Nocturnal fish utilization of a subtropical mangrove-seagrass ecotone
Abstract
Whereas diel fish migration between mangrove and seagrass habitats has been recognized for decades, quantitative studies have focused mainly on diurnal patterns of fish distribution and abundance. In general, previous studies have shown that fish abundances decline with increasing distance from mangroves; however, evidence for such a pattern at night, when many fishes are actively feeding, is scarce. The present study is the first to report nocturnal fish abundances along a continuous distance gradient from mangroves across adjacent seagrass habitat (0–120 m). Here, we used nocturnal seine sampling to test the null hypothesis (based on diurnal studies and limited nocturnal work) that fish abundance would decrease with increasing distance from shoreline. We focused on species and life-stage-specific abundance patterns of Lutjanus griseus, Sphyraena barracuda, Archosargus rhomboidalis, and Haemulon sciurus. Results indicated that assemblage composition and structure differed significantly by season, likely influenced by temperature. However, within each season, the fish habitat use pattern at both the assemblage and species-specific level generally failed to support our working null hypothesis. Species-specific analyses revealed that, for most species and life-stages examined, nocturnal abundance either did not change with distance or increased with distance from the mangrove-seagrass ecotone. Our results suggest that analyses where taxa are grouped to report overall patterns may have the potential to overlook significant species- and stage-specific variation. For fishes known to make nocturnal migrations, we recommend nocturnal sampling to determine habitat utilization patterns, especially when inferring nursery value of multiple habitats or when estimating fish production.
Problem
Several studies indicate that, during daylight hours, mangrove-lined shorelines can harbor high fish densities, with individuals presumably benefitting from reduced predation risk among the prop roots (e.g.Parrish 1989; Robertson & Blaber 1992; Laegdsgaard & Johnson 1995). Within mangrove shorelines, fish densities tend to be lower at night, as components of the assemblage disperse into adjacent habitats to forage, returning back to the prop roots before, or soon after, daybreak (Rooker & Dennis 1991; Nagelkerken et al. 2001; Ley & Halliday 2007). Based on evidence from gut contents (Randall 1967), stable isotope (Kieckbusch et al. 2004; Nagelkerken & van der Velde 2004a,b) and tagging investigations (Verweij & Nagelkerken 2007; Luo et al. 2009), seagrass beds are the prime nocturnal feeding destinations, especially for immature stages of snappers, grunts and other species that ultimately occupy coral reefs as adults.
Whereas diel fish migration between mangrove and seagrass habitats has been recognized for decades (Hobson 1965; Starck & Schroeder 1970; Rooker & Dennis 1991; Nagelkerken et al. 2001), quantitative studies have focused mainly on diurnal patterns of fish distribution and abundance at different distances from the mangrove-seagrass ecotone. Using various sampling techniques, these diurnal studies have consistently reported fish densities to be higher near to, as opposed to far from, the mangrove fringe (Thayer et al. 1987; Laegdsgaard & Johnson 1995; Nagelkerken et al. 2001; Newman & Gruber 2002; Christian 2003; de la Moriniere et al. 2004; Jelbart et al. 2007; Newman et al. 2007; Unsworth et al. 2008, 2009). However, interpreting fish utilization patterns in nearshore seagrass beds based on diurnal observations alone may be misleading, especially when inferring the nursery ‘value’ (Beck et al. 2001) of single or multiple habitat types, or for estimating secondary production of fishes (e.g.Valentine-Rose et al. 2007; Faunce & Serafy 2008a).
To date, only a few studies have reported nocturnal fish density patterns in seagrass beds at various distances from mangrove shoreline. Off the southwestern coast of Florida (USA), Christian (2003) compared nocturnal fish densities via visual surveys in seagrass beds 10 m versus 30 m from the mangrove fringe. Finding fish densities to be uniformly low and that density differences between sampling locations were insignificant, Christian (2003) concluded that nocturnal foraging migrations, if present, extended beyond the range of her sampling effort. Off Southwestern Australia, Jelbart et al. (2007) conducted diurnal and nocturnal seine sampling in seagrass beds <200 m versus >500 m from mangroves and found that fish densities decreased with increasing distance from shore by day as well as by night. Finally, off Hoga Island, Indonesia, Unsworth et al. (2008) compared diel fish densities using seine sampling in seagrass beds <50 m versus 3.5–6.5 km from shore and reported that by both day and night, fish abundance in close proximity to mangroves was at least twice that found in seagrass beds that were more distant from shore. However, resolving nocturnal fish abundance–mangrove proximity relationships in seagrass beds from the above studies is difficult given that (i) each study only compared two distances, (ii) distance differences may have been either too small (Christian 2003) or large (Jelbart et al. 2007; Unsworth et al. 2008) to reveal the nature of abundance-proximity relationships (e.g. linear or parabolic), if they existed, and (iii) results were not broken down, for the most part, at the species- and stage-specific level.
Because no studies have reported nocturnal fish abundances along a continuous distance gradient from mangrove edge across adjacent seagrass habitat, we conducted a study in subtropical Biscayne Bay, Florida, USA – a marine system rimmed by mangroves (mostly Rhizophora mangle) that transition into dense seagrass (mostly Thalassia testudinum). While several recent studies have examined seasonal and spatial patterns of fish use of Biscayne Bay’s mangrove habitats, all have been focused on diurnal observations along the mangrove fringe (Serafy et al. 2003, 2007; Faunce & Serafy 2008b). In the present study, we used nocturnal seine net sampling to investigate nocturnal fish habitat use patterns at 20-m intervals along a 120-m transect extending from the mangrove edge across adjacent seagrass habitat. We tested the hypothesis (based on diurnal studies and the nocturnal results of Jelbart et al. 2007 and Unsworth et al. 2008) that fish abundance would decrease with increasing distance from shoreline. Prior to this study we conducted some diurnal seine sampling within Biscayne Bay and also found that fish abundance patterns decreased with distance from shore (see Appendix 1). Our focus is on abundance and size information pertaining to four fishes: great barracuda (Sphyraena barracuda), seabream (Archosargus rhomboidalis), bluestriped grunt (Haemulon sciurus) and gray snapper (Lutjanus griseus). These fishes were selected because (i) they are among the most abundant and easily identified to species level, (ii) each is representative of a different trophic guild (great barracuda – piscivore, seabream – herbivore, bluestriped grunt – crustacean zoobenthivore, gray snapper – generalist omnivore), and (iii) two have economic importance (great barracuda and gray snapper), especially in the recreational fishery of the region.
Material and Methods
Study site
This study was conducted over two seasons (wet season: July–October, 2007; dry season: January–April, 2008) along the eastern boundary of Southern Biscayne Bay, Florida, USA, along the leeward side of Elliott Key between latitudes 25.427164° N and 25.406472° N (Fig. 1). Sampling was conducted along three 120-m-long transects that extended perpendicularly from shore and shared the following characteristics: (i) consistently high seagrass and macroalgae bottom cover (mean: 91% ± 8.0 SD); (ii) consistently shallow depths (mean: 88 cm ± 14 SD) out to 120 m from shore; and (iii) stable annual salinity due to its close proximity to oceanic waters (mean: 37 ppt ± 1.0 SD). Collectively, the above characteristics were chosen to reduce within- and between- transect variation in factors that might cloud relationships between fish abundance and distance from the mangrove shoreline. At these sites, water temperature varies somewhat between seasons (mean wet season: 32 °C ± 1.0 SD; dry season: 24 °C ± 2.0 SD); but within a season, water temperature is virtually identical within and between transects.
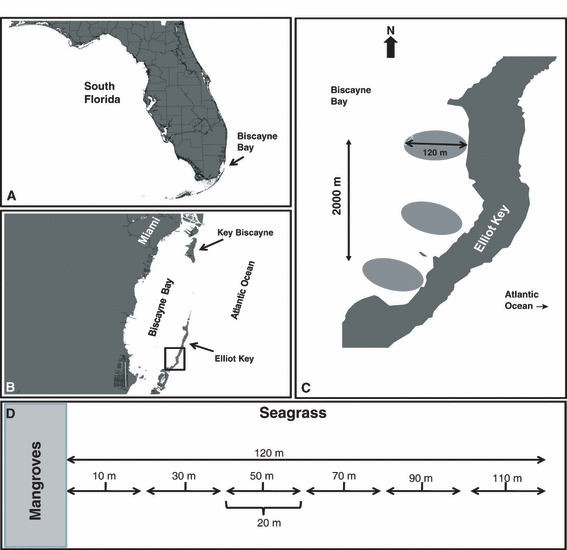
Study sites: (A) map of South Florida depicting location of Biscayne Bay; (B) position of study area (black square) on leeward side of Elliott Key within Biscayne Bay; (C) location of sampling transects within the study area; and (D) 120-m distance gradient with 20-m sampling intervals demarcated. The midpoints of the sampling intervals correspond with positions of beach seine bags.
Fish sampling
Center-bag seine nets (21.3 m long, 1.8 m high, 3 mm mesh) were used to sample fishes every 20 m along the three 120-m-long transects (Fig. 1D). Although no gear is without bias, seine nets were selected because this gear has been found to be an effective tool for examining the abundance patterns of the fishes under investigation in the current study (e.g.Newman et al. 2007). Moreover, previous diurnal studies using seine nets at this and other sites have found fish abundance patterns that decline with increasing distance from shore (e.g.Jelbart et al. 2007; Newman & Gruber 2002; Appendix 1). A 120-m transect was selected because 120 m was the maximum distance from shore where depths were consistently shallow enough to permit sampling with seine nets. Our seining technique followed Purtlebaugh & Rogers (2007), whereby nets are hauled parallel to shore, against the current (or wind if stronger) and pursed such that a standardized area of 142 m2 is sampled with each haul. At least two seine samples were collected simultaneously and the sequence at which each distance was sampled was chosen randomly. At each sampling event, seining began a half an hour after sunset in complete darkness, occurred within 2.5 h of low tide and all sampling took <2 h to complete. Each transect was visited on different days within each season to enable the collection of three to four seine samples for each transect–distance–season combination.
Life-history stage (LHS) designation
All collected fishes were identified to species and measured to the nearest mm total length (TL). Fish length information and published size–age relationships (de Sylva 1963; Billings & Munro 1974; Manooch & Matheson 1981; Stoner & Livingston 1984; Domeier et al. 1996) were used to assign all individuals to one of two life-history stages (LHS). Individuals measuring less than reported size at age 1 were designated as early juveniles, and those larger than age-one size but smaller than reported size at maturity were designated as late juveniles.
Data analysis
Assemblage level
Multivariate analysis was used to investigate potential differences in focal fish assemblage composition and structure among seasons, transects and distances from shore. Following the approach used by Ley & Halliday (2007), stepwise one-way analysis of similarity (ANOSIM) was employed to assess the influence of each factor (season, transect, distance) separately. Bray–Curtis similarity coefficients were generated based on fourth-root-transformed fish densities to create the similarity matrix (Clarke & Warwick 1994). Nonmetric multidimensional scaling (MDS) in conjunction with hierarchical agglomerative cluster analysis, incorporating group-average linking, was used to search for transect and distance groupings based on the similarity matrix generated (Clarke & Warwick 1994). All multivariate analyses were performed using Plymouth Routines in Marine Environmental Research (PRIMER) (copyright M. R. Carr and K. R. Clarke, Marine Biological Laboratory, Plymouth, UK; Clarke & Warwick 1994). Statistical significance was declared at the P < 0.05 level.
Species- and stage-specific level
Spatial patterns of fish density were examined by comparing seine catches of juvenile gray snapper, bluestriped grunt, seabream and great barracuda along the distance gradient. At the species- and LHS-specific level, density data were positively skewed and zero values predominated and thus were inappropriate for use in conventional parametric statistical analyses. Therefore, species- and stage-specific mean densities (per 142 m2) for each season–transect–distance combination were determined using a delta-distribution mean estimator (Fletcher et al. 2005), a measure of fish density that separately considers the proportion of samples positive for a given assemblage component (i.e. frequency of occurrence) and its mean density when present (i.e. concentration). This approach was previously used to examine mangrove fish density patterns in Biscayne Bay (Faunce & Serafy 2007, 2008a,b; Serafy et al. 2007). Among-transect differences in absolute abundances have the potential to obscure overall relative abundance-proximity patterns. Therefore, to reveal overall density patterns with distance, we expressed transect-specific fish densities as residuals about their transect-specific means (Winer 1971). Using SAS (SAS Institute, Cary, NC, USA) statistical software, these standardized, zero-centered values were then regressed against distance from shore by applying linear and quadratic models. Statistical significance was declared at the P < 0.05 level.
Results
General
A total of 134 nocturnal seine samples (62 wet season, 72 dry season) yielded 1706 specimens of the four focal species, which ranged in size from 3.5 to 30.0 cm TL (Table 1). For all four species, early-juvenile stages were composed of individuals less than 10 cm TL, and late-juvenile stages included fishes up to 20 cm TL for seabream, 25 cm TL for gray snapper and bluestriped grunt, and 30 cm TL for great barracuda. Of the 134 samples, the percentage of samples positive for the early stages of the focal species ranged from 22.4% to 91%; those positive for the late stages ranged from 32.8% to 65.7%. Other fishes captured include: redfin needlefish (Strongylura notata), pipefish (Syngnathidae sp.), bandtail puffer (Sphoeroides spengleri), checkered puffer (Sphoeroides testudineus), Gulf toadfish (Opsanus beta), yellow stingray (Urolophus jamaicensis) and striped mullet (Mugil cephalus). However, catches of these species were too sparse for statistical treatment.
| species | no. of fishes collected | total length (cm) | ||||
|---|---|---|---|---|---|---|
| wet | dry | total (early-juv; late-juv) | min | max | average | |
| gray snapper | 78 | 129 | 207 (155; 89) | 5.20 | 17.20 | 10.16 |
| bluestriped grunt | 619 | 474 | 1093 (841; 238) | 3.50 | 21.80 | 8.00 |
| great barracuda | 110 | 57 | 167 (88; 150) | 5.40 | 18.70 | 10.83 |
| seabream | 128 | 111 | 239 (91; 70) | 3.80 | 30.00 | 10.56 |
Assemblage level
Overall, multivariate analyses (cluster, MDS, ANOSIM) indicated that season exerted the strongest effects on the focal species assemblage composition and structure (ANOSIM, P < 0.001, Table 2); thus, subsequent analyses were conducted by season. Within the wet season, there was a significant difference between distances from shore (P < 0.003), but not among transects. Similarly, during the dry season, the assemblage differed significantly between distances from shore (P < 0.016), but not among transects. Both cluster and MDS analysis within each season (grouped by transect) revealed that assemblage composition and structure closest to the mangroves differed from the rest (Fig. 2A,B). Within both seasons, samples closest to the mangroves separated at about 80% similarity level, whereas the remaining five distances were similar at ≥90% similarity.
| step | grouping factor | factor analyzed | R% | P value |
|---|---|---|---|---|
| 1 | none | season | 38 | 0.001 |
| 1 | none | transect | 4.7 | 0.095 |
| 1 | none | proximity | 14.6 | 0.005 |
| 2 | season (wet) | transect | 10 | 0.100 |
| 2 | season (wet) | proximity | 33 | 0.003 |
| 3 | season (dry) | transect | 2.1 | 0.340 |
| 3 | season (dry) | proximity | 28 | 0.015 |
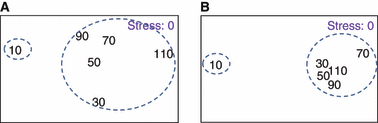
Nonmetric multidimensional scaling (MDS) plots revealing that samples nearest the mangroves separate from the rest in both the dry (A) and wet (B) season. Dashed lines indicate cluster analysis grouping of samples at about 80% similarity. Numerical values indicate distance from the mangrove-seagrass shoreline. Success of MDS is measured by a ‘stress coefficient.’ Stress <0.05 gives an excellent representation with no prospect for misinterpretation.
Species- and LHS-specific level
With two exceptions, our data failed to support our working hypothesis that nocturnal fish abundance would decline with increasing distance from shore (3, 4). The exceptions were dry season patterns of early juvenile seabream and great barracuda. Otherwise, each component’s abundance trend was either (i) uniform across the distance gradient, (ii) increased linearly or (iii) was parabolic. Among the early juveniles, the following density-distance patterns emerged: (i) uniform– gray snapper (dry and wet season), bluestriped grunt, seabream and barracuda (wet); (ii) negative linear (i.e. decrease with distance) – seabream and great barracuda (dry); and (iii) parabolic– bluestriped grunt (dry). The following density-distance patterns emerged for the late juveniles: (i) uniform- bluestriped grunt (wet), gray snapper and barracuda (dry); (ii) positive linear (i.e. increase with distance)– bluestriped grunt (dry), gray snapper and seabream (wet); and (iii) parabolic– seabream (dry) and great barracuda (wet).
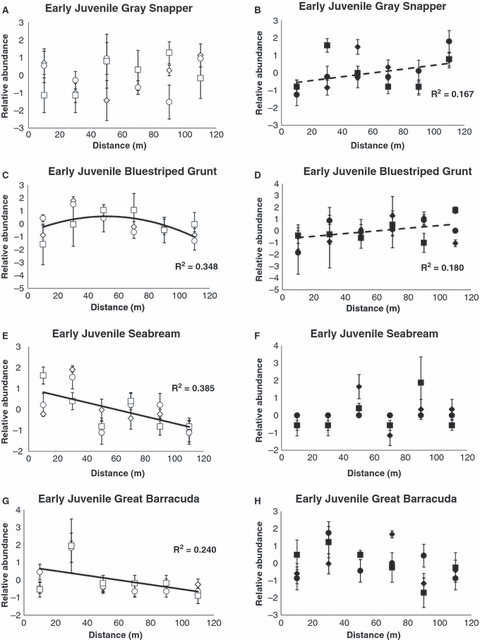
Relative density-distance patterns for early-juveniles: (A,B) gray snapper; (C,D) bluestriped grunts; (E,F) seabream; and (G,H) great barracuda. Open symbols indicate dry season and solid symbols wet season. Symbol shapes correspond with different transects. Values are standardized (zero-centered) transect-specific mean (±1 standard error) fish densities (per 142 m2). Solid lines and associated R2 values indicate significant distance patterns (P < 0.05). Dashed lines and associated R2 values indicate marginally significant distance trends (0.05 < P < 0.1).
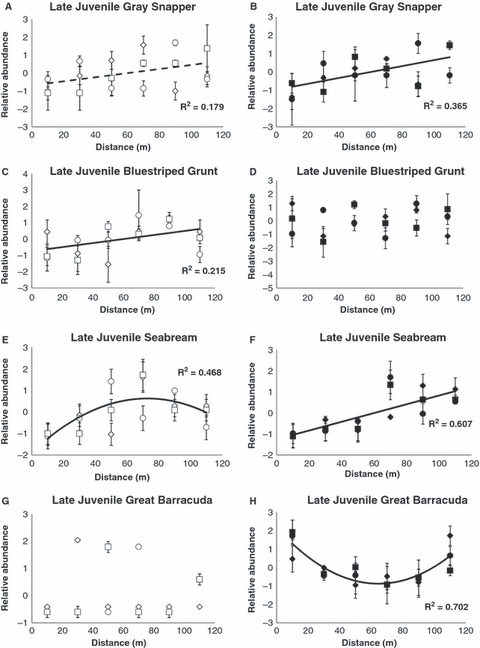
Relative density-distance patterns for late-juveniles: (A,B) gray snapper; (C,D) bluestriped grunts; (E,F) seabream; and (G,H) great barracuda. Open symbols indicate dry season and solid symbols wet season. Symbol shapes correspond with different transects. Values are standardized (zero-centered) transect-specific mean (±1 standard error) fish densities (per 142 m2). Solid lines and associated R2 values indicate significant distance patterns (P < 0.05). Dashed lines and associated R2 values indicate marginally significant distance trends (0.05 < P < 0.1).
Discussion
Nocturnal sampling along a distance gradient from mangrove edge across adjacent seagrass habitat (0–120 m) revealed fish habitat use patterns at the assemblage and the species- and LHS-specific level that diverge from the reported pattern of decreasing density with increasing distance from shore observed diurnally (and in two cases nocturnally: Jelbart et al. 2007 and Unsworth et al. 2008) in a wide range of tropical and subtropical systems. Within each season, the assemblage differed according to distance from shore, with the zone closest to the mangrove edge being significantly distinct. Species-specific analyses revealed mostly uniform patterns of abundance with distance from shore for early juveniles. Except for the piscivorous Sphyraena barracuda, the zone nearest the mangrove edge tended to harbor the lowest fish densities for late juveniles.
Our ability to contrast these results with other studies is limited because comparable investigations are lacking. Several authors also have found lower densities of gray snapper, bluestriped grunt, seabream and great barracuda within or near the mangroves at night versus day (Rooker & Dennis 1991; Nagelkerken et al. 2001; Christian 2003; Yeager & Ariaz-Gonzalez 2008). Recent acoustic tracking studies of juvenile gray snapper have demonstrated that at sunset these fishes migrate rapidly out of the mangroves in a synchronized fashion and do not forage in seagrass meadows nearest the mangroves, but rather offshore (Luo et al. 2009; S. Whitcraft, personal communication). However, these studies could not determine where or how far the snappers moved offshore due to the ∼500 m detection range of the acoustic receivers (a limitation of the technology). Without presenting data, Starck & Davis (1966) commented that gray snapper feed up to 1.6 km from diurnal resting areas on the reef. Working in Spanish Water Bay, Curacao, Verweij & Nagelkerken (2007) reported that French grunt (Haemulon flavolineatum) and bluestriped grunt moved a mean distance of 23 m from mangroves to adjacent seagrass beds in the afternoon, presumably to begin foraging at night. On a coral reef, Ogden & Ehrlich (1977) reported grunts migrating up to 300 m at night to feed, while Starck & Davis (1966) claimed that bluestriped grunt fed as much as 400 m from their nearest point of diurnal concentration.
From the present study, we can only speculate as to the mechanisms driving fish habitat use decisions; however, because fish are feeding at night, it seems likely that their abundance patterns are related to prey availability. For example, gray snapper and bluestriped grunt feed on a wide variety of benthic invertebrates, with late-stages consuming larger invertebrates and, in the case of gray snapper, also small fishes (Randall 1967; Starck & Schroeder 1970; de la Moriniere et al. 2003; Nagelkerken et al. 2006). Perhaps some of these species tended to avoid foraging near the mangrove-seagrass ecotone due to low prey supply there. This may result from fishes occupying mangrove shorelines during the day and overgrazing prey under or within meters of the prop roots (i.e. creating a halo affect) as has been found in reef systems (Hay 1984). But, working in different systems, Rodriguez & Villamizar (2000), Skilleter et al. (2005), Kopp et al. (2007) all found that abundance of invertebrate prey was highest (not lowest) nearest the mangroves and decreased with increasing distance from shore. Moreover, late-stage seabream are herbivorous, feeding primarily on aquatic vegetation (Randall 1967; Vaughan 1976; Stoner & Livingston 1984). However, their densities were also lowest nearest the mangroves where vegetation cover and seagrass canopy height were highest. With a few exceptions, early-juvenile fishes in both seasons generally followed a uniform distribution pattern with distance from shore. Vegetation cover and canopy height were relatively high across the distance gradient. Seagrass and macroalgae cover averaged 90% (minimum 66%, maximum 99%) in both seasons. Thus, the observed uniform abundance pattern of early juveniles may reflect sufficiently high vegetation cover and seagrass canopy height (above some threshold level) along the distance gradient, providing early juveniles with ample prey supply and shelter to forage. However, to adequately explore relationships between nocturnal fish utilization and prey supply in our study domain, gut content analysis and prey distribution studies are needed.
Nocturnal fish habitat use at this site may also be influenced by predation risk. For example, several fishes may be avoiding the mangrove-seagrass edge due to increased risk of predation there at night. Transition zones between refuges and feeding areas are potentially predictable ‘hot spots’ in space and time where animals are vulnerable to predation (Decamps & Naiman 1988;Sheaves 2005). At night, when secondary consumers leave the safety of the mangroves to feed on emerging benthic or epibenthic prey, tertiary predators may patrol the mangrove shoreline to ambush them. For example, great barracuda are piscivorous (de Sylva 1963; Schmidt, 1989) and they may be positioning themselves to ambush small fishes migrating about the mangroves and feeding offshore. Thus, the mangrove–seagrass interface and its surroundings may act as a gauntlet to fishes migrating to forage, especially between dawn and dusk, when predators have a visual advantage (Munz & McFarland 1973). Organisms may be at highest risk from predation when crossing ecotones between sheltered and feeding patches. For example, Shulman (1985) and Sweatman & Robertson (1994) provided experimental evidence that juvenile fishes avoided seagrass bordering the coral reef edge, along a reef-seagrass gradient, due to increased predator encounters. Exploring gradients of predation pressure to fishes along the distance gradient (e.g. via tethering experiments) would provide a means to quantify relative predation risk with mangrove proximity (Aronson & Heck 1995; Baker & Sheaves 2007).
Conclusions and Implications
Although it is well recognized that a large portion of fish feeding activity primarily occurs at night, studies examining nocturnal fish utilization of mangroves and seagrass beds are extremely limited. For example, Faunce & Serafy (2006) reviewed 111 studies published between 1955 and 2005 examining fish habitat use in mangrove systems; of these, only six (5%) were conducted at night. In a recent international symposium on mangroves as fish habitat (Serafy & Araujo 2007), only one of the 25 (4%) published studies reported on fish abundance at night (Ley & Halliday 2007). Our investigation of nocturnal fish habitat use across a mangrove–seagrass distance gradient revealed abundance-distance trends that varied according to season, species and life-stage. Further nocturnal investigations of fish habitat use in mangrove-seagrass systems would provide valuable insight into the ecology of nearshore fishes. In our study, the mangrove-seagrass ecotone generally harbored low densities of late-juvenile gray snapper, seabream and bluestriped grunt. Our results support the notion imparted by Ley & Halliday (2007) that progress toward identifying general trends in habitat selection of fishes might be achieved by focusing survey efforts on ecotones at a time (i.e. night) when feeding occurs.
It is worth considering that our results are based on sampling that took place relatively early in the night. Thus, it is possible that fish distributions during this period may not be the same as much later in the night or just before dawn the next morning. It is possible that as the night progresses, fishes may become satiated and return to the mangroves and exhibit declines in distribution with distance from shore. However, Luo et al. (2009) tracked gray snapper in Biscayne Bay and found that snapper left the mangroves at sunset to forage offshore and did not return until the following morning. We recommend that future work at this site investigate if and how fish distributions may change throughout the course of the night.
Previous diurnal studies investigating fish abundance in relation to mangroves have generally analyzed data where taxa were grouped by species, trophic level, and/or life-history stage. Here, density patterns clearly varied by species and life-history stage. This suggests that analyses where taxa are grouped to report overall patterns may have the potential to overlook significant species- and stage-specific variation. This has implications for fisheries management of economically important species, which typically operates at the species- and stage-specific level.
Recent research has focused on determining habitat-specific secondary production rates of nearshore fishes for conservation and management purposes, such as prioritizing areas for marine reserve planning (Mumby et al. 2004; Valentine-Rose et al. 2007), identifying nursery habitats (Beck et al. 2001) or effective juvenile habitats (Dahlgren et al. 2006), and characterizing essential fish habitats (Faunce & Serafy 2008a). As a result, several recent studies have generated secondary production rates for juvenile fishes occupying mangroves based on diurnal surveys. For example, Faunce & Serafy (2008a) reported production estimates for gray snapper in Florida Keys mangroves between 6 and 11 g·m−2·year−1. Similarly, Valentine-Rose et al. (2007) reported production rates for gray snapper ranging between 50 and 150 g·m−2·year−1 in mangroves within Bahamian tidal creeks. However, for realistic secondary production estimates, knowledge of the area utilized by the fishes is crucial. The present study reveals patterns of fish utilization of seagrasses at odds with those derived from daytime studies, with some species abundances steadily increasing out to 120 m from the mangrove-seagrass ecotone. Thus, secondary production rates calculated for mangrove-dwelling fishes based solely on diurnal studies may be overestimates as the full areal extent of seagrass use has not been taken into account. Moreover, attributing production to a single habitat (e.g. mangroves or seagrass) may be inappropriate since many fishes use multiple habitats over the diel cycle to survive and grow.
Our study system is likely to share features in common with other aquatic systems. For example, Dorenbosch et al. (2005) investigated diurnal reef fish abundance along a distant gradient from coral reef across adjacent seagrass. Adult densities of reef fish species were highest on the coral reef and decreased in adjacent seagrass with increasing distance from reef edge. At night, reef species like Lutjanidae and Haemulidae leave the reef to forage in habitats up to 1.6 km from diurnal resting areas (Starck & Davis 1966; Ogden & Ehrlich 1977; Burke 1995). This scenario is comparable to the fish habitat use patterns found in the present study. Thus, our approach and conclusions may be applicable to the study of other marine environments.
Acknowledgements
Funding support for this work was primarily provided by the Herbert W. Hoover Foundation, the Batchelor Foundation Inc., NOAA Living Marine Resource Cooperative Science Center, National Park Service (Biscayne National Park – BNP), the SeaStar Foundation, Save The Blue, YSI Inc., the University of Miami’s South Florida Student Shark Program (SFSSP) and Rosenstiel School of Marine and Atmospheric Science. We are indebted to the technical support of all SFSSP volunteers, primarily D. Ovando, A. Disilvestro, A. Morgan, L. Winn, E. Overstreet, J. Ross, C. Antal, A. Matulik, X. Serrano, K. Titley, D. Washington and the students of Palmer Trinity High School, South Broward High School, MAST Academy and the University of Miami. Special thanks to M. Heithaus, D. Lirman, L. Brand, T. Kellison, G. Thomas, D. Die, P.B. Teare, C. Peyer, T. Jackson, L. Hoover and R. Mann. For logistical support, thanks to M. Lewis, E. Alvear, R. Curry and H. M. Tritt of BNP. This work was conducted under BNP Permit # BISC-2008-SCI-0003.
Appendix
Appendix 1
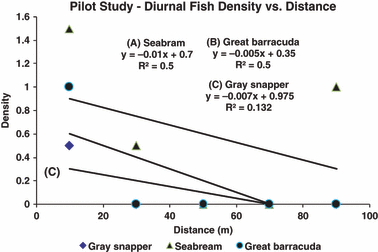
Results of diurnal seine sampling in Biscayne Bay, Florida (USA). Density-distance patterns of late-stage seabream, Archosargus rhomboidalis (A), great barracuda, Sphyraena barracuda (B) and gray snapper, Lutjanus griseus (C). Sampling revealed density declines with distance. See text for further details on sampling and analysis.