Aggregational behavior of the blue mussels Mytilus edulis and Mytilus trossulus: a potential pre-zygotic reproductive isolation mechanism
Abstract
The blue mussels Mytilus edulis and Mytilus trossulus occur sympatrically and are able to hybridize in populations on the eastern coast of Newfoundland, Canada, presenting an opportunity to study their aggregational behavior. Aggregation behavior may therefore provide insight into post-settlement interactions and pre-zygotic reproductive isolation between the species. Three treatments were designed using M. edulis and M. trossulus to investigate their intraspecific and interspecific spatial distribution patterns. With Ripley’s K-function and Monte Carlo simulation analysis, we found that in the single-species treatment, M. edulis aggregated significantly but not M. trossulus. Based on results of two-way ANOVAs, both the number of aggregations and the moving distance were significantly affected by the treatments (single-species or mixed-species treatment) and times (24, 48, 72 and 96 h). In further pairwise comparisons using Tukey’s test, M. edulis aggregated differently with or without M. trossulus occupying the same tank, suggesting that the aggregational behavior of M. edulis could be driven by species-specific chemical cues. The result that M. edulis aggregates intraspecifically may increase the probability of intraspecific fertilization of the spawned gametes and thus function as a pre-zygotic reproductive isolation mechanism maintaining the blue mussel hybrid zone.
Introduction
Grouping is considered to be the most common collective behavior of organisms (Jeanson et al. 2005). A wide range of taxa, including bacteria, arthropods, fish, birds and mammals, exhibit this behavior (Parrish & Hamner 1997; Parrish & Edelstein-Keshet 1999; Parrish et al. 2002). The terminology for such assemblages varies (schools, herds, pods, flocks, etc.), but Allee (1931) uses the term ‘aggregation’. Camazine et al. (2001) define an aggregation as ‘any assemblage of individuals that results in a higher density of individuals than in the surrounding area’. Sessile invertebrates such as blue mussels (including both Mytilus edulis and Mytilus trossulus) lack the ability to move in the adult stage and therefore can only aggregate over micro-geographical distances. Irrespective of whether the aggregation occurs at geographical or micro-geographical scales, there is a selective advantage to group membership (Buss 1981) based on fundamental evolutionary assumptions such as increased predator avoidance and reproductive success. Theoretically, broadcast spawning invertebrates that release their gametes into the water column usually have high gamete dispersal ability and thereby increase the possibility of non-assortative mating between heterospecific taxa co-occurring in the same area. However, since the gamete contact time required for successful fertilization is only a few minutes (Rosenthal et al. 1988; Levitan et al. 1991; Grubert et al. 2005; Kupriyanova & Havenhand 2005), the gametes may not have enough time to disperse far from their spawning environment before most eggs are fertilized. Therefore, segregation of heterospecific taxa caused by micro-geographical aggregation behavior will lead to assortative mating within each taxon-specific aggregation and thereby act as a pre-zygotic reproductive isolation mechanism in a natural hybrid zone (Erlandsson et al. 1999; Cruz et al. 2004). In addition, marine broadcast spawning organisms with external fertilization such as blue mussels do not have complex mating behaviors that may play an important role in reproductive isolation in most species with internal fertilization (Arita 1979; Gomez & Serra 1995; Price & Boake 1995; Yamada et al. 2008). Therefore, pre-zygotic reproductive isolation at the spatial level can be one of the most important reproductive isolation mechanisms in broadcast spawning blue mussels and other sessile marine invertebrates.
Speciation is a fundamental theme in evolutionary biology. It is generally accepted that the majority of speciation events occur when two populations are geographically isolated and diverge genetically through natural selection and/or genetic drift, eventually resulting in intrinsic reproductive barriers between populations (Dobzhansky 1937; Mayr 1963). Diverging populations may come into contact again and form a natural hybrid zone. Since reproductive isolation is often incomplete between populations that hybridize, there can be ongoing gene flow between populations, and therefore natural hybrid zones can be maintained by reproductive isolation mechanisms that counteract the homogenization effect of gene flow. Therefore, hybrid zones such as the blue mussel hybrid zone in Newfoundland (Canada) provide excellent opportunities for studying the evolution of reproductive isolation mechanisms and the speciation process (Barton 2001; Vines et al. 2003; Berrieman et al. 2005; Jones et al. 2006).
Blue mussels, M. edulis, M. trossulus and their hybrids coexist along Newfoundland coasts (Bates & Innes 1995; Innes & Bates 1999). In most cases, when two divergent populations come into contact to form a hybrid zone, a clinal change in the frequency of mixed hybrid genotypes is produced (Barton & Hewitt 1985, 1989). In contrast, M. edulis, M. trossulus and hybrid individuals are found along the entire shore of Newfoundland, forming a mosaic distribution pattern consisting of mixtures of both parental species and hybrids in close contact (Bates & Innes 1995). The existence of viable hybrids shows that there is incomplete reproduction isolation between M. edulis and M. trossulus (Miranda et al. 2010; Liu et al. 2011), which is consistent with studies on other hybridizing species (Harper & Hart 2005; Milne & Abbott 2008). The M. edulis and M. trossulus hybrid zone in Newfoundland provides a unique opportunity to investigate intraspecific and interspecific micro-geographical aggregational behavior and its potential implications for reproductive isolation. This study attempts to answer the following questions; (i) Do M. edulis and M. trossulus aggregate intraspecifically, interspecifically, or randomly? (ii) Do the aggregation patterns of the two species differ from each other? (iii) Is the intraspecific pattern maintained in the presence of other species?
Material and Methods
Animals
Adult blue mussels (Mytilus edulis and Mytilus trossulus) were collected from a mussel farm at Trinity, Trinity Bay, Newfoundland, Canada, in 2006 and were housed in flowing, 10 °C seawater. The mussels were fed daily with Commercial Instant Shellfish Diet 1800® (Reed Mariculture, Inc.) according to the instructions provided. Mussels were given 3 days to acclimatize to laboratory conditions before the labeling procedure.
Experiment set-up and execution
The shell length of each individual was measured to the nearest 0.05 mm with electronic calipers and only those individuals measuring 40–60 mm in length were used. The shell shape was used as a preliminary species identification (Innes and Bates, 1999). Each individual was labeled with a plastic tag stating species name and individual number, which was affixed to the shell using marine epoxy glue. A 1.0 × 0.8 m water tank was thoroughly cleaned with repeated washes of ethanol and filtered seawater. Once the tank was dry, a grid of 70 quadrats (each quadrat measuring 0.1 × 0.1 m, with total grid area 0.7 m2) was drawn onto the tank bottom with a permanent marker. The tank inflow–outflow area was not included in the experimental area.
Three different treatments were carried out; the first tank had 70 Mytilis edulis specimens, the second tank had 70 Mytilis trossulus specimens, and the third tank contained a mixture of 35 M. edulis specimens and 35 M. trossulus specimens. Each treatment was repeated twice. In each single-species treatment, a single individual was placed in the center of a quadrat, with all individuals being in the same orientation (Fig. 1, single-species treatment at time 0). For the mixed treatment, M. edulis and M. trossulus were centrally placed in alternating quadrats, a ‘checkerboard’ pattern (Fig. 1, mixed-species treatment at time 0). This arrangement ensured that for each experiment, all individuals were equally spaced and had an equal chance of encountering each other.
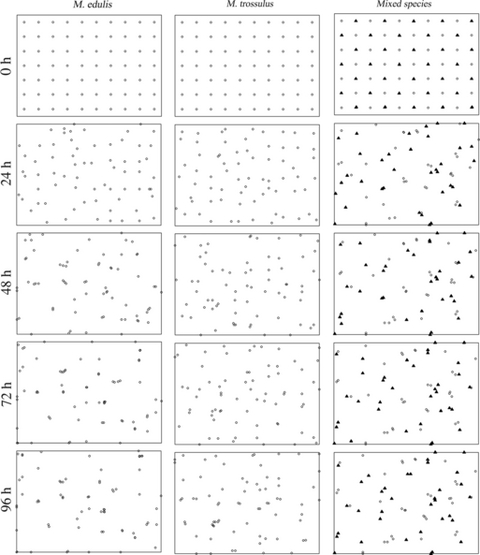
Examples of mapped spatial distribution of blue mussels in single-species treatment (the first two columns) and mixed-species treatment (the last column, in which circles indicate M. edulis individuals and triangles indicate M. trossulus individuals) at 0, 24, 48, 72 and 96 h.
Starting from initial set-up (time 0), the location of each individual was recorded as x- and y-coordinates every 24 h for 4 days. The point of mussel byssus attachment to the tank was the point recorded (Fig. 1).
Application of PCR-amplified DNA markers for species identity confirmation
Approximately 100 mg of mantle edge was removed from each individual, coarsely chopped and digested in 500 μL lysis buffer (50 mm Tris–HCl, pH 8.0; 1% SDS; 25 mm ethylenediaminetetraacetic acid, EDTA) with 200 μg of proteinase K (Sigma) at 37 °C overnight. The solution was then extracted twice with 24:24:1 phenol: chloroform:isoamyl alcohol followed by 95% ethanol precipitation at −20%. The extracted DNA was resuspended in 200 μL of purified distilled water.
Two PCR-based nuclear DNA markers were used to verify the species identity. The ME marker produces single diagnostic bands for each species. The length of the non-repetitive regions amplified by this reaction is specific to each species; 180 and 168 base pairs (bp) for Mytilus edulis and Mytilus trossulus, respectively. The primers used were ME15 (5′CCA GTA TAC AAA CCT GTG AAG-3′) and ME16 (5′-TGT TGT CTT AAT AGG TTT GTA AGA-3′) (Inoue et al. 1997). The ITS marker is a co-dominant marker that produces specific patterns; bands of 180 and 450 bp for M. edulis, bands of 180 and 280 bp for pure M. trossulus, and all three bands for hybrids. The primers used were ITS1 (5′-GTT TCC GTA GGT GAA CCT G-3′) and ITS2 (5′-CTC GTC TGA TCT GAG GTC G-3′) (Heath et al. 1995).
PCR amplifications were carried out in a 25-μL reaction volume containing DNA templates (5 μL for ME, 2 μL for ITS), 0.2 mm dNTPs, 2 mm MgCl2, 0.4 mm of each primer, 1 unit of Taq (Thermus aquaticus strain YT1) DNA polymerase, PCR buffer and sterile distilled water. For ITS amplifications, 5 μL of each amplified PCR product was digested with 0.5 units of the restriction enzyme Hhal at 37 °C for 12 h.
Genotyping was performed after the experiment and the preliminary species identification was shown to be 92% correct. From a total of 300 individuals used in the experiment, 138 were identified as M. edulis and 138 as M. trossulus.
Statistical analysis
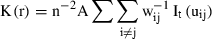
In this spatial point-pattern analysis, a circle of radius r was centered in each point and the number of individuals (‘neighbors’) with their byssus attachment point within the circle was counted. For n individual points in area A, the density (λ = n/A) gave the mean number of individuals per unit area. The function λK(r) gave the expected number of further points within radius r of an arbitrary point. If the points were randomly distributed, the expected value of K(r) should be equal to πr2 (Haase 1995).
Since all individuals were defined by a pair of x-, y-coordinates, the distance between two points i and j were calculated by: 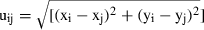 . This procedure was repeated for all point-to-point distances and It(uij) was summed for each distance r (Haase 1995).
. This procedure was repeated for all point-to-point distances and It(uij) was summed for each distance r (Haase 1995).
In the next step of analysis, K(r) was calculated from the data and Monte Carlo simulations were used to determine statistical significance of Ripley’s K-function results. This method simulated randomly generated plots of the same dimensions as the plot used in the experiments. The simulation was repeated 99 times and the lowest and highest value of K(r) for each r was used to define the upper and lower limits of a 99% confidence envelope (α = 0.01). The confidence interval for the null hypothesis could be defined by the number of simulations (n) and equals n/(n + 1) × 100 (Leemans 1991). Results of spatial pattern analysis using Ripley’s K-function were then presented as 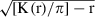 against r. This transformation meant that under the null hypothesis of complete spatial randomness, the derived function had an expectation of 0 for all values of r. This transformation allowed easy interpretation of spatial distribution (Leemans 1991) since a clumped distribution can be assumed when the deviation from the sample statistic from zero expectation was positive and a regular pattern can be assumed when the deviation was negative. If the sample statistic remains within the bounds of the confidence envelope, the null hypothesis of complete spatial randomness cannot be rejected.
against r. This transformation meant that under the null hypothesis of complete spatial randomness, the derived function had an expectation of 0 for all values of r. This transformation allowed easy interpretation of spatial distribution (Leemans 1991) since a clumped distribution can be assumed when the deviation from the sample statistic from zero expectation was positive and a regular pattern can be assumed when the deviation was negative. If the sample statistic remains within the bounds of the confidence envelope, the null hypothesis of complete spatial randomness cannot be rejected.
Aggregation formation was counted and distances moved were calculated across all treatments over time. A two-way analysis of variance for number of aggregations formed and distance moved over time and different treatments was performed using R statistical software. Tukey’s tests were subsequently conducted between all pairwise comparison groups. A P-value <0.05 was accepted as significant for all statistics.
Results
Aggregation behavior
According to the results of Ripley’s K-function and Monte Carlo simulation analysis (Table 1), for Mytilus edulis individuals alone there was evidence of a regular distribution between 4 and 10 cm at 24 h; however, a clumping distribution pattern was found from 48 to 96 h between 3 and 5 cm. For Mytilus trossulus alone there was a significant regular distribution pattern between 4 and 10 cm at 24 and 48 h. By 72 h, there was a random distribution, which continued into 96 h. Ripley’s K-function for two variables was applied to evaluate the species’ aggregational patterns as influenced by the interspecific set-up. For M. edulis, results suggest a clumped distribution between 4 and 6 cm at 24 and 48 h, respectively and a significantly clumped distribution at 5 cm for 72 and 96 h, respectively (Table 1). For M. trossulus, there was a random distribution pattern at 24, 48, 72 and 96 h for the first replicates; however, there was a significantly clumped distribution at 5 cm for results from the second replicate at 24, 48 and 72 h.
| Time (h) | Spatial distribution pattern of | |||
|---|---|---|---|---|
| In single-species treatment | In mixed-species treatment | |||
| M. edulis | M. trossulus | M. edulis | M. trossulus | |
| 24 | Regular | Regular | Clumping | Random/clumping |
| 48 | Clumping | Regular | Clumping | Random/clumping |
| 72 | Clumping | Random | Clumping | Random/clumping |
| 96 | Clumping | Random | Clumping | Random |
Number of aggregations formed and distances moved
The number of aggregations formed by blue mussel individuals changed with time (Table 2). When occupying the same tank, most aggregations were formed by Mytilus edulis alone. Clumps consisting of both species were the second most common and there were few aggregations consisting of only Mytilus trossulus (Table 2). Based on results of two-way ANOVAs, treatments (including three groups: M. edulis only, M. trossulus only, and mixed species), times (24, 48, 72 and 96 h) and their interactions all showed significant (P < 0.05) effect on the number of aggregations formed (Table 3). With further pairwise Tukey’s test, all tested treatment and time groups were significant by (P < 0.05) different from each other.
| Time (h) | Average number of aggregations formed by | Total | ||||
|---|---|---|---|---|---|---|
| In single-species treatment | In mixed-species treatment | |||||
| M. edulis | M. trossulus | M. edulis | M. trossulus | Mixed | ||
| 24 | 1 ± 0 | 1.5 ± 0.7 | 8.5 ± 0.7 | 3.5 ± 0.7 | 3.5 ± 0.7 | 15.5 ± 0.7 |
| 48 | 6.5 ± 0.7 | 3 ± 1.4 | 9 ± 1.4 | 4.5 ± 0.7 | 6 ± 1.4 | 19.5 ± 2.1 |
| 72 | 11.5 ± 0.7 | 7 ± 1.4 | 9 ± 0 | 4.5 ± 2.1 | 6.5 ± 2.1 | 20 ± 0 |
| 96 | 15.5 ± 0.7 | 10.5 ± 0.7 | 9 ± 1.4 | 4.5 ± 2.1 | 6.5 ± 2.1 | 20 ± 1.4 |
| df | F | P | |
|---|---|---|---|
| Treatments* | 2 | 341.15 | 2.67 × 10−11 |
| Times* | 3 | 86.95 | 2.08 × 10−8 |
| Interaction | 6 | 10.43 | 3.61 × 10−4 |
- *Significant (P < 0.05) difference for all pairwise comparisons with Tukey’s test.
Similar to that of aggregation numbers, the distances moved by blue mussel individuals also changed with time (Table 4) and were significantly affected by treatment (including four groups: M. edulis and M. trossulus in single-species and mixed-species treatments, respectively) and time (Table 5). According to pairwise comparison using Tukey’s test, the distances moved by M. edulis with or without the other species occupying the same tank were significantly different (P < 0.05); however, these were not significantly different (P = 0.1) for M. trossulus. In addition, based on Tukey’s test, distances moved significantly changed through time except for 72 and 96 h (P = 0.99).
| Time (h) | Average distance moved (mm) by | |||
|---|---|---|---|---|
| In single-species treatment | In mixed-species treatment | |||
| M. edulis | M. trossulus | M. edulis | M. trossulus | |
| 24 | 4.13 ± 0.20 | 3.67 ± 0.11 | 10.42 ± 0.61 | 9.40 ± 0.21 |
| 48 | 4.97 ± 0.34 | 4.30 ± 0.25 | 1.78 ± 0.04 | 2.13 ± 0.30 |
| 72 | 4.63 ± 0.11 | 3.15 ± 0.21 | 0.18 ± 0.04 | 0.71 ± 0.18 |
| 96 | 4.52 ± 0.28 | 3.56 ± 0.33 | 0.27 ± 0.04 | 0.38 ± 0.06 |
| df | F | P | |
|---|---|---|---|
| Treatments | 3 | 26.17 | 2.08 × 10−6 |
| Times | 3 | 309.31 | 2.08 × 10−14 |
| Interaction | 9 | 112.43 | 3.61 × 10−13 |
Discussion
Several studies have investigated the reproductive isolation mechanisms that maintain species identity in blue mussel hybrid zones (Bates & Innes 1995; Toro et al. 2004; Miranda et al. 2010; Liu et al. 2011). Although M. edulis and M. trossulus in Newfoundland show some differences in spawning time, their spawning periods overlap (Toro et al. 2004). Temporal differences in spawning time alone cannot provide strong reproductive isolation in Newfoundland because of the relatively short summer. However, there is some evidence for habitat segregation between the two species that may reduce opportunities for hybridization. For example, Mytilus edulis from the Nova Scotia hybrid zone prefer low salinity and less wave-exposed environments (Gartner-Kepkay et al. 1983). Similarly, Bates & Innes (1995) found that more than 90% of the individuals at two wave-exposed sites in Newfoundland were Mytilus trossulus, suggesting a potential for habitat segregation between the two species. Mytilus edulis from a Mediterranean hybrid zone prefers sheltered habitats for settlement when compared to Mytilus galloprovincialis (Bierne et al. 2003). This habitat segregation could result from both larval settlement and individual aggregational behavior (Bayne 1964), which may act as a pre-zygotic reproductive isolation mechanism at a spatial level.
Studies of aggregational behavior in intertidal snails show that aggregation can be affected by both individual size (Erlandsson et al. 1999) and time (mating or non-mating season) (Erlandsson & Kostylev 1995). In the present study, aggregational behavior of M. edulis and M. trossulus was investigated with controlled individual sizes and time. The fact that the majority of M. edulis is within 3–6 cm of the nearest neighbor, regardless of whether M. trossulus is present or absent, indicates that M. edulis aggregates intraspecifically to a significant extent. In laboratory experiments, more than 50% blue mussel eggs were reported to fertilize within 60 s of gamete interaction (Liu 2009). In this context, most blue mussel eggs are fertilized by sperm of individuals from the same micro-habitat. Therefore, if species-specific micro-geographical aggregation behavior does exist, it can lead to micro-habitat segregation and subsequently increase assortative mating within micro-habitat. Along with increasing intraspecific fertilization success, as suggested in other broadcast spawning organisms (Pennington 1985; Giese & Kanatani 1987; Pearse et al. 1988; Levitan 1998), this observed aggregational behavior may result in a reduction in hybridization, as reported in intertidal and sea snails (Johannesson et al. 1995; Rolán-Alvarez et al. 1999; Cruz et al. 2004). By clumping with other individuals of the same species, M. edulis increases intraspecific reproductive success by improving the chances that its own sperm will fertilize eggs of the same species (Levitan et al. 1992). Meanwhile, this behavior decreases mating between individuals from different species that occupy different micro-habitats and therefore decreases the formation of hybrids.
Given the insignificant Ripley’s K-function results for M. trossulus, individuals of this species did not aggregate significantly in this experimental set-up. It is also interesting to note that in the mixed-species treatment, aggregations containing both species were greater in number than those containing only M. trossulus. This suggests that M. trossulus may have a tendency for random distribution, as a regular pattern would likely coincide with an insignificant difference between numbers of aggregations that contain both species when compared to those consisting only of M. trossulus. Although not achieved here, a significant regular distribution pattern could be indicative of aggregation avoidance behavior. A random distribution pattern would indicate that individuals are neither avoiding nor actively participating in aggregation. As with any animal aggregation, there are costs and benefits to aggregating and living within a mussel bed. Okamura (1986) found that mussels in groups experienced growth reduction and that this effect was quite prominent in individuals in the center of the group. Fréchette & Bourget (1985) have documented that the ‘active feeding of many mussels may reduce the absolute amount of food available to any one individual, thereby limiting growth’. In addition to slow growth, shell distortion has been observed in natural mussels beds (Harger 1972). Therefore, the absence of significant aggregational behavior in M. trossulus could either be an adaptation to avoid the intense competition for resources that accompanies group life (Okamura 1986) or that adaptation in the form of either lifestyle (clumping/non-clumping) is not selectively advantageous.
Both species maintain the similar spatial pattern for single-species and mixed-species treatments. Of particular interest is that only M. edulis aggregated differently with or without interspecific individuals in their surrounding. Although no waterborne chemical inducer has been isolated and fully characterized, De Vooys (2003) has explored the effect of the synthetic tripeptide analogue glycine-glycine-arginine (GRR) on the aggregational behavior of M. edulis. It was shown that individual mussels were attracted to mussel concentrations and they moved actively in the direction of the concentrations. Given its demonstrated intraspecific aggregation pattern across treatments, M. edulis individuals may be especially reactive to the chemical cue of other M. edulis individuals. It is also possible that M. edulis could release a stronger aggregational chemical cue in the presence of M. trossulus and subsequently cause changes in aggregational behavior. Moreover, the chemical cue released by M. edulis may be species-specific and therefore not alter the aggregational behavior of M. trossulus.
Conclusion
In the present experiment M. trossulus did not aggregate significantly and was shown to exhibit a random distribution pattern. However, M. edulis aggregated intraspecifically and the presence of M. trossulus affected its aggregational behavior, which may be driven by a species-specific aggregating chemical cue. Considering that the fertilization process of blue mussels takes place within minutes, most eggs will be fertilized in close vicinity to the individual micro-habitat and therefore lead to high assortative mating, which can act as a pre-zygotic reproductive isolation mechanism in the blue mussel hybrid zone.
Acknowledgements
We wish to extend our thanks to Prof. Gonzalo Giribet for critical reviewing of the manuscript. We would like to thank Dr. Maria Cristina Gambi for her assistance and patience in manuscript processing. We thank Glean Gonsalves and Prof. Gonzalo Giribet for help in polishing the English. This research was funded by an NSERC grant to Prof. David Innes.




