Phytoplankton in a transitional ecosystem of the Northern Adriatic Sea and its putative role as an indicator for water quality assessment
Abstract
Pluriannual data on the phytoplankton spatial and seasonal distribution in the lagoon of Venice (North-western Adriatic Sea, Italy) were presented and analysed to suggest a possible tool for the transitional ecosystem water quality assessment. In order to meet the European Water Framework Directive 2000/60, data of phytoplankton distribution were processed together with environmental parameters to verify whether such a community may give indications of the water quality. Some evidence in this direction appeared to be provided by diatom biomass and cell abundance. The starting hypothesis was that the occurrence of opportunistic species (small cells with a high growth rate) is related to the worst environmental conditions, and the conservative species (large cells with a slow growth rate) to the best water quality areas. The method was validated in other Italian transitional systems (Sacca di Goro, Lesina Lagoon, Orbetello Lagoon, Marano-Grado Lagoon, Mar Piccolo of Taranto). The overall results seem to give quite a good description of the environmental status, but they also highlighted the limits of the phytoplankton community as an indicator and, hence, the necessity to conduct regular sampling throughout the year.
Problem
The European Water Framework Directive 2000/60 (WFD) paid particular attention to brackish and coastal water protection and safeguarding by stressing the importance of physical, chemical and biological monitoring. Lagoons are, generally, characterised by strong gradients of both physical and chemical variables such as depth, salinity and nutrient concentrations. Moreover, tidal excursions favour water mixing and renewal, further confusing the gradient. The presence of areas limited by wetlands and fishing ponds with reduced hydrological regimes does not necessarily class an environment as being of poor quality but it provides evidence that it is a different habitat. In such conditions lagoons appear as a puzzle of several microhabitats with different ecological relevance and with peculiar resiliency to human pressure. The heterogeneity increases with the enlargement of the transitional system and depends on river-inputs, seawater exchange and human exploitation. Considering the complexity of the transitional systems, a reliable classification of the different lagoons is difficult. Each lagoon may show several and different habitats whose conditions can be equally good in relation to their ecological use.
The use of microalgae as trophic indicators is widely applied in freshwater ecosystems (Kelly 1998; Prygiel et al. 1999; Dell’Uomo 2004) but little attention has been paid to coastal areas, probably because their heterogeneity on a small spatial scale makes classification difficult.
Chlorophyll a concentrations have often been used in combination with other parameters (i.e. nutrients, oxygen, turbidity, macrophytes) to classify the aquatic trophic status (Vollenweider et al. 1998; Borja et al. 2004; Giovanardi & Vollenweider 2004; Specchiulli et al. 2008; Giordani et al. 2009). Nevertheless, defining quality thresholds is difficult and sometimes definitions are only useful locally.
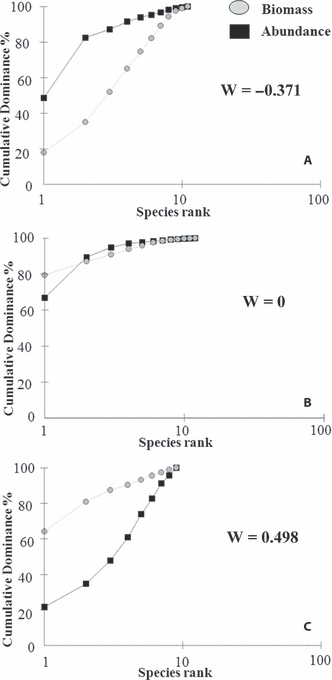
The main aim of this paper is to investigate the phytoplankton spatial distribution in a transitional ecosystem (Venice Lagoon) to verify its suitability as a bioindicator of water quality as required by the WFD. The phytoplankton is widely subject to abiotic factor variations and hence can be difficult to understand if the abundance and community fluctuations are related to natural variability or anthropic pressures. Nevertheless, we tried to ascertain possible relationships between the phytoplankton and the ecosystem status. Some results were achieved by calculation of the diatom biomass and abundance dominance curves. The comparison between biomass and abundance (W-statistic) of the macrozoobenthic community provided interesting results in distinguishing sites in relation to their pollution level (Clarke & Warwick 1994). On the basis of such results, we tried to verify whether that assumption could be extendible to the phytoplanktonic community. Data collected in the Venice Lagoon from 1998 to 2003 were used. Notwithstanding its great variability, phytoplankton (in general determined as chlorophyll a concentrations) has been often used to coarsely detect eutrophication, but beyond the use of an abundance or a concentration we think that the study of the community structure may furnish a more general approach which could also be applied to other transitional ecosystems. On the basis of previous experience with water status assessment (Sfriso et al. 2007, 2009) we suggest the possible use of the W-statistic as a simple tool that can be applied to samples routinely collected for monitoring programs. The suggested index was validated in other Italian transitional waters (Sacca di Goro, Lesina Lagoon, Orbetello Lagoon, Marano-Grado Lagoon, Mar Piccolo of Taranto).
Study area
Water samples were collected in six Italian transitional coastal ecosystems (Fig. 1A), although results refer mainly to the Venice Lagoon (North Adriatic) (Fig. 1E), a semi-enclosed water basin (mean depth c. 1.0 m) with a surface of c. 550 km2. During one tidal cycle (12 h), it exchanges c. 60% of its water body with the North-western Adriatic Sea through three inlets. The lagoon receives untreated wastewaters from Venice historical centre and untreated and treated sewages from the city of Mestre, its hinterland and other lagoon islands. Pollutants of different nature and cooling waters from Porto Marghera industrial zone also flow in the lagoon. Naval traffic is rather heavy, as two large and deep canals (the Malamocco-Marghera and Vittorio Emanuele canals, width: 100–200 m; depth: 12–20 m) were dug to allow the transit of commercial ships. The lagoon can be divided into three morphological basins (southern, central and northern), which are separated by the Malamocco-Marghera canal in the south and by the Burano salt-marshes in the north (see black lines in Fig. 1E).
Map of the study basins. Sampling sites are marked with letters and numbers. In the map of the Venice Lagoon only the sites which were sampled monthly are reported, than two black lines respresent an ideal separation of the three morphological basins.
The Marano-Grado Lagoon (Fig. 1D) and the Sacca di Goro (Fig. 1B) are also located in the Northern Adriatic Sea. The first has a surface of c. 160 km2, and receives some spring river flows and high amounts of mercury (Piani et al. 2005). Water exchange occurs through at least five inlets. The latter is part of the Po Delta lagoon system and it is c. 26 km2 wide with a high shellfish farming density. It has good seawater exchange even though it is heavily influenced by freshwaters.
The Lesina Lagoon (Fig. 1F) is located in the Southern Adriatic Sea with a surface of c. 51 km2. Although it is often considered a lake, it has two narrow and long inlets which connect it with the sea and hence it contains salty water. It receives minor freshwater inputs and its natural resources are scarcely exploited.
The Orbetello Lagoon (Fig. 1C) is located in the Tyrrhenian Sea with a surface of c. 27 km2. It is an almost closed basin with poor seawater circulation through three artificial inlets. The lagoon receives huge amounts of urban wastewaters from a highly touristed area and it has c. 100 fishermen making their living on the lagoon resources.
The Mar Piccolo of Taranto (Fig. 1G) is located in the Ionian Sea; its surface is c. 21 km2. It has a good water renewal and it is generally considered a marine bay. Most of the freshwater inputs come from submarine springs.
Material and methods
Sampling descriptions
Data on the main environmental factors and on the phytoplankton abundance, biomass and taxonomic composition were collected in the lagoon of Venice from 1998 to 2003. The sampling stations were located in submerged areas, out of the main canals, where the mean water depth is c. 1 m. Monthly campaigns were carried out in 10 stations located in the central area (Fig. 1E): Alberoni (St. B), Sacca Sessola (St. E), San Giuliano (St. H) and Fusina (St. F) from November 1998 to October 1999 (Facca et al. 2002), Santa Maria del Mare (St. A), Lido (St. D) and San Giuliano (St. I) from June 2000 to May 2001, and San Nicolò (St. C), Celestia (St. L) and Tresse (St. G) from July 2001 to June 2002 (Facca et al. 2004). In addition, 67 stations were sampled in the central part of the lagoon during the last week of June 2003, 79 in the southern area during the first 10 days of July 2003 and 19 in July 2003 in the northern area.
In the Sacca di Goro and Lesina Lagoon, two sampling campaigns were carried out in May and July 2004. A campaign was carried out in the Orbetello Lagoon in July 2005, one in the Mar Piccolo of Taranto in July 2006 and another in the Marano-Grado Lagoon in July 2007 (Table 1).
| Site acronyms | Lagoon | Salinity | Period | Frequency | No. of observations |
|---|---|---|---|---|---|
| A | Venice | E | From June 2000 to May 2001 | Monthly | 12 |
| B | Venice | E | From November 1998 to October 1999 | Monthly | 12 |
| C | Venice | E | From July 2001 to June 2002 | Monthly | 12 |
| D | Venice | E | From June 2000 to May 2001 | Monthly | 12 |
| E | Venice | E | From November 1998 to October 1999 | Monthly | 12 |
| F | Venice | P | From November 1998 to October 1999 | Monthly | 12 |
| G | Venice | P | From July 2001 to June 2002 | Monthly | 12 |
| H | Venice | P | From November 1998 to October 1999 | Monthly | 12 |
| I | Venice | P | From June 2000 to May 2001 | Monthly | 12 |
| L | Venice | E | From July 2001 to June 2002 | Monthly | 12 |
| * | Northern Venice | Both | July 2003 | Once | 19 |
| * | Central Venice | Both | June/July 2003 | Once | 67 |
| * | Southern Venice | Both | July 2003 | Once | 79 |
| G1 | Goro | P | May and July 2004 | Twice | 2 |
| G2 | Goro | P | May and July 2004 | Twice | 2 |
| G3 | Goro | P | May and July 2004 | Twice | 2 |
| G4 | Goro | P | May and July 2004 | Twice | 2 |
| L1 | Lesina | P | May and July 2004 | Twice | 2 |
| L2 | Lesina | P | May and July 2004 | Twice | 2 |
| L3 | Lesina | P | May and July 2004 | Twice | 2 |
| L4 | Lesina | P | May and July 2004 | Twice | 2 |
| O1 | Orbetello | P | July 2005 | Once | 1 |
| O2 | Orbetello | P | July 2005 | Once | 1 |
| O3 | Orbetello | P | July 2005 | Once | 1 |
| O4 | Orbetello | P | July 2005 | Once | 1 |
| T1 | Taranto | E | July 2006 | Once | 1 |
| T2 | Taranto | E | July 2006 | Once | 1 |
| 1 | Marano-Grado | P | July 2007 | Once | 1 |
| 2 | Marano-Grado | P | July 2007 | Once | 1 |
| 3 | Marano-Grado | P | July 2007 | Once | 1 |
| 4 | Marano-Grado | P | July 2007 | Once | 1 |
| 5 | Marano-Grado | P | July 2007 | Once | 1 |
| 6 | Marano-Grado | P | July 2007 | Once | 1 |
| 7 | Marano-Grado | P | July 2007 | Once | 1 |
| 8 | Marano-Grado | E | July 2007 | Once | 1 |
| 9 | Marano-Grado | E | July 2007 | Once | 1 |
| 10 | Marano-Grado | P | July 2007 | Once | 1 |
| 12 | Marano-Grado | E | July 2007 | Once | 1 |
| 13 | Marano-Grado | E | July 2007 | Once | 1 |
| 14 | Marano-Grado | E | July 2007 | Once | 1 |
| 15 | Marano-Grado | E | July 2007 | Once | 1 |
| 16 | Marano-Grado | E | July 2007 | Once | 1 |
| 17 | Marano-Grado | E | July 2007 | Once | 1 |
| 18 | Marano-Grado | P | July 2007 | Once | 1 |
| 19 | Marano-Grado | E | July 2007 | Once | 1 |
| 20 | Marano-Grado | P | July 2007 | Once | 1 |
- *During the June/July 2003 campaign, samples were collected in 165 sites in the whole lagoon but for reasons of space, the sites are not listed separately.
The SURFER mapping system (SURFER v. 7.02; Golden Software 2000, Golden, CO) was used to draw maps using the kriging method. Such data give a representation of the average summer conditions in the lagoons of Venice and Marano-Grado.
Environmental variables
Temperature, pH, redox potential, salinity, nutrient and chlorophyll concentrations, suspended particulate matter (FPM) and oxygen saturation were measured according to Facca et al. (2002). Water transparency was estimated by means of the Secchi disk. Because of the shallowness and the tidal changes, results are expressed as percentage of the water column visibility: 100% means that the Secchi disk was visible at the bottom level, whereas 50% means that it disappeared halfway through the water column.
At each station five to six water column aliquots were manually collected by means of a Plexiglas pipe (4 cm wide and 150 cm high) and carefully mixed to produce a sample representative of the entire water column. The pipe was immerged in the water column so that the sample was collected from the surface to 10/15 cm above the bottom. Particular attention was paid to avoiding the water/sediment interface.
Phytoplankton was estimated as cell abundance using Utermöhl’s method (Utermöhl 1958). Known aliquots (5–25 ml) of water preserved with formaldehyde solution neutralised with hexamethylenetetramine were allowed to settle in combined chambers after manual mixing. Depending on the settled volume, the sample analysis was performed 12–48 h later. The taxonomic identification of diatoms was carried out according to Peragallo & Peragallo (1897–1908), Hustedt (1927–1966), Tomas (1996), Vanlandingham (1967–1979) and Round et al. (1990). The other taxonomic groups were mainly identified following Tomas (1993). During the inverted light microscope analysis, cell sizes were measured to estimate carbon content (biomass) according to Edler’s formulation (Edler 1979). The chlorophyll a distribution is reported in Sfriso & Facca (2007).
Statistical analyses
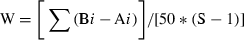
Examples of biomass and abundance dominance curves. The W-statistic minimum (A), zero (B) and maximum (C) values are plotted.
To attempt to meet WFD requirements (final score between 0 and 1; identification of reference conditions), the W-statistic values were normalized and referred to reference conditions. The choice of the reference sites was difficult because the studied ecosystems are very heterogeneous and polymorphous and often the literature on their environmental conditions and changes is poor. To facilitate this task the lagoons were grouped together in relation to salinity values, as suggested by the WFD. Two macro-areas were identified: polyhaline (salinity between 18 and 30) and euhaline (salinity >30). Polyhaline areas are mainly represented by Lesina Lagoon, Sacca di Goro and by the inner stations in the Venice and Marano-Grado Lagoons (Table 1). It is possible to find good water quality in Lesina Lagoon (Giordani et al. 2009; Sfriso et al. 2009), but most of the other sites display poor water quality because of the anthropic impact and freshwater inputs. Areas characterised by euhaline waters may have high water renewal, such as Mar Piccolo of Taranto and the seaward stations in the lagoons of Venice and Marano-Grado, or high evaporation rates, such as Orbetello Lagoon (Specchiulli et al. 2008) and the northern basin of the Venice Lagoon (Table 1).
For each of the two macro-areas the highest W-statistic values were used to normalize the results in the range between 0 and 1, to obtain the Ecological Quality Ratio (EQR). Each station was then classified according to the area it belonged to – polyhaline or euhaline. The range between 0 and 1 was subdivided into five equivalent classes (Bad: 0–0.20; Poor: 0.21–0.40; Moderate: 0.41–0.60; Good: 0.61–0.80; High: 0.81–1.0).
Pearson’s correlation coefficients and principal component analysis (PCA) were used to highlight the relationship between the physico-chemical variables and the W-statistic modified in the EQR. Those analyses were performed on the richest datasets (Venice and Marano-Grado); few observations were available for the other lagoons. The significant values were accepted when P < 0.05.
Results
Phytoplankton taxonomic composition and abundance
In all, 231 taxa were identified in the lagoons. They belonged to the divisions Bacillariophyta, Chlorophyta, Chromophyta and Dinophyta. Taxa were identified at least to genus level in 220 taxa. Their class distribution was as follows: 107 Bacillariophyceae, 51 Coscinodiscophyceae, 29 Fragilariophyceae (according to the classification in Round et al. 1990), 26 Dinophyceae, five Euglenophyceae, one Cryptophyceae and one Prasinophyceae. The other taxa were identified to class level, whereas the nanoflagellates do not represent a taxonomic group, as they are small (<5 μm) spherical cells whose classification is not possible using conventional optical microscopy. That group was very abundant in terms of cell abundance; in some cases, it represented up to 94% of the total phytoplankton, but in terms of biomass it was always <7%. Dinophyceae cell abundance was negligible (<1%) in Venice, Marano-Grado, Taranto and Lesina, c. 6% in Goro and close to 20% in Orbetello. In the Venice Lagoon the dinoflagellates were recorded only near the inlets. Table 2 shows the taxa with an abundance of >1% occurring in at least three lagoons. The taxa recorded for all the lagoons were 10 diatoms and one dinoflagellate; of them, only Cylindrotheca closterium abundance exceeded 1% everywhere.
| Venice | Marano | Goro | Lesina | Orbetello | ||
|---|---|---|---|---|---|---|
| Bacillariophyta | ||||||
| Amphiprora paludosa | Smith | 2/6/7/12 | 7 | 5/7 | ||
| Amphora exigua | Gregory | 1–12 | 7 | 5 | 5/7 | 7 |
| Amphora veneta | Kützing | 1–12 | 7 | 5/7 | ||
| Cerataulina dentata | Hasle | 5 | ||||
| Cerataulina pelagica | Hasle | 1/6/7/8 | 7 | 5/7 | ||
| Chaetoceros curvisetus | Cleve | 7 | ||||
| Chaetoceros mitra | Cleve | 7/8/10 | 7 | 7 | ||
| Chaetoceros simplex | Ostenfeld | 1/2/4/5 | 7 | |||
| Chaetoceros socialis | Lauder | 1–12 | 7 | 5/7 | 5/7 | 7 |
| Cocconeis molesta | Kützing | 1–12 | 7 | 5/7 | 5/7 | 7 |
| Cocconeis scutellum | Ehrenberg | 1–12 | 7 | 5 | 5/7 | 7 |
| Cylindrotheca closterium | Reimann et Lewin | 1–12 | 7 | 5/7 | 5/7 | 7 |
| Fragilaria sp. | 1–12 | 5 | ||||
| Gyrosigma attenuatum | Cleve | 7 | ||||
| Navicula arenaria | Donkin | 2/3/8/10 | 7 | 7 | ||
| Navicula cryptocephala | Kützing | 1–12 | 7 | 5/7 | 5/7 | 7 |
| Navicula lanceolata | Kützing | 1–12 | 7 | 5/7 | 5/7 | 7 |
| Navicula sp. <10 μm | 1–12 | 7 | 5/7 | 5/7 | 7 | |
| Navicula sp. <30 μm | 1–12 | 7 | 5/7 | 5/7 | ||
| Nitzschia dissipata | Grunow | 3/4/5/7/9/10/12 | 7 | 5 | 7 | |
| Nitzschia frustulum | Grunow | 1–12 | 7 | 5/7 | 5/7 | |
| Nitzschia lanceolata | Smith | 1–12 | 7 | 7 | 5/7 | |
| Nitzschia longissima | Grunow | 1–9/11/12 | 7 | 7 | 7 | |
| Nitzschia microcephala | Grunow | 1/4–11 | 7 | 7 | 7 | |
| Nitzschia sigma | Smith | 1/3–7/11/12 | 7 | 7 | ||
| Nitzschia sp. <15 μm | 1–12 | 7 | 5/7 | 5/7 | 7 | |
| Nitzschia sp. <30 μm | 1–12 | 7 | 7 | 7 | ||
| Psammodictyon panduriforme | Mann | 1/4/5/6/9/10/12 | 7 | 7 | ||
| Skeletonema marinoi | Sarno et Zingone | 1–12 | 7 | 5/7 | 7 | |
| Thalassionema nitzschioides | Van Heurck | 1–7/9–12 | 7 | 5/7 | 7 | |
| Thalassiosira sp. | 1–12 | 7 | 5/7 | 5/7 | 7 | |
| Chlorophyta | ||||||
| Indeterminate Chlorophyceae | 1–12 | 7 | 5/7 | 5/7 | 7 | |
| Euglena acusformis | Schiller | 7 | 7 | |||
| Eutreptia pertyi | Pringsheim | 7 | ||||
| Micromonas sp. | 4–10 | |||||
| Chromophyta | ||||||
| Indeterminate Cryptophyceae | 1–12 | 7 | 5/7 | 5/7 | 7 | |
| Indeterminate Prymnesiophyceae | 1–12 | 5 | ||||
| Dinophyta | ||||||
| Alexandrium insuetum | Balech | 2/6/9 | 7 | 7 | ||
| Alexandrium sp. | 5/9/12 | 7 | 7 | |||
| Gyrodinium estuariale | Hulburt | 3/5/7–10 | 7 | 7 | 7 | |
| Protoperidinium pellucidum | Bergh | 7 | 7 | |||
| Protoperidinium sp. | 1/8 | |||||
| Scripsiella trochoidea | Loeblich | 7 | 7 | |||
| Indeterminate Dinophyceae | 2/4–7/11 | 7 | 5/7 | 7 | ||
| Nanoflagellates | ||||||
| Indeterminate Nanoflagellates | 1–12 | 7 | 5/7 | 5/7 | 7 | |
- For each lagoon the sampling month is reported as a number: January is 1, February 2, etc. The slash (/) means ‘and’ and the dash (–) indicates ‘from…to’.
In summer 2003, in the Venice Lagoon, Thalassiosira sp. reached up to 30 × 106 cells·l−1 (79% of the phytoplankton community in that site) near the fishing ponds in the southern lagoon (Fig. 3C), whereas Nitzschia frustulum bloomed in the northern lagoon (Fig. 3A) with values up to 72 × 106 cells·l−1 (89% of phytoplankton community in that site). In the rest of the lagoon, values >106 cells·l−1 were observed only along the mainland and close to urban centres (Chioggia and Lido). The biomass (in term of carbon content) ranged from 3.1 to 1148 μg·C·l−1, displaying the highest values along the mainland, with a gradient quite similar to the abundance gradient (Fig. 3A–C).
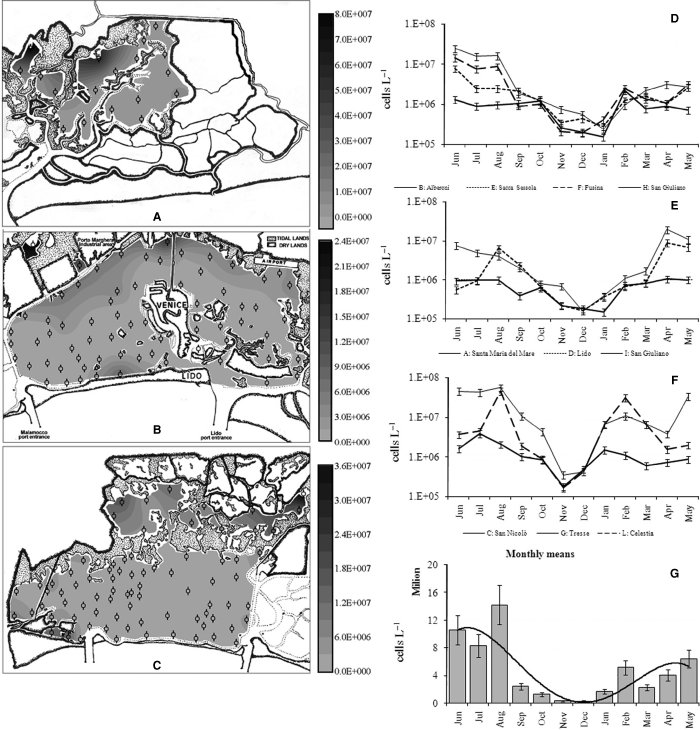
The maps of the northern (A), central (B) and southern (C) Venice Lagoon show the total cell abundance (cells l−1) distribution in summer 2003. The black marks in each map indicate the site locations. The cell abundance seasonal trends in the Venice Lagoon are plotted in graphs D (sites sampled in 1998–1999), E (sites sampled in 2000–2001) and F (sites sampled in 2001–2002). The cell abundance means for each month are represented in graph G.
In the Venice Lagoon, Skeletonema marinoi (Biddulphiales) bloomed in late winter (February and March), then Chaetoceros spp. (c. 4 × 106 cells·l−1) appeared in April/May and N. frustulum (55.9 × 106 cells·l−1) and C. closterium (20.5 × 106 cells·l−1) dominated during summer (Figs 2D, F). In September, cell abundances decreased drastically. The monthly mean abundance (Fig. 3G) calculated in the 10 stations, highlighted that the number of cells hardly exceeded 106 cells·l−1 in the period from October to January. Some taxa constituted a constant background presence in the community, such as Navicula cryptocephala and Navicula lanceolata, whereas others (such as Cryptophyceae or Micromonas sp.) were occasionally dominant. Large pelagic and colonial diatoms occurred only near the inlets, whereas the inner areas were characterised by small benthic species. The abundance increased while moving from the sea to the mainland, as shown by both map distribution and seasonal values (Fig. 3). The biomass trends were quite similar to those of abundance (Fig. 4D–G). The minimum (2.1 μg·C·l−1) and maximum (844 μg·C·l−1) biomass values were both recorded at St. L (Fig. 4F).
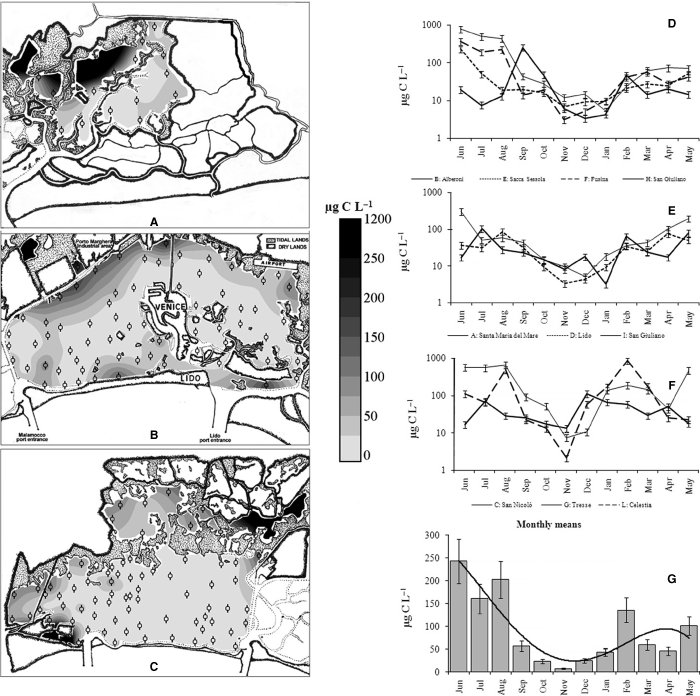
The maps of the Northern (A), Central (B) and Southern (C) Venice Lagoon show the biomass (μg·C·l−1) distribution in summer 2003. The black marks in each map indicate the site locations. The biomass seasonal trends in the Venice Lagoon are plotted in graphs D (sites sampled in 1998–1999), E (sites sampled in 2000–2001) and F (sites sampled in 2001–2002). The biomass means for each month are represented in graph G.
In the Marano-Grado Lagoon, most of the stations displayed cell abundances between 0.6 and 2.2 × 106 cells·l−1. Blooms up to 18 × 106 cells·l−1 were only recorded close to the main river outflows (Fig. 5A). The blooming species were C. closterium and to a minor extent N. frustulum. The biomass ranged between 9.4 and 68.8 μg·C·l−1 in the stations with the lowest abundance and it peaked at 217 μg·C·l−1 in the areas with C. closterium (Fig. 5B).
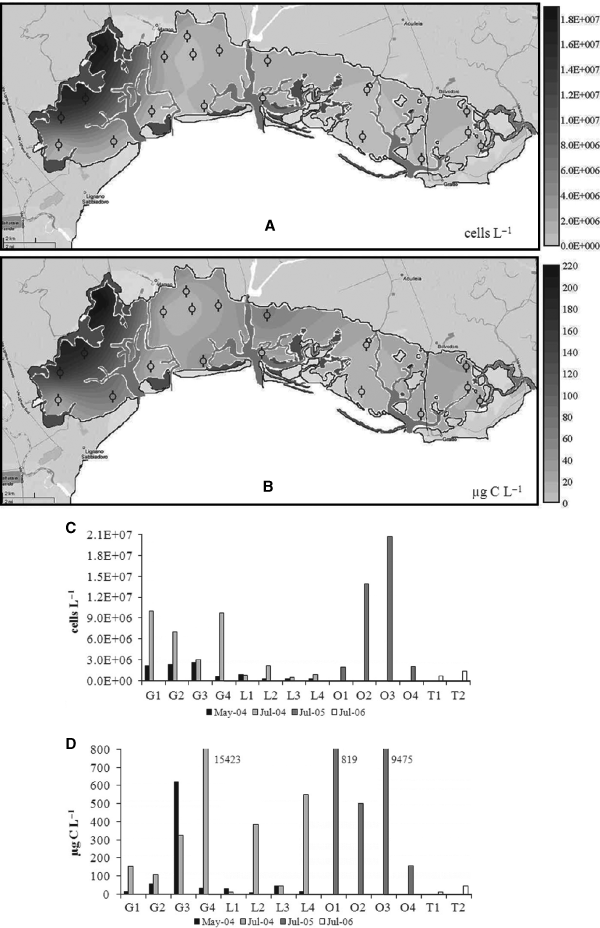
Map A shows the cell abundance distribution in the Marano-Grado Lagoon (July 2007) and map B the biomass. In the histograms the phytoplankton cell abundance (C) and biomass (D) in Sacca di Goro (stations with G), Lesina (stations with L), Orbetello (stations with O) and Taranto (stations with T) lagoons are presented. The series are arranged according to the sampling dates.
A high variability in terms of both cell abundance and biomass was observed in the other lagoons (Fig. 5C, D). At Lesina the variations in cell abundance were less evident, but marked biomass increases were observed at the sites L2 and L4 in July 2004, due to the blooms of large size Coscinodiscus, Eutreptia and some Dinophyceae. High biomass fluctuations were also recorded at Goro, where the occurrence of Protoperidinium pellucidum was determined as >15 mg·C·l−1. The Orbetello Lagoon also displayed important Dinophyceae biomasses, with values up to 9.5 mg·C·l−1.
Biomass-abundance curves as water quality indicators
At the beginning, two datasets were considered to calculate the W-statistic and hence the EQR: one including only diatom species and the other using both diatoms and dinoflagellates. To evaluate which elaboration better fitted the ecological status of the environments, results were compared with those obtained by Sfriso et al. (2009), who applied the Macrophyte Quality Index to the same stations and in the same period. The comparison was made using the data from the Sacca di Goro (polyhaline) and the Orbetello Lagoon (euhaline), where some Dinophyceae blooms occurred (Table 3). Even though a clear pattern in the EQR variations was not evident, most of the observations were comparable with those of Sfriso et al. (2009) when only diatoms were considered. We preferred to base EQR only on diatom abundance and biomass because: (i) dinoflagellates are generally negligible in the study areas, (ii) some species are potentially harmful and hence may damage the environmental equilibrium and represent a risk to human health, and (iii) their inclusion in the elaboration may lead to an overestimation of the water quality (Table 3).
| Dinoflagellate relative abundance (%) | Dinoflagellate relative biomass (%) | W-statistic (only diatoms) | EQR (only diatoms) | Quality class (only diatoms) | W-statistic (diatoms + dinoflagellates) | EQR (diatoms + dinoflagellates) | Quality class (diatoms + dinoflagellates) | MAQI (Sfriso et al. 2009) | |
|---|---|---|---|---|---|---|---|---|---|
| Orbetello (confined basin) | |||||||||
| O1 | 63.2 | 95.9 | 0.332 | 0.833 | High | 0.294 | 0.795 | Good | Good |
| O2 | 1.12 | 5.23 | 0.028 | 0.528 | Moderate | 0.010 | 0.510 | Moderate | Poor |
| O3 | 44.4 | 97.5 | 0.063 | 0.563 | Moderate | 0.287 | 0.788 | Good | Bad |
| O4 | 64.3 | 77.0 | 0.283 | 0.784 | Good | 0.076 | 0.576 | Moderate | Good |
| Goro (non-confined basin) | |||||||||
| G1 | 0.00 | 0.00 | 0.039 | 0.540 | Moderate | 0.039 | 0.540 | Moderate | Bad |
| G2 | 0.52 | 6.66 | −0.046 | 0.453 | Moderate | −0.097 | 0.400 | Poor | Poor |
| G3 | 17.3 | 89.3 | 0.016 | 0.516 | Moderate | 0.352 | 0.862 | High | Poor |
| G4 | 38.5 | 99.2 | −0.077 | 0.421 | Moderate | 0.277 | 0.785 | Good | Poor |
The highest W-statistic values, used as reference conditions, were recorded for both polyhaline and euhaline waters in the Venice Lagoon (−0.486 and −0.498, respectively). For polyhaline waters a similar but slightly lower value was observed in the Lesina Lagoon.
By applying the EQR to the Venice Lagoon (data collected in summer 2003; Fig. 6A), results show that ‘Good’ and ‘High’ quality areas were mainly concentrated in the proximity of the sea-inlets, along the main canals and in the southern basin. Most of the northern part of the lagoon was assigned ‘Poor’ quality status, whereas the sites close to the industrial area and urban discharges shifted from a ‘Poor’ to a ‘Moderate’ status. Also in the southern basin, several sites between ‘Poor’ and ‘Moderate’ were recorded above all close to Chioggia centre and the fishing areas (Orel et al. 2000). However, this was a single determination and we have to take into consideration that, on an annual basis, important fluctuations occur. Depending on the sampling period the planktonic diatom communities shifted from one quality class to another. However, the mean EQR values for the 10 stations, which were sampled monthly for 1 year and which are representative of the central lagoon conditions, showed fluctuations in the ‘Moderate’ class (Fig. 7A). Similarly, averaging EQR for each site, it was possible to observe variations in the ‘Moderate’ class (Fig. 7B).
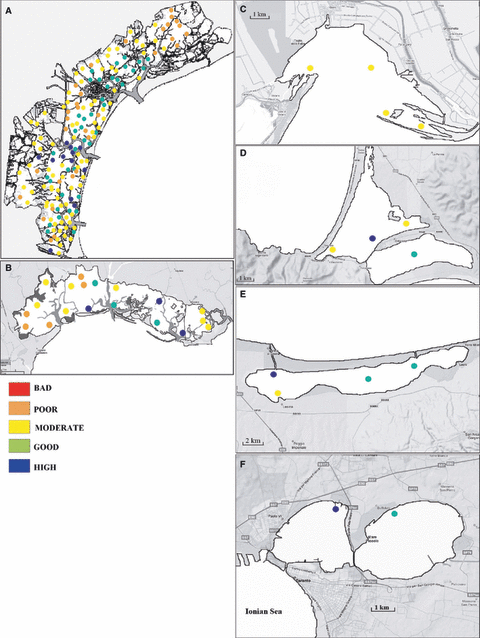
Lagoon assessment on the basis of the Ecological Quality Ratio (EQR) calculated starting from W-statistic values of diatom abundance and biomass. The data used for all lagoons were collected in July. (A) Venice Lagoon; (B) Marano-Grado Lagoon; (C) Sacca di Goro; (D) Orbetello Lagoon; (E) Lesina Lagoon; (F) Mar Piccolo of Taranto.

Graph A presents the monthly mean values of the 10 stations which were sampled seasonally in the Venice Lagoon. Graph B shows the annual mean of the 10 stations which were sampled seasonally in the Venice Lagoon.
In the Marano-Grado Lagoon, ‘Good/High’ conditions were mainly recorded along the inlets, whereas the areas with freshwater outflows were characterised by ‘Poor’ to ‘Moderate’ (Fig. 6B). Sacca di Goro was classified as ‘Moderate’ (Fig. 6C), Orbetello alternated between ‘High’ and ‘Moderate’ (Fig. 6D), Lesina was mainly characterised by ‘Good/High’ (Fig. 6E) and the Mar Piccolo of Taranto displayed ‘High/Good’ status (Fig. 6F).
In Table 4, abiotic data synoptically collected with the diatom samples are reported. The data displayed a high variability in all the sampling sites. For example, in the Venice Lagoon, dissolved inorganic nitrogen (DIN) concentrations varied between 0.66 and 86.5 μm and reactive phosphorus between 0.10 and 11.2 μm. Despite such significant fluctuation, the EQR values were mainly correlated with water transparency (Secchi disk extinction depth), reactive phosphorus, nitrates and salinity (Table 5). Examples of the relationship between EQR and the environmental variables are also displayed by PCA in Fig. 8. The EQR loadings were in the same direction as water transparency and salinity, whereas they were in the opposite direction to nutrient concentrations.
| T°C | pH | Eh | S | O2 | Secchi Disk | FPM | Si-SiO4 | P-PO4 | N-NH4 | N-NO2 | N-NO3 | DIN | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | mg·l−1 | μm | μm | μm | μm | μm | μm | |||||
| Venice St. A (12 monthly observations) | |||||||||||||
| Mean | 18.3 | 8.52 | 363 | 33.7 | 138 | 100 | 23.6 | 4.29 | 0.69 | 6.80 | 0.83 | 6.68 | 14.3 |
| SD | 7.50 | 0.30 | 37.4 | 1.4 | 40.9 | 0.00 | 14.3 | 2.59 | 0.61 | 6.02 | 0.47 | 6.94 | 7.31 |
| Min | 6.90 | 7.77 | 305 | 31.0 | 90.8 | 100 | 8.70 | 1.06 | 0.13 | 1.50 | 0.31 | 0.14 | 4.91 |
| Max | 27.7 | 8.98 | 420 | 35.9 | 205 | 100 | 53.0 | 10.3 | 2.35 | 17.4 | 2.06 | 21.1 | 25.8 |
| Venice St. B (12 monthly observations) | |||||||||||||
| Mean | 15.8 | 8.20 | 312.9 | 32.5 | 131 | 100 | 50.8 | 6.8 | 0.45 | 3.69 | 0.92 | 11.1 | 15.8 |
| SD | 7.95 | 0.14 | 27.4 | 2.1 | 15.3 | 0.00 | 19.7 | 3.67 | 0.25 | 3.02 | 0.57 | 6.02 | 6.77 |
| Min | 4.60 | 8.03 | 287 | 28.5 | 113 | 100 | 27.0 | 3.04 | 0.14 | 0.13 | 0.30 | 3.00 | 5.70 |
| Max | 28.2 | 8.57 | 370 | 35.8 | 162 | 100 | 85.0 | 14.6 | 0.91 | 10.4 | 1.93 | 22.00 | 26.3 |
| Venice St. C (12 monthly observations) | |||||||||||||
| Mean | 17.5 | 8.01 | 326 | 33.7 | 128 | 97.3 | 29.6 | 4.66 | 0.75 | 9.82 | 0.66 | 7.82 | 18.3 |
| SD | 7.60 | 0.19 | 52.1 | 1.7 | 25.9 | 6.32 | 12.1 | 5.53 | 0.65 | 10.7 | 0.37 | 3.31 | 11.7 |
| Min | 6.40 | 7.70 | 220 | 30.9 | 100 | 83.3 | 13.6 | 0.95 | 0.10 | 3.08 | 0.17 | 1.34 | 7.12 |
| Max | 28.2 | 8.40 | 392 | 36.5 | 180 | 100.0 | 51.2 | 17.2 | 2.00 | 35.6 | 1.65 | 14.4 | 45.2 |
| Venice St. D (12 monthly observations) | |||||||||||||
| Mean | 17.7 | 8.23 | 347 | 32.7 | 137 | 90.2 | 32.5 | 4.84 | 0.74 | 8.02 | 1.02 | 9.37 | 18.4 |
| SD | 8.86 | 0.15 | 42.1 | 2.0 | 41.6 | 14.6 | 18.9 | 3.14 | 0.53 | 4.85 | 0.47 | 9.25 | 8.95 |
| Min | 6.40 | 7.89 | 268.0 | 28.8 | 86.9 | 60.0 | 10.1 | 1.14 | 0.11 | 1.20 | 0.16 | 0.57 | 7.63 |
| Max | 29.1 | 8.45 | 410 | 35.5 | 215 | 100.0 | 73.8 | 9.42 | 2.11 | 17.9 | 1.64 | 26.1 | 36.6 |
| Venice St. E (12 monthly observations) | |||||||||||||
| Mean | 15.9 | 8.18 | 305 | 31.4 | 133 | 87.5 | 66.2 | 9.48 | 0.62 | 8.02 | 1.36 | 14.5 | 24.0 |
| SD | 8.05 | 0.18 | 32.7 | 2.7 | 25.3 | 25.0 | 33.3 | 5.97 | 0.39 | 7.31 | 0.75 | 6.37 | 8.05 |
| Min | 3.40 | 7.94 | 270 | 26.0 | 102 | 25.0 | 26.4 | 2.63 | 0.23 | 1.69 | 0.28 | 3.00 | 8.60 |
| Max | 28.3 | 8.57 | 367 | 35.0 | 181 | 100.0 | 133.5 | 20.3 | 1.51 | 26.3 | 2.97 | 26.0 | 34.8 |
| Venice St. F (12 monthly observations) | |||||||||||||
| Mean | 17.4 | 8.00 | 300 | 29.9 | 119 | 63.8 | 72.0 | 12.1 | 1.07 | 14.7 | 1.71 | 20.1 | 35.1 |
| SD | 6.85 | 0.22 | 34.8 | 2.6 | 15.9 | 22.3 | 25.7 | 5.58 | 0.46 | 9.46 | 0.77 | 6.28 | 8.73 |
| Min | 7.30 | 7.68 | 260 | 25.5 | 96.0 | 35.7 | 29.2 | 4.62 | 0.31 | 3.69 | 0.72 | 9.00 | 17.4 |
| Max | 28.2 | 8.50 | 371 | 33.4 | 149 | 100.0 | 123.0 | 21.2 | 1.83 | 36.7 | 3.25 | 27.0 | 47.4 |
| Venice St. G (12 monthly observations) | |||||||||||||
| Mean | 18.1 | 7.93 | 325 | 29.6 | 107 | 63.9 | 51.6 | 8.69 | 1.85 | 17.8 | 1.95 | 20.8 | 40.5 |
| SD | 7.36 | 0.22 | 54.5 | 2.2 | 26.4 | 24.0 | 24.7 | 7.63 | 3.01 | 11.1 | 0.68 | 10.1 | 17.3 |
| Min | 6.80 | 7.65 | 244 | 25.5 | 70.0 | 20.0 | 25.2 | 0.55 | 0.11 | 4.33 | 1.25 | 0.32 | 10.4 |
| Max | 29.2 | 8.31 | 402 | 32.9 | 157 | 100.0 | 112.9 | 26.5 | 11.18 | 44.7 | 3.45 | 35.0 | 76.8 |
| Venice St. H (12 monthly observations) | |||||||||||||
| Mean | 15.1 | 8.18 | 322 | 26.2 | 113 | 72.9 | 67.7 | 22.7 | 0.86 | 19.0 | 2.34 | 20.4 | 41.8 |
| SD | 7.42 | 0.29 | 42.5 | 4.7 | 17.1 | 22.7 | 23.4 | 28.6 | 0.60 | 18.5 | 1.53 | 9.53 | 24.7 |
| Min | 4.40 | 7.58 | 280 | 18.6 | 85.0 | 40.9 | 27.0 | 2.10 | 0.31 | 3.25 | 0.14 | 2.00 | 9.00 |
| Max | 26.7 | 8.68 | 403 | 34.7 | 149 | 100.0 | 112.6 | 105 | 2.48 | 53.6 | 5.27 | 32.0 | 86.5 |
| Venice St. I (12 monthly observations) | |||||||||||||
| Mean | 17.5 | 8.12 | 365 | 28.8 | 94.3 | 57.8 | 65.5 | 9.77 | 1.46 | 11.9 | 1.49 | 18.2 | 31.6 |
| SD | 8.48 | 0.17 | 21.5 | 3.0 | 17.9 | 29.4 | 60.1 | 6.81 | 0.70 | 5.05 | 0.52 | 11.1 | 13.8 |
| Min | 6.40 | 7.86 | 335 | 23.8 | 61.5 | 16.7 | 12.4 | 0.95 | 0.58 | 5.80 | 0.91 | 2.84 | 9.73 |
| Max | 29.0 | 8.34 | 403 | 32.5 | 124 | 100.0 | 229.1 | 22.0 | 2.62 | 23.2 | 2.53 | 40.5 | 56.0 |
| Venice St. L (12 monthly observations) | |||||||||||||
| Mean | 17.8 | 7.97 | 322 | 32.4 | 114 | 77.6 | 39.4 | 6.52 | 0.93 | 9.11 | 0.87 | 7.43 | 17.4 |
| SD | 8.12 | 0.22 | 52.8 | 1.7 | 24.9 | 22.6 | 21.1 | 6.11 | 1.05 | 7.84 | 0.40 | 4.02 | 7.28 |
| Min | 6.00 | 7.69 | 252 | 28.4 | 68.9 | 23.1 | 12.9 | 0.59 | 0.17 | 2.64 | 0.13 | 1.17 | 3.94 |
| Max | 28.5 | 8.33 | 399 | 35.0 | 160 | 100.0 | 88.0 | 17.6 | 3.58 | 31.2 | 1.80 | 13.6 | 33.7 |
| Venice polyhaline area (20 observations in summer 2003) | |||||||||||||
| Mean | 14.8 | 6.10 | 264 | 22.5 | 86.8 | 54.5 | 1.97 | 16.8 | 1.58 | 14.3 | 30.0 | ||
| SD | 9.24 | 3.51 | 141 | 12.4 | 48.7 | 33.3 | 2.68 | 15.0 | 1.35 | 12.6 | 25.0 | ||
| Min | 4.40 | 0.17 | 21.5 | 1.70 | 17.1 | 16.7 | 0.11 | 2.64 | 0.13 | 0.32 | 3.94 | ||
| Max | 29.2 | 8.7 | 403.0 | 35.0 | 160 | 100 | 11.2 | 53.6 | 5.27 | 40.5 | 86.5 | ||
| Venice euhaline area (145 observations in summer 2003) | |||||||||||||
| Mean | 27.4 | 8.23 | 341 | 34.3 | 116 | 91.0 | 0.18 | 2.78 | 0.37 | 4.16 | 7.28 | ||
| SD | 1.79 | 0.53 | 20.3 | 2.35 | 29.6 | 16.8 | 0.17 | 2.20 | 0.22 | 3.97 | 5.53 | ||
| Min | 24.0 | 7.48 | 282 | 30.0 | 15.7 | 31.3 | 0.01 | 0.20 | 0.11 | 0.22 | 0.66 | ||
| Max | 32.2 | 9.33 | 378 | 43.1 | 214 | 100 | 1.75 | 16.2 | 1.24 | 17.4 | 26.2 | ||
| Marano-Grado polyhaline area (10 observations in summer 2007) | |||||||||||||
| Mean | 26.7 | 9.79 | 0.24 | 9.21 | 0.58 | 16.8 | 26.6 | ||||||
| SD | 3.87 | 4.62 | 0.23 | 4.54 | 0.26 | 14.0 | 15.9 | ||||||
| Min | 19.6 | 3.85 | 0.13 | 3.34 | 0.33 | 4.59 | 8.26 | ||||||
| Max | 30.0 | 14.8 | 0.87 | 18.6 | 1.01 | 51.1 | 63.8 | ||||||
| Marano-Grado euhaline area (9 observations in summer -2007) | |||||||||||||
| Mean | 33.4 | 7.2 | 0.1 | 8.3 | 0.3 | 10.5 | 19.1 | ||||||
| SD | 2.03 | 3.05 | 0.06 | 4.50 | 0.15 | 13.6 | 16.7 | ||||||
| Min | 30.2 | 3.70 | 0.04 | 3.63 | 0.18 | 3.09 | 7.09 | ||||||
| Max | 35.8 | 14.3 | 0.27 | 17.0 | 0.68 | 45.9 | 60.3 | ||||||
| Sacca di Goro (3 observations in summer 2005) | |||||||||||||
| Mean | 25.3 | 8.13 | 318 | 23.7 | 202 | 83.3 | 26.9 | 0.36 | 6.92 | 1.38 | 12.3 | 20.7 | |
| SD | 1.93 | 0.16 | 48.3 | 3.006 | 41.6 | 19.1 | 12.8 | 0.19 | 1.89 | 0.97 | 8.18 | 7.63 | |
| Min | 23.0 | 7.82 | 256 | 19.9 | 155 | 54.5 | 10.0 | 0.23 | 3.97 | 0.40 | 3.70 | 11.1 | |
| Max | 27.5 | 8.33 | 391 | 29.0 | 261 | 100 | 50.1 | 0.81 | 9.75 | 3.41 | 25.4 | 30.6 | |
| Lesina (8 observations in summer 2005) | |||||||||||||
| Mean | 23.7 | 8.56 | 256 | 16.5 | 131 | 87.6 | 38.8 | 0.44 | 12.7 | 0.51 | 10.2 | 23.4 | |
| SD | 2.30 | 0.81 | 17.6 | 4.13 | 60.1 | 26.1 | 48.5 | 0.42 | 11.0 | 0.34 | 15.8 | 22.2 | |
| Min | 21.1 | 7.72 | 230 | 11.5 | 0.00 | 25.0 | 4.62 | 0.11 | 3.90 | 0.18 | 0.79 | 5.02 | |
| Max | 26.3 | 9.93 | 281 | 23.0 | 214 | 100 | 117 | 1.11 | 35.8 | 1.06 | 41.6 | 65.2 | |
| Venice | |||||
|---|---|---|---|---|---|
| Lagoon | Central | Southern | Northern | Seasonal trends | Grado-Marano |
| No. of cases | 67 | 79 | 19 | 120 | 19 |
| Temperature | −0.24 | ||||
| Salinity | 0.40 | 0.19 | 0.68 | ||
| Secchi disk | 0.32 | 0.20 | |||
| Suspended matter | −0.22 | ||||
| Reactive phosphorus | −0.25 | −0.20 | |||
| Silicate | −0.46 | ||||
| Ammonium | |||||
| Nitrites | −0.39 | −0.53 | |||
| Nitrates | −0.24 | ||||
| Macrophyte biomass | 0.30 | ||||
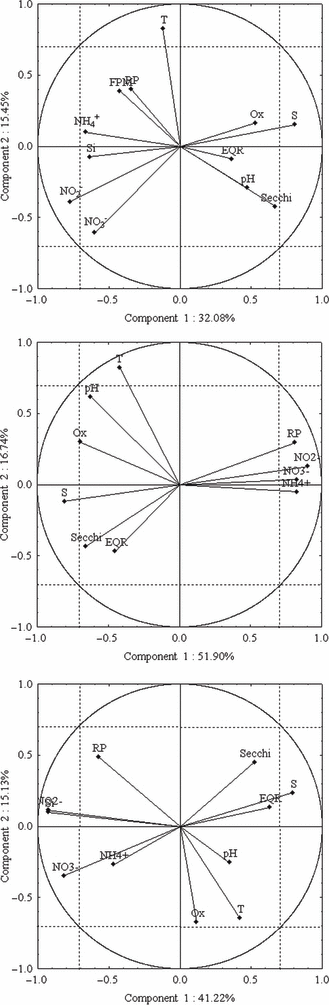
Principal component analysis (PCA) results. The upper plot was made using the dataset of the Venice Lagoon seasonal trends (120 samples). The middle plot was performed with the dataset from the central area of the Venice Lagoon (67 samples). The lower plot was made with the Marano-Grado Lagoon dataset (19 samples). The explained variance is reported in the title of each axis. Loadings: T , temperature; Secchi, Secchi disk extinction depth; NO3−, nitrate concentration; NO2−, nitrite concentration; S, salinity; Ox, oxygen saturation; NH4+, ammonium concentration; RP, reactive phosphorus concentration; Si, silicate concentration; FPM, suspended particulate matter; EQR, Ecological Quality Ratio as water quality indicator.
Discussion
The use of phytoplankton to assess water quality in complex ecosystems might appear inadequate because of the cell movements which depend on tidal currents and winds and the short life cycle. Nevertheless, it has already been observed that phytoplankton can provide important information on the ecosystem status (Nogueira 2000; Cabecinha et al. 2009). Moreover, it has been recorded that in a zone of high productivity, small classes of nano/micro-phytoplankton tended to be dominant (Sabetta et al. 2008 and references within). Diatoms, in particular, are recognised to be useful indicators of river eutrophication (Kelly 1998; Prygiel et al. 1999). However, the transitional ecosystems can not be compared with freshwaters, particularly with rivers, where the current flows in a unique direction. The tide, the weather conditions and the morphological structure determine, in the transitional areas, complex hydrodynamics and water mixing. Data on benthic diatom distribution and taxonomical composition have provided some good results in the Venice Lagoon, but the environmental assessment has tended to be overestimated (Facca & Sfriso 2007). On the other hand, phytoplankton is more widely studied than benthic microalgae and there are a lot of data that can be used in the event of comparisons.
The study of the diatom community structure in terms of the ratio between biomass and cell abundance (W-statistic) as described by Clarke & Warwick (1994) provided results that merit some attention. It was, in fact, observed that the areas affected by anthropic impacts (not only wastewaters, but also sediment resuspension) tended to be characterised by opportunistic species. On such a basis we tried to distinguish the sites in relation to the EQRs: in the Venice Lagoon, Sts A, B and C displayed higher values than Sts G and H, according to the main environmental conditions of the lagoon described in the literature (Sfriso et al. 2003a; Frignani et al. 2005; Rossini et al. 2005; Secco et al. 2005). However, the classification appeared quite flattened if the annual means were considered, as all the stations then fell into the ‘Moderate’ class. This is probably due to the tide, which favours lagoon water renewal and mixing and hence the spreading of planktonic cells. To overcome such difficulties, a binomial classification is proposed (Sfriso et al. 2009) for the values which fall close to the borderlines of adjacent classes. This would make it possible to reduce the uncertainty produced by the sampling frequency and to make a better distinction among the sites and their trend: Sts A, B and C would then be classified as ‘Moderate/Good’ and the others as ‘Moderate/Poor’. The seasonal changes represent a critical point, but if the samples are repeated at least every 2–3 months, the assessment might appear reliable enough. In fact, the results showed significant negative correlations with the nutrient concentrations, confirming that the EQR based on the W-statistic may give an indication of trophic status. Moreover, the correlations which take into account the suspended matter and water transparency can also supply information on the water ecological status. The resuspension phenomena, which reduces water transparency in the shallow areas, can in fact seriously damage the ecosystem (Pranovi & Giovanardi 1994; Sfriso et al. 2003b, 2005).
The complete map of the Venice Lagoon shows a fairly good environmental assessment even though a single sampling is not sufficient for this scope and can furnish only a qualitative and instantaneous description. In fact, Goro, Lesina, Taranto and Orbetello Lagoon samples were not sufficient for a valid statistic elaboration but the results made it possible to assign them to the same classes as the ones obtained using the Macrophyte Quality Index (Sfriso et al. 2007, 2009). Some important discrepancies were noticed in St. L2 in the Lesina Lagoon and in Sacca di Goro. However, the general classification of the two ecosystems is in accordance with the observations of Giordani et al. (2009), who assigned a better water quality to Lesina Lagoon than to Sacca di Goro. Specchiulli et al. (2008) describe a trophic gradient of the Orbetello Lagoon which is quite similar to the present results, above all for Sts O1 and O4.
Identifying a formulation, and hence a number, able to estimate the environmental conditions is, in general, an enormous task, and it is even greater using the phytoplankton community. However, the comparison with the evaluations from other authors makes it possible to estimate the potential that is provided, in this case, by diatoms, bearing in mind that such a classification may represent more of a support to other methods than an independent tool.
Even though the suggested elaboration requires some good taxonomical preparation it should be considered that phytoplankton abundance and taxonomical composition are data collected routinely by many marine research centres, so new technologies or expertise are not necessary. Moreover, W-statistic calculation is quite easy and can be performed by PRIMER software, which is well known among researchers. The phytoplankton sampling is based on an acknowledged protocol and in some cases past data can also be retrieved to make comparisons. On the other hand, frequent monitoring and observations are a prerequisite for a reliable description of the community.
Conclusions
The results of the present work provide some evidence for considering phytoplankton a reliable biological quality element to assess ecological conditions in transitional systems. The community structure and, in particular, the relationship between biomass and abundance (e.g. the W-statistic) is proposed as a biological index which is of primary interest for coastal area assessment. The W-statistic can supply information on the general status of the environment because the cell size depends on different factors. It requires data routinely collected by a monitoring agency but does not require long elaborations. The main problem could be represented by the temporal variability of microalgae, but this can be overcome by monthly samplings. In fact, the annual means were well correlated to some important environmental parameters. This reduces the seasonal variability, and interesting information on the environment status can be obtained and integrated with observations from other communities.
Acknowledgements
Part of the data were collected in the framework of the project ‘Ecological integrity and trophic state indices for coastal and transition marine areas based on macroalgal taxonomic ratios, target species and nutrient concentrations’ co-funded by the Italian Ministry of Education, University and Research (MIUR). The project was one of the five projects co-funded in the general plan ‘NITIDA’ (New trophic state and ecological integrity indicators of coastal marine and transitional environments) (in Italian) in 2003—prot. 2003051023_005. Particular thanks go to Prof. Alberto Basset and to the anonymous reviewers for the useful suggestions and adjustments of the paper and to Dr Orietta Zucchetta for the English editing.




