that these structures might serve as propagules, as in the case of Acanthophora nayadiformis (Agardh 1863; as Acanthophora delilei J.V. Lamouroux), Chondria crassicaulis (Okamura 1903) and Polysiphonia furcellata (Bornet 1892). At other times, they misinterpreted their role as tetrasporangial branchlets, e.g. in Alsidium corallinum (Falkenberg 1901; as Alsidium lanciferum Kützing) and again P. furcellata (Harvey 1846). Feldmann & L’Hardy-Halos (1977) were the first to focus on the study of multicellular propagules. Santelices (1990) only mentioned vegetative reproduction by multicellular propagules in his exhaustive treatment of reproduction, dispersal and recruitment of macroalgae. In a comprehensive review of reproductive strategies of Rhodophyta, Hawkes (1990) reported only a few species reproducing in this way.
In the last few years, studies on the life histories of alien species have shown that several of them, e.g. Asparagopsis taxiformis (Mairh 1977), Heterosiphonia japonica (Husa & Sjøtun 2006) and Hypnea cornuta (Cecere et al. 2004), reproduce by multicellular propagules exclusively or in addition to other ways.
Definition of Multicellular Propagules
Before discussing the subject, it is necessary to explain what we mean by the word ‘propagule’. In current approaches, every vegetative, meiotic and sexual diaspore in both plants and animals (e.g. fragment, specialized vegetative structure, spore, gamete, zygote, germling and larva), which gives rise to a new individual is called a ‘propagule’ (Hoffmann 1987; Santelices 1990; Amsler et al. 1992; Clayton 1992; Norton 1992; Kinlan et al. 2005). Even perennial holdfasts were considered propagules by Womersley (1979). In contrast, in our definition, a multicellular propagule is a vegetative multicellular structure which detaches from the parent thallus and gives rise to a new individual.
Origin and Morphological Features of Multicellular Propagules
As far as we know, multicellular propagules as defined above, with various origins and morphologies, were reported from 43 species of Rhodophyta (Tables 1–3). They may derive from:
| species | morphology | plants bearing propagules | starch | role | character of taxonomic significance | additional kinds of reproductiona | references |
|---|---|---|---|---|---|---|---|
| Acanthophora nayadiformis (Delile) Papenfuss | Highly modified apical parts, cone-shaped, 1.5–2 mm long, basally constricted | Tetrasporophytes; gametophytes | Yes | Checked | Yes | Sexual; asexual (tetrasporangia, fragmentation) | Delile inAgardh (1823, 1863), Preda (1909), Cecere & Perrone (2002), Perrone et al. (2005), Cecere et al. (2007) |
| Alsidium corallinum C. Agardh | Modified, simple or branched, also tetrasporic, up to 6 mm long, stalked | Tetrasporophytes; gametophytes | Yes | Checked | Yes | Sexual; asexual (tetrasporangia, fragmentation) | Agardh (1863), Falkenberg (1901), Preda (1909), Cecere et al. (2002a), E. Cecere & A. Petrocelli, unpublished data |
| Anisoschizus propaguli Huisman & Kraft | Highly modified, ovoid, two-celled, 200 μm long, stalked | Tetrasporophytes; gametophytes | Yes | Checked in culture | Yes | Sexual; asexual (polysporangia) | Huisman & Kraft (1982), Huisman & Gordon-Mills (1994), Womersley (1998) |
| Asparagopsis armata Harvey | Basal harpoon-like branches | Gametophytes | No | Checked in culture | Yes | Sexual; asexual (tetrasporangia, fragmentation) | Codomier et al. (1977, 1978) |
| Asparagopsis taxiformis (Delile) Trevisan de Saint-Léon | Modified, tendrils | Gametophytes | No | Checked | No | Sexual; asexual (tetrasporangia, fragmentation) | Dixon (1965), Mairh (1977) |
| Centroceras sp. [as Centroceras clavulatum (C. Agardh) Montagneb] | Unmodified, 350 μm long, basally constricted, rhizoidsc | n.a. | No | Checked | No | Unknown | Lipkin (1977) |
| Chondria bulbosa Harvey (as Chondria suprabulbosa Gordon-Mills & Womersley) | Highly modified, ovoid, about 2 mm long, stalked | Tetrasporophytes | Yes | Supposed | Yes | Sexual, asexual (tetrasporangia) | Gordon-Mills & Womersley (1987), Womersley (2003) |
| Chondria capillaris (Hudson) M.J. Wynne | Tetrasporangial branches, 2.5 mm long, markedly basally constricted | Tetrasporophytes | n.a. | Supposed | No | Sexual, asexual (tetrasporangia) | Maggs & Hommersand (1993), E. Cecere & A. Petrocelli, personal observation |
| Deucalion levringii (Lindauer) Huisman et Kraft | Highly modified, ovoid, three-celled, 240 μm long, stalked | Tetrasporophytes | Yes | Checked in culture | Yes | Sexual (only in lab-cultures); asexual (polysporangia) | Huisman & Kraft (1982), Womersley (1998) |
| Griffithsia corallinoides (Linnaeus) Trevisan de Saint-Léon (as G. corallina C. Agardh) | Unmodified branches | n.a. | No | Checked in culture | No | Sexual; asexual (fragmentation) | Chemin (1928), Maggs & Hommersand (1993) |
| Guiryella repens Huisman & Kraft | Highly modified, ovoid, two-celled, up to 225 μm long, stalked | Tetrasporophytes; sterile plants | Yes | Checked in culture | Yes | Sexual, asexual (tetrasporangia) | Huisman & Kraft (1992), Womersley (1998) |
| Hypnea chordacea Kützing | Unmodified tetrasporangial branches, 0.6–2 mm long | Tetrasporophytes | No | Checked | No | Sexual, asexual (tetrasporangia) | Mshigeni (1976), Bangmei & Yongqiang (1997) |
| Hypnea cornuta (Kützing) J. Agardh | Highly modified, star-shaped, 3–6 rays, 250–900 μm long, basally constricted | Tetrasporophytes; female gametophytes;d sterile plants | Yes | Checked | Yes | Sexual;d asexual (tetrasporangia,d fragmentation) | Agardh (1852), Bangmei & Yongqiang (1997), Masuda et al. (1997b), Lipkin & Silva (2002), Cecere et al. (2004) |
| Hypnea musciformis (Wulfen) J.V. Lamouroux | Modified, tendrils | Tetrasporophytes | No | Checked | No | Sexual; asexual (tetrasporangia) | Preda (1909), Rama Rao (1977) |
| Hypnea stellulifera (J. Agardh) Yamagishi et Masuda | Highly modified, star-shaped, 3–8 rays, 0.2–3 mm × 70–250 μm, basally constricted | Tetrasporophytes | No | Supposed | No | Sexual, asexual (tetrasporangia) | Yamagishi et al. (2003) |
| Laurencia caduciramulosa Masuda et Kawaguchi | Unmodified, 100–600 μm long, basally constricted, | n.a. | No | Checked | Yes | Unknown | Masuda et al. (1997a, 2001), Furnari et al. (2001), Klein & Verlaque (2005) |
| Laurencia decidua E.Y. Dawson | Unmodified, 1 mm long, basally constricted | Tetrasporophytes | No | Supposed | Yes | Asexual (tetrasporangia) | Dawson (1954, 1963) |
| Laurencia subcorymbosa E.Y. Dawson | Unmodified, 150 μm long, basally constricted | n.a. | No | Supposed | No | Unknown | Dawson (1963) |
| Melanothamnus somalensis Bornet et Falkenberg | Modified, spindle-shaped, 200 μm × 68–84 μm, stalked, rhizoidsc | n.a. | n.a. | Supposed | No | Unknown | Falkenberg (1901), Wynne & Banaimoon (1990) |
| Polysiphonia adamsiae Womersley | Unmodified | n.a. | Supposed | Checked | No | Sexual, asexual (tetrasporangia) | Womersley (2003), Mei & Schiel (2007) |
| Polysiphonia furcellata (C. Agardh) Harvey | Modified, branched, 1–1.8 mm long, basally constricted | n.a. | Yes | Checked | Yes | Sexual, asexual (tetrasporangia) | Harvey (1846), Bornet (1892), Preda (1909), Feldmann (1942), Maggs & Hommersand (1993) |
| Polysiphonia propagulifera Womersley | Modified, spindle-shaped, 400–500 μm long, stalked, rhizoidsb; tendrils | n.a. | n.a. | Checked | Yes | Unknown | Womersley (1979) |
| Polysiphonia stricta (Dillwyn) Greville | Unmodified | n.a. | Supposed | Checked | No | Sexual, asexual (tetrasporangia) | Maggs & Hommersand (1993), Mei & Schiel (2007) |
| Solieria chordalis (C. Agardh) J. Agardh | Unmodified, 1–4.5 cm long | n.a. | No | Checked | No | Sexual, asexual (tetrasporangia) | Bornet & Thuret (1880), Gabrielson & Hommersand (1982) , Floc’h et al. (1987) |
| Womersleyella setacea (Hollenberg) R. Norris (as Polysiphonia setacea Hollenberg) | Modified | Unknown | Supposed | Checked in culture | No | Asexual (fragmentation, tetrasporangia) | Hollenberg (1968), Rindi et al. (1999) |
| Yuzurua poiteaui (J.V.Lamouroux) Martin-Lescanne [as Laurencia poiteaui (J.V. Lamouroux) M.A. Howe] | Unmodified, 2 mm long, basally constricted, rhizoidsc | n.a. | No | Checked | No | Sexual, asexual (tetrasporangia) | Schneider & Searles (1991), Cruz Adames & Ballantine (1996), Littler & Littler (2000), Martin-Lescanne et al. (2010) |
- aAdditional to vegetative reproduction by multicellular propagules.
- bAccording to Won et al. (2009), Centroceras clavulatum is restricted to the Pacific Ocean, so that Lipkin’s Red Sea alga bearing propagules must belong to a different species.
- cRhizoids issuing before propagule shedding.
- dReproductive organs reported by Lipkin & Silva (2002) from the Dahlak Archipelago without distinction of the two varieties (var. cornuta and var. stellulifera) later erected to the species rank (Yamagishi et al. 2003).
- n.a. = data not available.
| species | morphology | plants bearing propagules | starch | role | character of taxonomic significance | additional kinds of reproductiona | references |
|---|---|---|---|---|---|---|---|
| Chondria crassicaulis Harvey | Highly modified, derived from trichoblasts, to 1 mm long, stalked | Tetrasporophytes | Yes | Supposed | No | Sexual; asexual (tetrasporangia) | Okamura (1903, 1907), Lee & Yoon (1996) |
| Chondria decidua Tani et Masuda | Highly modified, derived from trichoblasts, 450 μm long, stalked | n.a. | No | Supposed | Yes | Unknown | Tani et al. (2003) |
| Heterosiphonia japonica Yendo | Unmodified, derived from pseudolaterals, 4–16-celled, apical rhizoidsb | n.a. | No | Checked | No | Sexual; asexual (tetrasporangia fragmentation) | Noda (1987), Choi (2001), Bjærke & Rueness (2004), Husa & Sjøtun (2006) |
| Heterosiphonia plumosa (J. Ellis) Batters | Unmodified, experimentally abscissed pseudolaterals | n.a. | No | Checked in culture | No | Sexual; asexual (tetrasporangia) | Boney (1975), Maggs & Hommersand (1993) |
| Lophocladia lallemandii (Montagne) F. Schmitz | Unmodified, experimentally abscissed trichoblasts | Female gametophytes | No | Checked in culture | No | Sexual; asexual (tetrasporangia) | Feldmann & Feldmann (1938), Cormaci & Motta (1985) |
| Neosiphonia ferulacea (Suhr ex J. Agardh) S.M. Guimarães et M.T. Fuji [as Polysiphonia ferulacea Suhr ex J. Agardh] | Modified, derived from trichoblasts, spermatangial branch-like | Tetrasporophytes; gametophytes | No | Checked in culture | No | Sexual; asexual (tetrasporangia) | Kapraun (1977) |
| Polysiphonia fibrillosa (Dillwyn) Sprengel | (i) modified, derived from trichoblasts, cone-shaped, to10 mm long(ii) unmodified trichoblasts, or unfertilized carpogonial branches, gall-like shaped, to 200 μm broad, stalked | (i) male gametophytes(ii) female gametophytes | No | Checked in culture | No | Sexual; asexual (tetrasporangia) | Koch (1986), Maggs & Hommersand (1993) |
| Spyridia filamentosa (Wulfen) Harvey | Unmodified brachyblasts, 385 μm long | Tetrasporophytes; male gametophytes | No | Checked in culture | No | Sexual; asexual (tetrasporangia) | West & Calumpong (1989), Maggs & Hommersand (1993) |
- aAdditional to vegetative reproduction by multicellular propagules.
- bRhizoids issuing before propagule shedding.
- n.a. = data not available.
| species | morphology | plants bearing propagules | starch | role | character of taxonomic significance | additional kinds of reproductiona | references |
|---|---|---|---|---|---|---|---|
| Batrachospermum breutelii Rabenhorst | Derived from gonimoblast filaments, club-shaped, 3–6-celled, stalked | Female gametophytes | Yes | Checked in culture | Yes | Sexual | Skuja (1933), Sheath & Whittick (1995) |
| Botryocladia pyriformis (Børgesen) Kylin | Originated from cortical cells, small mounds | n.a. | n.a. | Checked in culture | No | Sexual; asexual (tetrasporangia) | Ballantine (1989) |
| Fosliella b paschalis (Me. Lemoine) Setchell et N.L. Gardner | Originated from hypothallial cells, discoid, to 45 μm long (100 μm in Canary Islands), stalked | n.a. | No | Checked | No | Asexual (bisporangia, tetrasporangia) | Hollenberg (1970), Afonso-Carrillo (1989) |
| Gracilaria gracilis (Stackhouse) Steentoft, Irvine et Farnham | Originated from germination discs, spherical | Tetrasporophytes | n.a. | Checked in culture | No | Sexual; asexual (tetrasporangia) | Polifrone et al. (2006) |
| Gracilaria sp. | Originated from axes, spherical | Tetrasporophytes | n.a. | Checked in culture | No | Sexual; asexual (tetrasporangia) | Plastino & de Oliveira Filho (1988) |
| Hildenbrandia rivularis (Liebmann) J. Agardh | Originated from hypothallial cells, spherical | n.a. | Yes | Checked in culture | No | Asexual (fragmentation) | Starmach (1952), Nichols (1965) |
| Hydrolithon farinosum (J.V. Lamouroux) D. Penrose et Y.M. Chamberlain [as Fosliella farinosa var. farinosa and var. solmsiana (Falkenberg) Foslie] | Originated from hypothallial cells, triangular, to 90 μm long, stalked | n.a. | No | Checked in culture | No | Sexual; asexual (bisporangia, tetrasporangia) | Solms-Laubach (1881), Falkenberg (1901), Coppejans (1978, 1983), Bressan & Babbini (2003) |
| Hydrolithon sp. (as Fosliellab sp.) | Originated from hypothallial cells, circular, to 125 μm long, stalked | n.a. | No | Checked | No | Unknown | Woelkerling (1988) |
| Kappaphycus striatus (F. Schmitz) Doty ex P.C. Silva | Originated from axis, wart-like, to 1 mm in diameter | Asexual plants | No | Checked in culture | No | Sexual; asexual (tetrasporangia, fragmentation) | Doty & Santos (1978), Mairh & Tewari (1994) |
- aAdditional to asexual reproduction by multicellular propagules.
- bThe genus Fosliella M.A. Howe (1920) is a later synonym of HydrolithonFoslie (1909) (Irvine & Chamberlain 1994).
- n.a. = data not available.
- 1
any part of the thallus;
- 2
indeterminate and determinate branches of any order, even fertile ones (usually tetrasporic branches) (Fig. 1);
- 3
brachyblasts, pseudolaterals and trichoblasts, even fertile ones (Fig. 2);
- 4
any other part of a thallus (e.g. gonimoblast filaments, cortical cells, hypothallial cells) (Fig. 3).
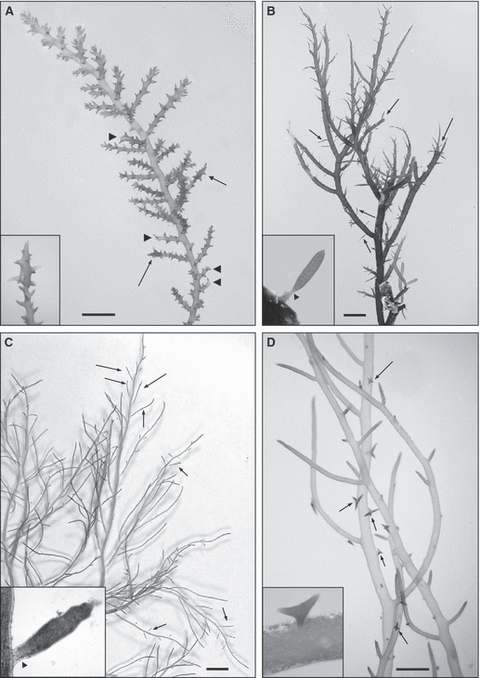
Some propagules derived from the transformation of branches. (A) Acanthophora nayadiformis (Mar Piccolo of Taranto, Italy). Apical propagules (arrows and inset image) and beheaded branches after propagule detachment (arrowhead). Scale bar: 10 mm. (B) Alsidium corallinum (Mar Piccolo of Taranto, Italy). Propagules on the thallus (arrows and inset image). The magnification of the propagule shows the abscission zone (arrowhead) as a basal and less pigmented constriction. Scale bar: 5 mm. (C) Chondria capillaris (Mar Piccolo of Taranto, Italy). Propagules on the thallus (arrows and inset image). The magnification of the propagule shows the abscission zone (arrowhead) as a basal and less pigmented constriction. Scale bar: 1 cm. (D) Hypnea cornuta (Mar Piccolo of Taranto, Italy). Stellate propagules on the thallus (arrows and inset image). Scale bar: 5 mm.
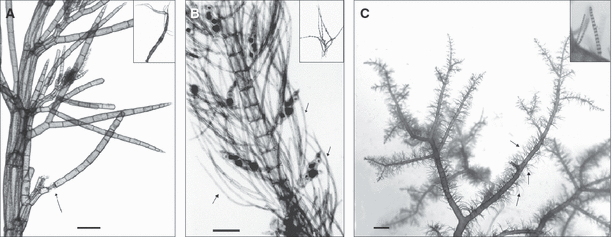
Examples of propagules derived from the transformation of brachyblasts, pseudolaterals or trichoblasts. (A) Heterosiphonia japonica (Bergen, Norway; photographs by Kjersti Sjøtun, by courtesy of Vivian Husa). Thallus with detaching pseudolateral (arrow). The inset image shows a detached pseudolateral bearing distal rhizoids. Scale bar: 40 μm. (B) Lophocladia lallemandii (Ibiza, Balearic Islands), tetrasporophyte with stichidia and brachyblasts (arrows). Scale bar: 100 μm. The inset image (Catania, Italy, by courtesy of Mario Cormaci) shows a detached brachyblast bearing basal rhizoids. (C) Spyridia filamentosa (Mar Piccolo of Taranto, Italy). Unattached thallus with brachyblasts (arrows and inset image). Scale bar: 4 mm.
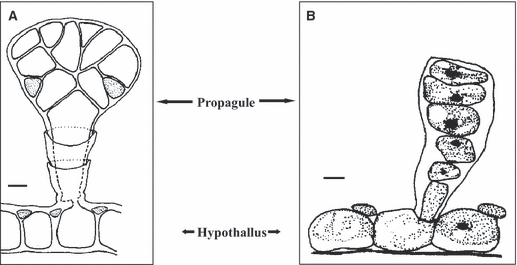
Examples of propagules derived from the transformation of other parts of the thallus. (A) Hydrolithon farinosum. Propagule growing from the hypothallial cells (Copyright © 1978 Royal Botanical Society of Belgium. From Bulletin de la Société Royale de Botanique de Belgique as Fosliella farinosa, by Coppejans. Reprinted by permission of Royal Botanical Society of Belgium). Scale bar: 10 μm. (B) Fosliella paschalis. Propagule growing from the hypothallus (Copyright © 1989 International Phycological Society. From Phycologia, by Afonso-Carrillo. Reprinted by permission of Allen Press Publishing Services). Scale bar: 5 μm.
Concerning their morphology, they may:
- 1
be simple proliferations of cortical cells;
- 2
be simple branchlets;
- 3
have a slightly modified morphology in comparison with the branch from which they derive;
- 4
have a morphology that is so different from their branch of origin that they become highly specialized structures.
Among all the multicellular propagules described in Rhodophyta, the most specialized are those of Acanthophora nayadiformis. These are apical parts which become a darkly pigmented, swollen spiked cone where spikes represent stolons, the most proximal of which curve downwards from their earliest growth (Cecere & Perrone 2002). They are very similar to the turions of aquatic dicotyledons, which are highly modified shoot apices, spherical to club-shaped, consisting of dwarf dark green leaves filled with storage substances and representing winter dormant organs (Sculthorpe 1967).
The vegetative reproduction of Rhodophyta through multicellular propagules is probably much more frequent than shown by Tables 1–3. Many species, particularly foliaceous species (e.g. Grateloupia spp., Halymenia spp., Kallymenia spp. Schizymenia spp.), produce marginal proliferations constricted at the base that are often able to give rise to new plants when they break away. In other species, the thallus is formed by the alternation of large complanate segments joined by thin cylindrical axes [e.g. Gloiocladia microspora (Bornet ex J.J. Rodriguez y Femenias) Sánchez & Rodríguez-Prieto, Rhodymenia spp., Schottera nicaeensis (J.V. Lamouroux ex Duby) Guiry & Hollenberg], and the vegetative reproduction occurs by the necrosis of old segments that release the younger ones, which are frequently rich in floridean starch granules. Such a phenomenon can be generalized to all the perennial species in which the oldest portions seasonally disappear, releasing free-living fragments in the water.
Tendrils can also function as multicellular propagules and not only as structures which enable thalli to get entangled with neighbouring specimens (Fritsch 1965). Indeed, in Asparagopsis taxiformis (Mairh 1977) and Hypnea musciformis (Rama Rao 1977) tendrils were observed to detach themselves from the parent thallus and to develop into young plantlets. Rhizoids are often developed from pericentral cells on the concave side of Polysiphonia propagulifera’s tendrils (Womersley 1979). Similar adaptation can be expected in other Rhodophyta producing tendrils or hooks such as Acrosorium venulosum (Zanardini) Kylin, the gametophytes of Bonnemaisonia hamifera Hariot, Calliblepharis jubata (Goodenough & Woodward) Kützing, Halopithys incurva (Hudson) Batters, Plocamium spp., and Portieria spp.
Other specialized attachment-structures such as the basal harpoon-like branches of Asparagopsis armata Harvey can function as multicellular propagules (Codomier et al. 1977, 1978). Likewise, it is not uncommon that secondary multicellular attachment-structures (rhizoids, holdfasts) regenerate a new individual after separation from the thallus (Westbrook 1927; Fritsch 1965).
Finally, the multicellular propagule concept can be broadened to a whole generation. A considerable number of perennial Rhodophyta are capable of surviving after detachment from the substratum and multiplying only by vegetative means since most of them are sterile (Fritsch 1965; Petrocelli et al. 2009). It is also illustrated by the Rhodophyta possessing a heteromorphic life history with alternation of fixed erect gametophytes and a loose lying filamentous tetrasporophyte. As a whole, cotton-wool-like tufts of the ‘Falkenbergia’ and ‘Trailliella’ phases of Asparagopsis spp. and Bonnemaisonia hamifera, respectively, can be regarded as multicellular propagules since, through intensive vegetative reproduction, they generate large free-floating populations that efficiently contribute to the short- and long-range dispersal of species (see Hawkes 1990 and references therein).
Adaptive Characteristics of Multicellular Propagules
Multicellular propagules may share one or several adaptive characteristics, as follows:
- 1
Presence of either a basal constriction or a stalk, which acts as an abscission zone (e.g. Acanthophora nayadiformis, Alsidium corallinum, Guiryella repens, Hydrolithon spp., Hypnea cornuta, Laurencia spp.);
- 2
Presence of high levels of floridean starch granules (e.g. Acanthophora nayadiformis, A. corallinum, Chondria bulbosa, Polysiphonia furcellata);
- 3
Capability to form other multicellular propagules in their turn (e.g. Acanthophora nayadiformis, A. corallinum, Guiryella repens, H. cornuta, Polysiphonia fibrillosa);
- 4
Possibility of in situ germination (e.g. A. nayadiformis, A. corallinum, Batrachospermum breutelii, Chondria bulbosa, Hydrolithon farinosum, H. cornuta);
- 5
Capability to form rhizoids before abscissing (e.g. Centroceras sp., Heterosiphonia japonica, Yuzurua poiteaui).
- 6
Capability to attach by rhizoids issuing from both proximal and distal ends (e.g. H. japonica, Hydrolithon farinosum, Lophocladia lallemandii, Solieria chordalis).
Concerning the first point, anatomic investigations showed that basal constrictions or stalks are weak points of the thallus structure (Cecere et al. 2002a; Perrone et al. 2005), which make it possible for multicellular propagules to be detached as the result of gentle wave action. However, in Griffithsia corallinoides, Chemin (1928) observed that thalli shed branchlets even in calm waters. Polysiphonia propagulifera and Chondria bulbosa are described as deep-water species living down to 36 m depth (Womersley 1979; Gordon-Mills & Womersley 1987). At such depths, thalli are unlikely to be subject to the effects of waves, and the falling of multicellular propagules must be spontaneous or due to other mechanical causes (e.g. deep currents or fauna activities). The studies performed on H. japonica, an alien species on the Norwegian coasts, showed that pseudolateral detachment is usually due to the necrosis of the cell above the suprabasal cell (Husa & Sjøtun 2006). Therefore, the existence of a more complex process similar to the abscission of green land plants cannot be excluded.
There have been no careful anatomical investigations of either the basal constrictions or the stalks after propagule detachment. Most papers report only that the falling of multicellular propagules leaves a scar on the mother plant, as in Laurencia caduciramulosa (Klein & Verlaque 2005). However, in H. japonica the remains of abscissed pseudolaterals were observed to develop new shoots (Husa & Sjøtun 2006). Similarly, in A. corallinum, the small pigmented axis subtending the propagule regenerates a new propagule after the first one falls (Cecere et al. 2002a). Therefore, there may be a mechanism of wound healing and regeneration involving hormones, similar to that described for some species of Griffithsia (see Lobban & Harrison 1994 and references therein).
Production of Multicellular Propagules as a Taxonomic Feature
In several taxa, the production of multicellular propagules is a species-specific feature, which can thus be used as a taxon-specific diagnostic character (Tables 1–3). Indeed, on the basis of the presence of multicellular propagules, several species [e.g. Chondria bulbosa (Gordon-Mills & Womersley 1987), Chondria decidua (Tani et al. 2003), Guiryella repens (Huisman & Kraft 1992), Hypnea stellulifera (Yamagishi et al. 2003), Laurencia caduciramulosa (Masuda et al. 1997a), Laurencia decidua (Dawson 1954), Polysiphonia propagulifera (Womersley 1979)] and genera, i.e. Deucalion and Anisoschizus (Huisman & Kraft 1982), have been described, and a tribe, Monosporeae, has been resurrected (Huisman & Gordon-Mills 1994).
For the most part, multicellular propagules are not homologous and they have evolved in a wide variety of taxa. Therefore, the evolutionary pressure to increase vegetative reproduction seems to be universal. Molecular studies might bring new insight into their origin.
Once observed in a species, multicellular propagules have been found on conspecific specimens collected in different geographical localities at different times, e.g. Acanthophora nayadiformis [De Jong et al. 1999; Cecere & Perrone 2002; Perrone et al. 2006 (holotype); E. Cecere, personal observations; see Table 1), Alsidium corallinum (Falkenberg 1901; Cecere et al. 1995, 2000a, 2002b), Hypnea cornuta (Mshigeni & Chapman 1994; Bangmei & Yongqiang 1997; Chiang 1997; Lewmanomont 1997; Yamagishi & Masuda 1997; Lipkin & Silva 2002) and L. caduciramulosa (Masuda et al. 1997a, 2001; Furnari et al. 2001; Klein & Verlaque 2005).
Reproduction by Multicellular Propagules versus Other Forms of Reproduction
Of the 43 species listed (Tables 1–3), eight (corresponding to 18.6%) (i.e. Centroceras sp., Chondria decidua, Hildenbrandia rivularis, Hydrolithon sp., Laurencia caduciramulosa, Laurencia subcorymbosa, Melanothamnus somalensis, Polysiphonia propagulifera) seem to reproduce only vegetatively by means of multicellular propagules or fragmentation (Tables 1–3). However, for these taxa further studies are necessary to confirm the absence of sexual or tetrasporophytic reproduction. Indeed, pluri-annual monitoring of their populations has never been carried out. There is also a need for more thorough research on all the species for which the propagule-bearing phase of the plants has not been reported.
Thirty-two species (74.4%) are able both to form multicellular propagules and reproduce sexually. For 22 species, the simultaneous occurrence of both vegetative reproduction by multicellular propagules and other kinds of reproduction is certain, as propagules were observed on fertile gametophytes and/or tetrasporophytes. Multicellular propagules form on both gametophytes and tetrasporophytes in certain species, only on female gametophytes in Lophocladia lallemandii and Batrachospermum breutelii, and only on tetrasporophytes in other species (e.g. Chondria capillaris, Hypnea musciformis), even though gametophytes are also present (Tables 1–3).
In some species, reproduction by multicellular propagules occurs simultaneously with other forms of asexual reproduction, i.e. fragmentation, meiosis, apomeiosis and probably also parthenogenesis (Tables 1–3).
In the case of propagule-bearing plants that are exclusively tetrasporophytes, we can argue that tetrasporophytes are usually more numerous than gametophytes (see Santelices 1990 and references therein) making observation of multicellular propagules easier, even though they may also be present on gametophytes.
What are the Triggers of Vegetative Reproduction by Multicellular Propagules?
Hitherto, vegetative multiplication by fragmentation and propagules was thought to be a secondary alternative to reproduction by gametes and spores in a sexual or asexual reproductive pattern (alternation of generations) that can occur on certain occasions and under certain environmental conditions. In the light of information summarized in Tables 1–3 and considering the importance of multicellular propagules as a diagnostic character, such a mode of vegetative reproduction might be genetically programmed rather than an alternative to the usual life cycle pattern. In Yuzurua poiteaui from Puerto Rico, multicellular propagules (ultimate branchlets) are released prior to death (Cruz Adames & Ballantine 1996), but further studies are required to ascertain whether the subsequent death of the parent is a programmed event. Most species continue to grow after the release of multicellular propagules and, in some species (e.g. Alsidium corallinum), propagule production even occurs many times in the same plant, attesting to its good health (Cecere et al. 2002a).
Another hypothesis is that vegetative reproduction and sexual reproduction would occur at low and at high population densities, respectively (Bell 1982; Hawkes 1990). Some species reproduce vegetatively when the environmental conditions are not favourable for sexual reproduction, as for instance at the beginning of the growing season, and shift to sexual reproduction later (Hawkes 1978), as well as at the limits of species distribution range. Wart-like propagules of Kappaphycus striatus and spherical propagules of Hildenbrandia rivularis may represent a case of vegetative reproduction occurring under stress conditions, as their formation was observed in old cultures (Nichols 1965; Mairh & Tewari 1994). The lack of sexual reproduction in alien species in the localities where they have been introduced might be regarded as a case of a species at the limits of its geographical range. For example, Hypnea cornuta shows sexual reproduction in the Southern Red Sea (Lipkin & Silva 2002) but reproduces only by multicellular propagules in the Mar Piccolo of Taranto, a coastal, enclosed Mediterranean basin (Cecere et al. 2004), which represents its northern geographical limit. Likewise, the Asiatic species Heterosiphonia japonica does not show sexual reproduction on the Norwegian coasts (Husa & Sjøtun 2006).
What Might be the Advantages of the Alternative Vegetative Reproduction by Multicellular Propagules?
It has long been recognised that sexual reproduction results in an increase in genetic variability, which offers a greater range of genotypes on which selective pressures, such as environmental stresses, may act. In contrast, vegetative reproduction gives rise to individuals that are genetically identical to the parents, and, consequently, to populations with low or no genetic diversity, rendering them more vulnerable to environmental changes (see Hawkes 1990 for a general overview; Santelices 1990). However, several authors have offered theoretical considerations regarding the possible advantages of alternative vegetative reproduction.
According to Edyvean & Ford (1984) and Bierzychudek (1985), the species implementing such an alternative adaptive strategy would be able both to generate genetic diversity by sexual reproduction and to replicate a successful genotype at different times. Studies carried out on extensive populations of Acanthophora nayadiformis (Cecere et al. 2000b), Alsidium corallinum (Cecere et al. 2002a), Hypnea cornuta (Cecere et al. 2004) and Polysiphonia furcellata (Maggs & Hommersand 1993) showed that propagule formation is not restricted to a limited period but lasts for the whole growing season, occurring profusely in all the collected plants. Such an extended period of reproduction is probably a feature common to all forms of asexual reproduction; indeed, it was observed that reproduction by spores also occurs over a longer period than sexual reproduction in most of the species in which both forms of reproduction are present (see Brawley & Johnson 1992 and references therein).
The survival of any diaspore depends on its viability and the time necessary for its attachment (Vadas et al. 1992). Although certain spores may have delayed development, they usually do not have dormancy capabilities and their photosynthetic apparatus seems to be either not fully functional (see Santelices 1990 and references therein) or relatively limited (Amsler & Neushul 1991). By contrast, multicellular propagules, even simple branchlets, have a higher probability of survival than any spore or diaspore that lacks a protective outer covering and is relatively susceptible to microbial infections (Amsler et al. 1992), even though fewer propagules are made than spores. Since a large size is seen as a defence against predation, and since achieving a large size usually takes time (see Vadas et al. 1992 and references therein), multicellular propagules, which are already large when they detach from the mother plant, are also more likely to escape most filter-feeders as well as zooplanktonic and small benthic grazers compared with any spore or zygote. Moreover, multicellular propagules, especially those with already developed rhizoids, need less time to attach to the substrate.
Field experiments have proved that thallus fragments are more tolerant to sedimentation than spores (Mei & Schiel 2007); this is probably also true for the biggest multicellular propagules, due to the fact that they are larger and less easily buried (Eriksson & Johansson 2005).
Certain multicellular propagules can have also an overwintering or resting function (see below), the germination being delayed until a subsequent favourable period. In this way, even later multicellular propagules are not wasted, as they are also able to give rise to new individuals. This feature should not be undervalued in that it has been recognized that other diaspores whose germination is delayed are exposed to a higher risk of mortality (Vadas et al.1992).
Due to the traits described above, multicellular propagules have a higher survival probability and competitivity than zygotes, meiotic or apomeiotic spores, or their early post-settlement stages, which are usually subject to high mortality (see Vadas et al. 1992 for overview). All these considerations lead us to infer that multicellular propagules are more efficient in increasing local populations even when another kind of asexual reproduction (e.g. apomeiosis or fragmentation) occurs, for example at the limits of species distribution (Dixon 1965; Rueness 1978).
Moreover, by increasing the number of individuals, multicellular propagules may contribute, in dioecious species, to increased fertilization success, which depends on the density of fertile gametophytes (Santelices 1990; Brawley & Johnson 1992).
Finally, multicellular propagules can also enlarge the dispersal capacity of perennial species, which are sometimes overwhelmed by the blooms of opportunistic species. Indeed, it is known that spores and zygotes of perennial, long-lived species usually have a weak dispersal ability, whereas those of ephemeral, opportunistic species have a high dispersal ability. Moreover, the spores of opportunistic species are more viable than those of the long-lived, perennial species and can retain their attachment capability even several hours after their falling (Amsler & Searles 1980). Since it has been experimentally proven that the limited dispersal of perennial species can be overcome by increasing the number of source thalli (see Santelices 1990 and references therein), multicellular propagules may give perennial taxa a selective advantage.
Multicellular Propagules as Overwintering Organs
Many multicellular propagules contain floridean starch granules (Tables 1–3), which suggests that they can delay their germination. Late autumnal propagules of Acanthophora nayadiformis and Alsidium corallinum were observed to survive the winter in a steady-state and to resume growth in the next spring (Cecere & Perrone 2002; Cecere et al. 2002a). It has been experimentally proven that in overwintering multicellular propagules of A. nayadiformis, stolons resume growth as uprights in response to the same trigger (i.e. long-day regime) that stimulates the sprouting of uprights from the overwintering prostrate system. By contrast, in conditions of short-day regime and low irradiance (winter), they do not transform themselves into upright apices and do not even grow as stolons (Perrone et al. 2005). However, in the induction of both dormancy and germination of A. nayadiformis propagules, endogenous factors (hormones) might play an important role, as observed in the overwintering turions of some carnivorous plants (Adamec 1999). However, this hypothesis needs confirmation. Moreover, like the multicellular propagules of aquatic cormophytes (turions, winter buds and scions), the propagules of A. nayadiformis contain phenolic compounds (Petrocelli & Felicini 1995). Such compounds are known to function as a deterrent to grazing in macroalgae including some Ceramiales (Ragan & Craigie 1978), as well as in aquatic flowering plants (Spencer & Ksander 1994). These phenolic compounds, not yet identified, probably give A. nayadiformis propagules an extra chance of survival.
In Hypnea cornuta, multicellular propagules were never observed settled on rock and stones in winter, probably due to their very small size (E. Cecere & A. Petrocelli, unpublished data).
Although they do not contain starch (Husa & Sjøtun 2006), abscissed pseudolaterals of Heterosiphonia japonica probably behave as overwintering multicellular propagules, as laboratory culture tests showed that they can survive at 4 °C in short-day conditions, which are the average winter conditions off most of the Norwegian coast (Bjærke & Rueness 2004).
Could Multicellular Propagules Be Resting Organs?
Multicellular propagules are probably able to act as resting organs and survive, albeit for a limited period, in environments characterized by unstable conditions. The possibility that multicellular propagules may act as resting organs has been especially considered in alien species, to verify whether they have a higher dispersal capacity. Such studies started only recently and are still ongoing; therefore, most of the results have not yet been published.
Studies of the photosynthetic activity of A. nayadiformis and Hypnea cornuta introduced into the Mar Piccolo of Taranto showed that the maximum photosynthesis values, in terms of O2 produced, registered for multicellular propagules were lower than those registered for whole thalli (Petrocelli & Felicini 1997; Petrocelli & Cecere 2006), as usually occurs in the resting organs of aquatic angiosperms (Adamec 1999).
Multicellular propagules of A. nayadiformis and H. cornuta were able to survive in culture at extreme salinity values (i.e. 10 and 50 PSU), showing a better response (in terms of survival) to the highest value (A. Petrocelli & E. Cecere, unpublished data). Multicellular propagules of A. nayadiformis resumed growth after having been exposed to air for 48 h at high temperatures (28–39 °C), showing that they are able to survive desiccation (Delle Foglie 1999), and those of H. cornuta were able to regenerate in culture after being kept in natural seawater in darkness for 20 days (Cecere & Petrocelli 2004; A. Petrocelli & E. Cecere, unpublished data). Similar behaviour was seen in Heterosiphonia japonica introduced in Norway, as the multicellular propagules (mostly detached pseudolaterals) settled at the intertidal level during neap tides; those that remain entrapped in tidal pools have to withstand emersion and extreme values of both salinity and temperature (Husa & Sjøtun 2006). Only the spores and zygotes of very few species, i.e. the upper intertidal species, and a few recruits can overcome these abiotic extremes, as aerial exposure is usually responsible for high mortality among algal diaspores (Vadas et al. 1992).
Are Multicellular Propagules Useful for Increasing Local Populations or for Long-distance Dispersal?
Macroalgae tend to exhibit quite restricted dispersal ability (Santelices 1990; Kinlan et al. 2005). This is especially true in the case of multicellular propagules of Rhodophyta, which are usually large and heavy; for this reason, they tend to sink promptly after detachment. As recognized by Norton (1992), multicellular propagules perform their main duty efficiently, which is species perpetuation, while the colonization of new sites is merely incidental.
Usually, upon contact with the substrate, every kind of multicellular propagule produces rhizoids (Womersley 1979; Huisman & Kraft 1982; Floc’h et al. 1987; Cecere et al. 2002a), if rhizoids are not already present before abscission. Furthermore, the ability to develop rhizoids from both proximal and distal ends after detachment shows a strong tendency to ensure prompt attachment onto the substrate. In a few species (Coppejans 1978; West & Calumpong 1989; Cecere et al. 2004; Husa & Sjøtun 2006), propagule ‘germination’ has been described in detail. In the highly specialized multicellular propagules of A. nayadiformis, following contact with the substrate, the most proximal buds of the pre-existing stolons curve downward and produce rhizoids, while the distal stolons become apices of upright fronds (Cecere et al. 2007). Therefore, such propagules grow in a polar fashion, like the scions of vascular plants. Propagule-derived plantlets were sometimes observed to form multicellular propagules in their turn very precociously (Perrone et al. 2005). In sheltered basins, multicellular propagules of A. nayadiformis were observed to remain on the sea bottom near the base of the parent thallus, where they formed large cushion-like heaps, but along coasts exposed to high wave motion and strong currents, such formations were never observed, supporting the hypothesis that they disperse, albeit probably over a short distance (Cecere & Perrone 2002).
All these features make multicellular propagules, both the specialized and the simple branchlets, more effective in increasing local populations than in favouring long-distance dispersal. Indeed, reproductive diaspores can normally be dispersed over great distances only if they concentrate at the sea surface, where wind can induce rapid movements (see Clayton 1992 and references therein), with the exception of multicellular propagules fixed or entangled with mobile organisms (e.g. crustaceans, molluscs) or man-made devices (e.g. anchoring, fishing gear).
The contribution of multicellular propagules to the increase of local populations is probably considerable, although it has not been quantified. Pluri-annual research into the phenology of some species showed that all plants profusely form multicellular propagules in the growing season (Cecere et al. 2000b, 2002a). The only quantitative study of recruitment by fragments was carried out for Heterosiphonia japonica; a net value of 89 germlings per 10 cm2 during 1 year was observed and most fragments were detached pseudolaterals (Husa & Sjøtun 2006). In Lophocladia lallemandii, another alien species introduced into the Mediterranean Sea, propagation by multicellular propagules may account for its invasive behaviour (Cebrian & Ballesteros 2010).
Surprisingly, even though a strong tendency to attach has been observed, multicellular propagules are also able to exist as local free-living populations. This phenomenon was observed in the Mar Piccolo of Taranto. Here, multicellular propagules of A. nayadiformis and Hypnea cornuta sometimes remain entrapped in dense free-floating multi-species algal assemblages and are therefore unable to come in contact with the substrate; under these conditions, they give rise to unattached plants, usually with a ball-like habit with axes radially arranged around the original propagule (Cecere et al. 1994, 2004, 2007). Laboratory cultures of the aegagropilous plantlets of A. nayadiformis showed that after 1 month, the cone-shaped propagule body decayed and each radially arranged stolon became an independent free-living plant (E. Cecere & A. Petrocelli, unpublished data). Such free-living plants can be easily dispersed by currents and other vectors.
As far as long-range dispersal is concerned, multicellular propagules, as resting organs, may also be effective. Indeed, due to their ability to survive darkness, they can be transported by ballast waters, a vector of introduction of exotic algae until now considered effective only for the resting stages of phytoplankton and opportunistic species (Flagella et al. 2007). Moreover, the ability to overcome desiccation may allow some multicellular propagules to be transported on board ships (e.g. by fishing gear, ropes and anchorages), during shellfish transfers, or mixed with fishing bait. Therefore, the real possibility that some Rhodophyta might be dispersed over long distances and introduced into new areas by multicellular propagules makes it necessary to take into account possible new human-mediated introduction routes for species. The ecological implications of such a scenario are obviously relevant.
Conclusions
Dixon (1965) and Feldmann & L’Hardy-Halos (1977) first realized the importance and the extent of vegetative reproduction in the life histories of macroalgae in general, and in Rhodophyta in particular. Hawkes (1990) studied the subject extensively, while underlining the need for further investigations to clarify the ecological and evolutionary aspects of sexual versus vegetative reproduction in marine algae. The same author pointed out that most Rhodophyta are capable of vegetative reproduction by means of either monospores or other dispersible propagules. He specified that in ‘lower’ Rhodophyta (such as Compsogonales, Bangiales, Acrochaetiales and Nemaliales), monospore production is common, whereas it is rare or absent in Gigartinales, Rhodymeniales and Ceramiales.
The present review has shown that vegetative reproduction is also common in these orders, not by monospores, but via multicellular, simple or specialized, propagules.
This paper, far from having dealt with this matter exhaustively, should mainly serve to show how little is known of it. There is indeed a need for further multidisciplinary studies on vegetative reproduction by multicellular propagules from many points of view (physiological, ecological, demographic) to learn more about all the aspects of this phenomenon and to better understand its importance, implications and evolutionary meaning. Many questions need answering, including the following:
- 1
Can multicellular propagules also form on gametophytes in those species in which they have been observed only on tetrasporophytes?
- 2
In what phases do multicellular propagules form in those species for which the propagule-bearing phases have not yet been identified?
- 3
Does reproduction via multicellular propagules only observed in culture occur also in the field?
- 4
Is it necessary for a thallus to reach a certain age and/or size to form multicellular propagules?
- 5
What are the effects of environmental factors (mainly temperature, irradiance, photoperiod and water motion) on formation, dormancy and germination of multicellular propagules?
- 6
What is the role of endogenous factors in multicellular propagule formation, falling, dormancy and germination?
- 7
What are the analogies with the multicellular propagules of aquatic angiosperms?
- 8
What are the effects of both intra- and interspecific competition on the settlement and the germination of multicellular propagules?
- 9
What is the role of multicellular propagules in determining macroalgal assemblages?
- 10
What is the role of multicellular propagules in the long-range dispersal of species, mainly alien species?
Last but not least, a question of terminology arises; as mentioned above, at present, all diaspores are referred to as ‘propagules’ regardless of their origin and morphology. Fritsch (1965) considered only the propagules of Batrachospermum breutelii, Hydrolithon boreale (as Melobesia solmsiana) and Polysiphonia furcellata to be ‘specialized’. In 1965, Nichols distinguished two different methods of vegetative reproduction in Hildenbrandia rivularis, the one by means of multicellular spherical propagules (called ‘gemmae’) and the other by fragmentation of any part of the plant (Nichols 1965). Later, Guiry (1978) suggested that the asexual reproductive structures of the genus Monosporus and other Ceramiaceae be called unicellular propagules rather than monosporangia, and Huisman & Kraft (1982) supported this recommendation as a result of their studies. According to the above-mentioned authors, we suggest using the term ‘multicellular propagule’ only for the multicellular vegetative propagating structures as defined above. Any vegetative part of the thallus giving rise to a new individual could simply be called ‘vegetative fragment’. The more generic term ‘diaspore’ is more appropriate for any general structure forming a new individual. In such a way, as these terms are usually reported as key words, bibliographical searches would also be facilitated and better addressed.
In conclusion, multicellular propagules seem to allow species to increase populations, to cope with unpredictable environmental changes, to survive in conditions that would be lethal for whole thalli, and to reach new habitats. Therefore, propagule-forming species are probably more competitive than taxa that do not produce propagules, and this needs to be considered in future studies of both the ecology and demography of macroalgae.
Acknowledgements
We are grateful to Dr Mike Hawkes for critically reading the manuscript and for improvements; to Drs Mario Cormaci and Giovanni Furnari for kindly providing several old publications; to M. Perret-Boudouresque for bibliographical assistance; to Michael Paul for correcting the English. Dr Vivian Husa is also acknowledged for providing photos of Heterosiphonia japonica from Norway and Mario Cormaci for the photo of the brachyblast of Lophocladia lallemandii. Dr Julio Afonso Carrillo with Allen Press Publishing Services and Prof. Eric Coppejans with the Royal Botanical Society of Belgium were very kind in allowing the reproduction of drawings of propagules of Fosliella paschalis and Fosliella farinosa.




