Ecological assessment to detect imminent change in coral reefs of Admiral Cockburn Land and Sea National Park, Turks and Caicos Islands
Abstract
Coral reefs of the Turks and Caicos Islands (TCIs) (Caribbean Sea) constitute some of the few pristine coral reef systems in the world and play a crucial role in the islands’ economy because they support rich fisheries catches and tourism development. Ambitious development plans involving increase in fishing and tourism pressures are about to bring changes in coastal zone resources of the TCIs associated with increased sediments and nutrients and reduced predation by herbivorous fish on coral reefs. Understanding change is critical when attempting to protect the resources that these coral reefs support and to adopt proper management strategies. Yet, an environmental assessment program to detect imminent human-induced changes on the surrounding reefs of the TCIs is lacking. Thus, (i) we obtained baseline data on benthic composition and coral community structure at seven reef sites of representative reefs of the TCIs within the Admiral Cockburn Land and Sea National Park (ACLSNP) of South Caicos Island and (ii) performed a priori statistical power analysis to calculate replication requirements for safely and confidently detecting small (δ = 0.1), medium (δ = 0.3), and large (δ = 0.5) effect sizes for a number of relevant to anticipated changes, univariate, benthic indices and for power β = 0.95. The platforms of the margin reefs studied (9–12 m depth) appeared rather variable regarding benthic composition but quite homogeneous regarding hard coral community structure. Mean percent cover of algal functional groups was 0.1 ± 0.3 (mean ± sd) percent for coralline algae and Halimeda, 0.1 ± 0.6 (mean ± sd) percent for macroalgae, 21.7 ± 33 (mean ± sd) percent for turf algae and 4.8 ± 4.0 (mean ± sd) percent for hard coral cover. The dominant benthic component, however, was carbonate substrate (mean ± sd = 30.4 ± 34.3), thus indicating an accreting reef framework. Mean hard coral density, colony size and recruit density were 5.5 ± 1.8 (mean ± sd) corals per 20-m line transect, 13.0 ± 2.3 (mean ± sd) cm maximum colony diameter, and 1.3 ± 1.4 (mean ± sd) recruits per square foot, respectively. Due to high natural variance, hard coral colony size and density were practically the most sensitive indices in detecting even small size changes on benthos. Also, the geometric mean of log-transformed colony size-frequency distributions of the most abundant hard coral taxa, i.e. Montastrea annularis, Agaricia spp., Siderastrea spp. and Porites asteroides were practically sensitive for the same purpose. We hope that the study will optimize the spatial component of a necessary environmental impact assessment program on coral reefs of the TCIs once the natural spatial variability of the system has been assessed and sensitive, benthic, univariate indices have been identified for representative reference coral reef sites of the TCIs.
Problem
Coral reefs play a crucial role in the Turks and Caicos Islands (TCIs) economy because they support fisheries and tourism development (Anonymous 2005). In addition, they constitute some of the few pristine coral reef systems in the world within a region hard hit by (Buddemeier et al. 2003) and particularly sensitive to (Buddemeier & Fautin 2002; Hughes et al. 2003) anthropogenic effects leading to either ‘top-down’ (reduction in herbivory) or ‘bottom-up’ (increase in nutrients) induced ‘phase shifts’ from coral to algal reefs (Littler & Littler 1984). Furthermore, change in coastal zone resources of the TCIs is imminent due to ambitious development plans involving increase in fishing and tourism pressures (TCI Government 2004; Anonymous 2007).
Expected alterations in the ambient waters of coral reefs of the TCIs due to programmed development involve elevated levels of sediments and nutrients and decreased herbivory. Appropriate indicators of expected alterations should involve relevant structural aspects of the reef framework-building hard coral communities (Dikou & van Woesik 2006a) and functional aspects of the benthos (Littler et al. 2006). Scleractinian, or hard corals are sensitive to alterations in nutrient and sediment status (Pastorok & Bilyard 1985; Rogers 1990; Fabricius 2005) and in herbivore abundance (McManus et al. 2000; Mumby et al. 2006) of their ambient waters. Benthic composition, on the other hand, may be a more informative indicator of ecosystem health as it incorporates more organisms than studies that focus on hard coral species only (Brown & Howard 1985). Benthic composition determines the maximum level of coral recruitment (Hughes et al. 2000), especially for self-seeded reefs (Black et al. 1991; Roberts 1997), as well as the substrate available for hard corals in the competition for bare space with the faster growing algae (Bak & Engel 1979; Sammarco 1980; Miller & Hay 1998; McCook et al. 2001; Birrell et al. 2004), and therefore the capacity for growth and recovery after perturbation (Connell 1997; Miller et al. 2000; Gardner et al. 2003). The importance of analyzing the composition of benthos on coral reef systems in relation to past, present and future anthropogenic effects has been noted (Bak & Luckhurst 1980; Hughes et al. 1987). Furthermore, there have already been documented coral to algal whole reef ‘phase shifts’ due to anthropogenic effects such as increase in sediment load due to land clearing and subsequent runoff, dredging, and coastal construction (Sanders & Baron-Szabo 2005; Dikou & van Woesik 2006b); in nutrients load due to sewage discharge (Maragos et al. 1985); and trophic cascades associated with overfishing of herbivorous fish and quincidental mass mortality of the grazer sea urchin Diadema antillarum (Jackson et al. 2001).
Understanding and detecting imminent change on coral reefs of the TCIs is critical when attempting to protect the resources that these ecosystems support and to adopt proper management strategies. A priori statistical power analysis is a useful tool for study design as it allows the ad-hoc selection of the most sensitive indicators and the calculation of the sample size needed to detect a biologically/ecologically/environmentally meaningful magnitude of change in context (Fairweather 1991; Morrisey 1993). Its application in environmental impact assessments, however, has been rather limited so far (e.g. Ferraro & Cole 1990), supporting a false sense of security against public and environmentally costly Type II errors (Peterman 1990a,b).
Yet, an environmental assessment program to detect imminent human-induced changes on the surrounding reefs of the TCIs is lacking. Such a program requires a sampling design that would separate impacts from natural (spatial and temporal) variability through series of sampling times ‘Before’ and ‘After’ the disturbance, at the ‘Impacted’ site and at multiple ‘Control’ (reference) sites (BACI designs, Green 1979; Underwood 1992). This design should have adequate statistical power (Fairweather 1991; Benedetti-Cecchi 2001) to detect effects that are real, biologically/ecologically/environmentally important, and relevant to future anthropogenic interventions associated with development of the TCIs. The impact is defined as a change from ‘Before’ to ‘After’ the disturbance (the temporal component of the program) in the difference between the ‘Impacted’ and the average of the ‘Control’ sites (the spatial component of the program) and is detected as the interaction between the factors space (with two levels: ‘Impacted’ and ‘Control’ conditions) and time (with two levels: ‘Before’ and ‘After’ conditions). This study aims to optimize the spatial component of an environmental impact assessment program for coral reefs of the TCIs by providing power estimates of the difference between sites in a number of relevant benthic, univariate, community descriptors, which would correspond to the main effect of ‘Impacted’versus‘Control’ condition in a real environmental impact assessment study. In particular, it provides: (i) ‘before’ impact baseline data on benthic composition and coral community structure that allow the assessment of the natural spatial variability of the system and (ii) relevant and sensitive benthic indicators based on replication requirements for confidently detecting changes of small, medium and large size, using assessments at reef sites of representative reefs of the TCIs within the Admiral Cockburn Land and Sea National Park (ACLSNP) of South Caicos Island. The procedure is readily applicable to the assessment of anthropogenic impacts on coral reefs in general.
Material and Methods
The TCIs are located at 21°53′N, 71°47′W in the Caribbean Sea. They consist of eight islands and roughly 40 cays situated among two banks; the Caicos and Turks Banks (Fig. 1). These islands are surrounded by over 300 km of coral reefs and, due to the generally low human population, they are considered to be in a literally pristine condition. The island of South Caicos lies on the southeastern end of the Caicos Island chain along the western edge of the Columbus or Turks Island Passage (Fig. 1). All reef sites surveyed were situated along reef flats on the outer reefs of the Caicos Bank; five reef sites were located on the southeast side of Long Cay (Maze, Troy’s Dream, Warhead, Plane, Grotto) and another two were located south of East Bay (Fishbowl, Arch) (Fig. 1).
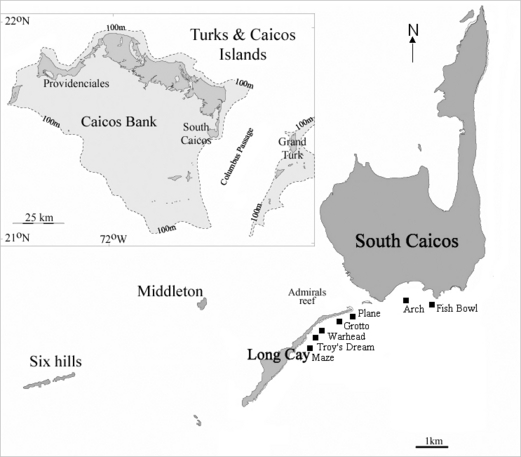
Map of South Caicos showing the location of the seven study sites. Maze: 21°28′160″N, 71°33′031″W; Troy’s Dream: 21°28′853″N, 71°32′157″W; Plane: 21°28′831″N, 71°31′020″W; Grotto: 21°28′876″N, 71°32′031″W, Warhead: 21°28′815″N, 71°32′236″W; Arch: 21°28′961″N, 71°32′031″W; Fishbowl: 21°29′101″N, 71°30′505″W.
Surveys in November and December 2006 and in April and May 2007 with SCUBA provided three types of data: benthic cover, hard coral colony size, and hard coral recruits. Cover by main benthic components included live hard coral, filter feeders (sponge, soft coral and other alcyonaria, zoanthids), macroinvertebates (molluscs, echinoids), turf algae (including encrusting fleshy algae), coralline algae (and Halimeda), calcareous substrate, macroalgae, dead hard coral, coral rubble, rock, and sand. For benthic cover and hard coral colony size data we used time-efficient replicate 20-m-long line transects (Loya 1972). Transects were laid haphazardly at each reef site, parallel to the coast (Bouchon 1981) to ensure homogeneity of ecological conditions along each transect, and at depths between 9 and 12 m. At each site, 9–14 replicate transects were used depending on time and weather constraints. Data on hard coral recruits were obtained at regular intervals along each line transect using three replicate 0.09 square meter quadrats and a magnifying lens; coral recruits were defined to be <50 mm in diameter for large-size hard coral species and <20 mm for small-size species (Miller et al. 2000). All authors participated in field assessments after training by the same person (A. Dikou).
Univariate indices were extracted from the aforementioned data to describe and compare the community structure of the seven coral reef sites: percent cover of main benthic components, hard coral colony cover (percent), density (number of corals per 20-m line transect), Pielou’s evenness (based on species cover, J’C) and size, (maximum diameter in centimetres) and hard coral recruit density (number of recruits per 0.09 square meter). To test for significant differences in percent live coral cover, hard coral colony density, Pielou’s evenness, size, and recruit density among the seven reef sites, one-way analysis of variance (ANOVA) was employed. Data on percent live coral cover and on coral colony size needed a double square root and a logarithmic transformation, respectively, to satisfy the assumptions of normality and homogeneity of variance of ANOVA. Data on hard coral evenness and recruit density could not satisfy both assumptions and therefore its non-parametric equivalent, the Kruskal–Wallis test, was employed.
Relationships among benthic substratum components were explored using Spearman rank correlations (rs) on means of the seven reef sites and (n = 7) and on the whole dataset (n = 81) to detect even small-size relationships. Ordination of reef sites in two dimensions reflecting their similarity/dissimilarity in their benthic composition was accomplished by employing multidimensional scaling (MDS) on an underlying triangular similarity matrix based on Bray–Curtis similarity of raw data (percent cover by different benthic components). Significant differences in mean percent cover of benthic components among reef sites were tested with a permutation procedure, the one-way analysis of similarity (ANOSIM). Clustering (based on group-average) was used to group sites into relatively homogeneous groups. MDS, ANOSIM and clustering were performed using PRIMER 5.0 (Clarke & Warwick 2001). All other statistical tests were carried out using STATISTICA 5.0.
Also, log-transformed colony size-frequency distributions (based on maximum colony diameter) of Agaricia spp., Siderastrea spp., Favia fragum, Porites asteroides and Montastrea annularis were constructed. These taxa abound in shallow waters of the studied reefs and of Caribbean reefs, in general. It is speculated that the majority of Agaricia spp. colonies were Agaricia agaricites with a small contribution of Agaricia humilis. All Siderastrea spp. colonies were either Siderastrea radians or Siderastrea siderea but in situ classification was difficult to perform. Logarithmic transformation of coral-size data produces a better view of population structure in scleractinian corals (Bak & Meesters 1998) and provides species- and site-specific responses to alterations in environmental conditions (Meesters et al. 2001). Parameters characterizing the aforementioned distributions were the geometric mean size, skewness (describes the asymmetry around the mean), kurtosis (describes the peakedness near the central mode), the 95th percentile (an indication of the maximum colony size), standard deviation, and the probability that the sample is from a normal distribution (Sokal & Rohlf 1995).
Power represents the probability that a test will result in the correct rejection of the null hypothesis (H0,) and is thus expressed as 1-β. Type II error is the result of accepting H0 when an impact exists. Type II error, therefore, is the failure to detect an impact, and occurs with a probability of β (Toft & Shea 1983; Rotenberry & Wiens 1985; Andrew & Mapstone 1987; Simberloff 1990). Power is dependent upon the level of significance (α), the sample size (n), the effect size (ES), and the inherent variability in data. The higher the power of a test, the more statistically likely it is to show an effect that actually exists (Toft & Shea 1983; Rotenberry & Wiens 1985; Osenberg et al. 1994). The number of replicates, or n, is directly related to power; as n increases, so does power. The effect size is the magnitude of change in a parameter. A system with small natural variance will require a smaller n to detect a given effect size at a certain power, and as that variance increases, so does the required number of replicates (Osenberg et al. 1994). Sample sizes necessary to detect small, medium and large effect sizes corresponding to a 10%, 30% and 50% change, respectively, were calculated for all reefs collectively. The equation described in Clarke & Green (1988) was used to estimate sample sizes for each parameter: n > (k + 1) + √(k2 + 1); k = (σ/δ)2(2 + φ−1(P))2, where σ2 is the variance of the population, δ is the size of the biological change and φ−1(P) is the inverse of the normal distribution function. This equation is an approximation of the sample size acceptable for n > 4 (Clarke & Green 1988). The effect size (ES) was calculated for each parameter by multiplying its grand mean (mean of all seven reef sites) by (δ=) 0.1, 0.3 and 0.5 for small, medium and large effect sizes, respectively. Sample sizes were calculated for level of power equal to 0.95 [φ−1(0.95) = 1.64] and for level of significance (α) equal to 0.05.
Results
Dominant benthic components were calcareous substrate, turf algae, and dead coral, while mean live coral cover ranged between 2.3 ± 1.19 (mean ± sd) and 6.9 ± 5.70 (mean ± sd) percent of benthic space (Fig. 2). Given the replication used, the relative cover of the main benthic components differed among sites, albeit to a small degree (one-way ANOSIM, Global R = 0.083, P = 0.0080). At 30% dissimilarity, three clusters of similar sites were formed by Warhead, Fishbowl and Arch; Maze and Troy’s Dream; Plane and Grotto (Fig. 3). Percent turf algal cover correlated negatively and significantly with percent dead coral cover (rs=−0.79, P = 0.0362, n = 7). There was also a marginally significant negative relationship between percent calcareous substrate cover and percent sand cover (rs=−0.75, P = 0.0522, n = 7). Furthermore, weak, yet significant, relationships were detected among algal functional groups on the reefs studied when all replicates, and not just means of reef sites, were used. In particular, percent turf algal cover correlated negatively with percent hard coral cover (rs=−0.28, P = 0.0102, n = 82) but positively with percent reef-building algal cover (rs=0.28, P = 0.0101, n = 82) and percent macroalgal cover (rs=0.35, P = 0.0013, n = 82).
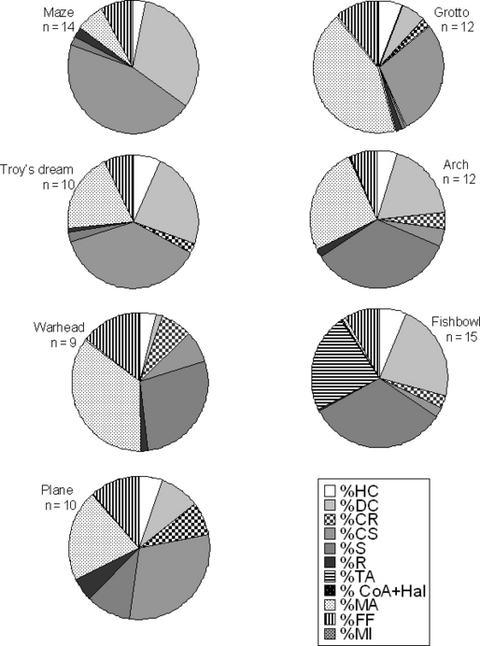
Benthic partitioning at seven reef sites within the ACLSMP using 20-m line transects. HC, hard coral; DC, dead coral; CR, coral rubble; CS, calcareous substrate; S, sand; R, rubble; TA, turf algae; CoA + Hal, coralline algae + Halimeda; MA, macroalgae; FF, filter feeders; MI, macroinvertebrates.
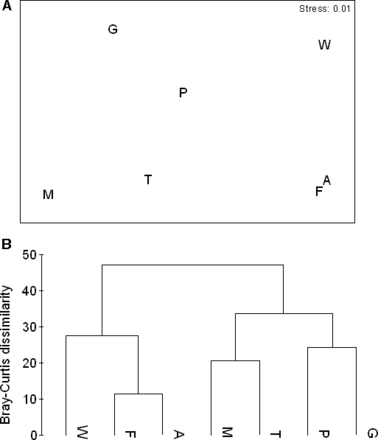
(A) 2-Dimensional MDS and (B) dendogram based on Bray–Curtis dissimilarity among means of 11 benthic variables for seven reef sites within the ACLMP. M, Maze; T, Troy’s dream; P, Plane; G, Grotto; W, Warhead; F, Fishbowl; A, Arch. Lines of different line patterns indicate different groups. Benthic variables and replication are presented in Fig. 2.
Double square root-transformed percent of live coral cover (F6,81 = 1.2492, P = 0.2913; mean ± sd = 4.8 ± 4.0), hard coral colony density (F6,81 = 1.4659, P = 0.2019; mean ± sd = 5.6 ± 1.8), and log-transformed mean hard coral colony size (F6,81 = 1.3484, P = 0.2469; mean ± sd = 13.0 ± 5.9) did not differ among the seven reef sites (Fig. 4). Also, hard coral recruit density did not differ among the seven reef sites (Kruskal–Wallis H6,386 = 6.7320, P = 0.3460) or between the two sampling seasons (Kruskal–Wallis H1,386 = 0.5980, P = 0.5980; mean ± sd = 1.3 ± 1.4) (Fig. 4). There was no pronounced dominance (Pielou’s evenness, Kruskal–Wallis H6,80 = 1.9323, P = 0.9258) of any of the scleractinian coral taxa encountered in any of the seven reef sites, and although patterns were not consistent within the seven reef sites, Agaricia spp., M. annularis, and Siderastrea spp. showed the highest relative cover as a percent of the total live hard coral cover when all reef sites were considered (Table 1).
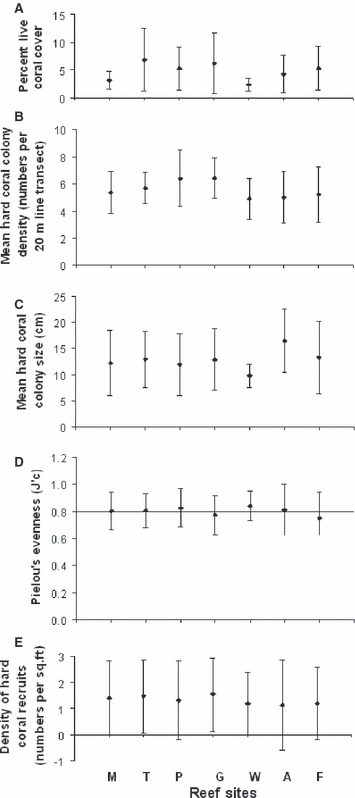
Variation (mean ± sd) in (A) percent live hard coral cover, (B) colony density, (C) colony size, (D) eveness, and (E) recruitment density in seven reef sites within the ACLMNP, 9–12 m depth. Reef sites as in Fig. 3.
| Reef sites | ||||||||
|---|---|---|---|---|---|---|---|---|
| Hard coral species | Maze (n = 14) | Troy’s Dream (n = 10) | Plane (n = 10) | Grotto (n = 12) | Warhead (n = 9) | Arch (n = 12) | Fishbowl (n = 14) | Grand (n = 81) |
| Acropora cervicornis | 3.3 ± 10.5 | 0.8 ± 2.4 | 0.5 ± 3.8 | |||||
| Agaricia spp. | 8.9 ± 11.3 | 15.5 ± 10.3 | 18.6 ± 18.5 | 9.9 ± 9.3 | 23.0 ± 17.2 | 27.0 ± 25.3 | 18.9 ± 20.1 | 17.1 ± 17.5 |
| Colpophyllia natans | 1.5 ± 3.1 | 1.1 ± 3.9 | 0.4 ± 1.9 | |||||
| Dendrogyra cylindricus | 2.2 ± 7.0 | 1.0 ± 3.0 | 3.1 ± 10.8 | 2.0 ± 7.3 | 1.2 ± 5.7 | |||
| Dichocoenia stokesii | 0.5 ± 1.9 | 3.4 ± 8.6 | 0.4 ± 1.4 | 0.6 ± 1.8 | 1.0 ± 2.7 | 0.8 ± 3.4 | ||
| Diploria strigosa | 1.9 ± 5.1 | 0.1 ± 0.4 | 0.3 ± 2.2 | |||||
| Diploria labyrinthiformis | 3.5 ± 5.9 | 0.9 ± 2.8 | 1.6 ± 4.5 | 0.8 ± 3.0 | ||||
| Diploria stokesii | 10.7 ± 17.6 | 0.7 ± 2.1 | 0.4 ± 1.1 | 0.3 ± 1.0 | 7.9 ± 16.1 | 2.9 ± 7.7 | 2.9 ± 6.1 | 3.8 ± 10.3 |
| Eusmilia fastigiata | 1.7 ± 4.6 | 1.1 ± 2.1 | 2.0 ± 4.6 | 0.7 ± 2.6 | ||||
| Favia fragum | 1.1 ± 2.6 | 1.9 ± 3.2 | 7.9 ± 12.6 | 5.8 ± 6.9 | 1.5 ± 1.9 | 2.7 ± 5.7 | 3.2 ± 5.8 | 3.4 ± 6.5 |
| Isophyllastrea rigida | 3.1 ± 5.9 | 2.5 ± 4.5 | 1.2 ± 4.2 | 1.2 ± 3.6 | 1.1 ± 3.6 | |||
| Madracis mirabilis | 1.9 ± 4.5 | 6.5 ± 22.5 | 2.3 ± 8.6 | 1.6 ± 9.4 | ||||
| Manicina areolata | 0.6 ± 2.4 | 0.1 ± 1.0 | ||||||
| Meandrina meandrites | 0.6 ± 1.2 | 3.4 ± 6.6 | 12.2 ± 16.4 | 0.5 ± 1.9 | 2.0 ± 6.9 | |||
| Millepora alcicornis | 0.2 ± 0.2 | 0.2 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.2 ± 0.3 | 0.1 ± 0.2 | 0.2 ± 0.4 | 0.2 ± 0.3 |
| Millepora complanata | 1.4 ± 5.3 | 0.3 ± 1.2 | 0.3 ± 2.2 | |||||
| Montastrea annularis | 16.6 ± 22.3 | 31.7 ± 28.1 | 16.3 ± 14.7 | 14.1 ± 18.7 | 4.9 ± 14.2 | 14.5 ± 17.6 | 18.5 ± 21.0 | 6.8 ± 20.6 |
| Montastrea cavernosa | 3.6 ± 6.1 | 3.8 ± 5.3 | 9.1 ± 15.6 | 7.1 ± 12.0 | 0.4 ± 1.7 | 0.5 ± 3.8 | ||
| Porites astreoides | 24.3 ± 22.4 | 5.3 ± 6.3 | 14.1 ± 13.5 | 10.3 ± 11.3 | 7.0 ± 12.1 | 9.9 ± 8.9 | 7.7 ± 7.8 | 11.7 ± 14.1 |
| Porites porites | 4.2 ± 9.3 | 5.2 ± 8.4 | 10.3 ± 24.9 | 8.3 ± 11.8 | 11.1 ± 11.4 | 0.9 ± 3.2 | 8.4 ± 11.3 | 6.7 ± 12.5 |
| Siderastrea spp. | 8.9 ± 9.7 | 18.6 ± 16.9 | 10.4 ± 11.0 | 28.9 ± 21.4 | 16.6 ± 26.9 | 15.2 ± 18.0 | 15.3 ± 21.9 | 16.1 ± 18.9 |
| Stephanocoenia michilini | 2.3 ± 7.3 | 5.0 ± 8.5 | 5.0 ± 6.7 | 1.1 ± 3.0 | 2.5 ± 8.7 | 4.0 ± 9.6 | 2.9 ± 7.2 | |
Logarithmically transforming colony-size data of Agaricia spp., Siderastrea spp., M. annularis, F. fragum and P. asteroides greatly improved normality of frequency distributions with four of five distributions being statistically non-significantly different from a normal distribution (Table 2, Fig. 5). Favia fragum was the smallest species, with an average colony size of 1.5 cm maximum colony diameter, while M. annularis, the largest species, averaged around 17 cm maximum colony diameter. Ninety-fifth percentiles of these two species were 9 cm and 65 cm maximum colony diameter, respectively. These two species also exhibited the largest standard deviations around the geometric mean colony size (Table 2). None of the coral-size distributions examined was symmetrical around the mean, but they were negatively skewed. This indicated colony-size distributions with relatively fewer colonies in the smaller size classes and a predominance of larger colonies. Of the five distributions in the dataset, two displayed negative kurtosis and three were positive with no clear pattern.
| Hard coral species | μ | SD | g1 (± SE) | g2 (± SE) | 95% | Pnorm | n |
| Agaricia spp. | 2.1 ± 0.11 | 0.71 | −0.0573 (± 0.1919) | −0.4850 (± 0.3815) | 3.47 | 0.0740 | 160 |
| Montastrea annularis | 2.8 ± 0.15 | 0.95 | −0.6539 (± 0.2000) | 0.3584 (± 0.3975) | 4.17 | 0.2000 | 147 |
| Favia fragum | 0.58 ± 0.30 | 1.32 | −2.6433 (± 0.2774) | 7.6573 (± 0.5482) | 2.08 | 0.0100 | 75 |
| Porites astreoides | 0.84 ± 1.69 | 0.30 | −0.7960 (± 0.2315) | 3.0143 (± 0.4590) | 1.26 | 0.2000 | 109 |
| Siderastrea spp. | 0.93 ± 0.07 | 0.39 | −0.1304 (± 0.2101) | −0.0696 (± 0.4171) | 1.60 | 0.2000 | 133 |
- μ = geometric mean size (cm); g1 = skewness (± SE); g2 = kurtosis (± SE); 95% = 95th percentile; Pnorm = probability that data follow a normal distribution (Kolmogorov–Smirnov test using Lilliefors adjusted probability); n = total number of colonies measured.
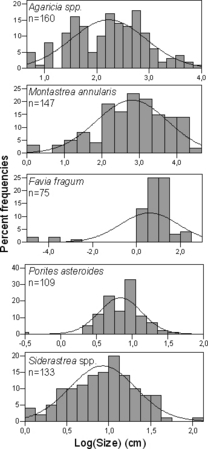
Size-frequency distributions (based on maximum colony diameter) of measured hard coral colonies with overlaid normal distributions for five taxa and seven reef sites within the ACLSMP.
Due to high natural variance, even the large ES specified (δ = 0.5) lay within the natural variation of all the specified benthic and hard coral community parameters, with the exception of hard coral colony density. Thus, a large number of replicates are required to detect even a 50% change in the mean of the selected parameters (Table 3a). We consider the medium effect size (δ = 0.3) specified for all parameters of great biological/ecological importance on the studied coral reefs (see Discussion). The most sensitive parameter in detecting even small effect sizes was hard coral colony size (as maximum colony diameter) followed by hard coral colony density, whilst the least sensitive parameter in detecting even large effect sizes was percent coralline algae and Halimeda cover (Table 3a). The geometric mean of log-transformed colony-size frequency distributions (based on maximum colony diameter) was a far more practically sensitive index in detecting small size effects for all hard coral taxa examined, except F. fragum, compared to skewness (Table 3b).
| Indices | Grand mean | Grand √σ2 | δ = 10% | δ = 30% | δ = 50% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| small ES | k | n | medium ES | k | n | large ES | k | n | |||
| (a) Benthic partition | |||||||||||
| Live coral cover (%) | 4.8 | 4.0 | 0.48 | 940.6 | 1882 | 1.43 | 104.51 | 210 | 2.4 | 37.6 | 76 |
| Dead coral cover (%) | 16.4 | 26.8 | 1.64 | 3548.2 | 7097 | 4.91 | 394.25 | 789 | 8.2 | 141.9 | 285 |
| Turf algae (%) | 21.7 | 33.0 | 2.17 | 3063.1 | 6127 | 6.51 | 340.34 | 682 | 10.8 | 122.5 | 246 |
| Macroalgae (%) | 0.1 | 0.6 | 0.01 | 24346.5 | 48694 | 0.04 | 2705.17 | 5411 | 0.1 | 973.9 | 1949 |
| Coralline algae and Halimeda (%) | 0.1 | 0.3 | 0.01 | 24447.0 | 48895 | 0.02 | 2716.34 | 5434 | 0.0 | 977.9 | 1957 |
| Filter feeders (%) | 8.3 | 9.0 | 0.83 | 1557.9 | 3117 | 2.49 | 173.10 | 347 | 4.1 | 62.3 | 126 |
| Coral colony density (number of corals per 20-m line transects) | 5.5 | 1.8 | 0.55 | 134.1 | 269 | 1.65 | 14.90 | 31 | 2.8 | 5.4 | 12 |
| Coral recruit density (number of corals per square foot) | 1.3 | 1.4 | 0.13 | 1580.9 | 3163 | 0.39 | 175.65 | 352 | 0.7 | 63.2 | 127 |
| Coral colony size (maximum diameter in cm) | 13.0 | 2.3 | 1.30 | 41.5 | 84 | 3.90 | 4.61 | 10 | 6.5 | 1.7 | 5 |
| b) Log-transformed colony size frequency distributions | |||||||||||
| Agaricia spp. (μ) | 2.10 | 0.7 | 0.2 | 151.5 | 304 | 0.63 | 16.83 | 35 | 1.1 | 6.1 | 13 |
| Montastrea annularis (μ) | 2.80 | 0.9 | 0.3 | 152.5 | 306 | 0.84 | 16.95 | 35 | 1.4 | 6.1 | 13 |
| Favia fragum (μ) | 0.58 | 1.3 | 0.1 | 6862.7 | 13726 | 0.17 | 762.52 | 1526 | 0.3 | 274.5 | 550 |
| Porites astreoides (μ) | 0.84 | 0.3 | 0.1 | 169.0 | 339 | 0.25 | 18.78 | 39 | 0.4 | 6.8 | 15 |
| Siderastrea spp. (μ) | 0.93 | 0.4 | 0.1 | 233.0 | 467 | 0.28 | 25.89 | 53 | 0.5 | 9.3 | 20 |
| Agaricia spp. (g1) | −0.06 | 2.4 | 0.0 | 2377735.0 | 4755471 | −0.02 | 264192.78 | 528387 | 0.0 | 95109.4 | 190220 |
| Montastrea annularis (g1) | −0.65 | 2.4 | −0.1 | 18220.4 | 36442 | −0.20 | 202.49 | 4050 | −0.3 | 728.8 | 1459 |
| Favia fragum (g1) | −2.64 | 2.4 | −0.3 | 1094.4 | 2190 | −0.79 | 121.60 | 244 | −1.3 | 43.8 | 89 |
| Porites astreoides (g1) | −0.80 | 2.4 | −0.1 | 12215.3 | 24432 | −0.24 | 1357.26 | 2716 | −0.4 | 488.6 | 978 |
| Siderastrea spp. (g1) | −0.13 | 2.4 | 0.0 | 457 458.1 | 914917 | −0.04 | 50828.68 | 101658 | −0.1 | 18298.3 | 36598 |
- μ = geometric mean (in cm); g1 = skewness; σ2 = variance; k = (σ/δ)2(2 + φ−1(P))2 as in Clarke & Green (1988).
- Data were pooled from seven reef sites.
Discussion
The platforms of the margin reefs of South Caicos studied appeared quite homogeneous regarding hard coral community structure but rather variable regarding benthic composition, even when this was portioned into living (mean ± sd = 35.0 ± 29.8) and non-living components (mean ± sd = 65.0 ± 29.8). Benthic partition is in accordance with descriptions of comparable sites at the TCIs (Sullivan et al. 1994; Riegl et al. 1999) and in the broader Caribbean three decades ago (Bak & Luckhurst 1980) but is in sharp contrast to more recent studies in the same region documenting the dominance of benthos by macroalgae (Andres & Witman 1995; Chiappone et al. 1997). According to the relative dominance paradigm of Littler & Littler (1984), Littler et al. (2006), reef-building corals would be dominant over reef-building (coralline and encrusting algae including Halimeda) and palatable algae (turf and macro algae) in a high herbivory but low nutrient environment. Although mean turf algal cover (mean ± sd = 21.7 ± 33.0) was the highest among the four algal functional groups on the reef sites studied, the dominant benthic component was carbonate substrate, thus indicating an accreting reef framework. Despite the lack of urchins on these reef flats (only one specimen was recorded under the total of 82, 20-m line transects and only a handful were observed inside crevices adjacent to the line transects), herbivorous, large-size fish apparently abound in ambient waters (Hoshino et al. 1999) because local fisheries are still largely based on the gasteropod Strombus gigas and the spiny lobster Panulirus argus (Rudd 2003). Numerous Diadema antillarum, as well as other urchin species, however, are found on shallower reefs around South Caicos (J. Rohde, personal observations). On these relatively pristine Caribbean reefs, a weak negative relationship between turf algal cover and hard coral cover and a weak positive relationship between reef-building algal cover and macroalgal cover were detected when a large number of replicates was used (n = 82). In the low herbivory and high nutrient environment of Jamaican fore reefs, in contrast, there was a strong, negative relationship (rs = −0.94, P = 0.0048, n = 6) between coralline and macroalgal cover (Andres & Witman 1995) with macroalgal cover averaging 79 ± 2.9 (mean ± sd) percent.
On a benthos largely dominated by carbonate substrate, at the beginning of the reef, and at relatively shallow waters (9–12 m depth), percent live coral cover was low. Percent live coral cover progressively increases towards the reef crest, after which live corals of large size dominate the benthos up to 50 m depth (A. Dikou, personal observations), similar to classic accounts of coral reef zonation (Loya 1972; Porter 1972; Bouchon 1981; Done 1982). The four most abundant hard coral species found in this study were in agreement with other studies conducted at the TCIs and the greater Caribbean (Loya 1976; Edmunds et al. 1990; Andres & Witman 1995; Riegl et al. 1999). In terms of cover, Agaricia spp. was the most abundant taxon followed by the reef-builder M. annularis, Siderastrea spp. and P. asteroids, and the small-size F. fragum was ubiquitous (73 colonies under 82, 20-m line transects). Mean recruitment rate of hard corals (mean ± sd = 20.8 ± 22.4 recruits·m−2) was double than that documented using same methods and considerations at Florida Keys, FL, USA (Edmunds et al. 1998; Miller et al. 2000).
Expansion of tourism and fisheries at the TCIs in the near future is likely to induce ‘bottom-up’ (due to increase in sediment and nutrient loads) and ‘top-down’ (due to reduction in fish herbivory) alterations in reef community structure and trophic dynamics similar to other developed islands in the Caribbean (Sullivan 2004). Although increase in sediments and nutrients usually take place in concert, a recent review has highlighted that an increase in turbidity and/or sedimentation impairs mainly growth, recruitment and survival of hard corals, while an increase in nutrients impairs mainly reproduction of hard corals (Fabricius 2005). Also, hard corals’ competitive inferiority over algae and filter feeders for space and light is further enhanced in reduced herbivory and/or increased nutrients environments (McManus et al. 2000; McCook et al. 2001). Thus, individual and/or synergistic effects are expected to be demonstrated, among others, through reduction in abundance of herbivorous fish in ambient waters, relative increase of palatable algal cover over reef-building algal cover (including scleractinian corals), increase in abundance of filter feeders, decrease in coral colony density, decrease in coral recruitment rates, and relative increase of sediment-resistant over sediment-intolerant hard corals along with concomitant changes in their colony-size frequency distributions. McClanahan & Obura (1997) found that genera that were more abundant under high sediment load conditions had larger mean size colonies compared with those in clearer waters, whereas genera more abundant on ‘reference’ reefs had smaller mean size colonies. Thus, differences in the direction and magnitude of changes in colony-size distributions of the abundant, sediment-resistant Siderastrea spp., P. asteroides and Agaricia spp. (Loya 1976; Cortes & Risk 1985) compared to that of the sediment-intolerant but nutrients-benefiting M. annularis (Loya 1976; Tomascik & Sander 1985) are expected. Meesters et al. (2001) evaluated the sensitivity of the log-transformed colony size-frequency distributions (based on colony aerial cover in square centimetres) of 13 hard coral species as indicators of heavy coastal urbanization on fringing reefs of Curaçao, Netherland Antilles, by comparing degraded reefs to upstream control areas. They concluded that Colpophyllia natans and Diploria labyrinthiformis were relatively sensitive to environmental conditions. Both species are present, though in low abundance, on the studied reefs.
While the direction of anticipated change in the variables studied on these reefs may be projected, the magnitude of a biologically/ecologically/environmentally important change is more difficult to decipher. The latter depends on the specific variable-indicator employed; the nature, severity, frequency and synergy of impacts; the ecology of the coral reef system these impacts are inflicted upon; and on what policy/public consider a un/acceptable amount of change for the coral reefs of interest. For example, chronic pollution by sewage along the west coast of Barbados was manifested, among others, through a 36.4% and 90.4% increase in suspended particulate matter concentration and chlorophyll a concentration, respectively, which, in turn, led to an 89.3%, 60.8%, 61.4%, and 79.5% decrease in skeletal linear extension rate of M. annularis, hard coral colony density, percent live coral cover, and hard coral recruit density, respectively, and a 413.1% increase in percent fleshy algal cover at the vicinity of the sewage discharge compared to a reference site approximately 50 km away from the sewage discharge (reef flat zones of stations BR and GS of Tomascik & Sander 1985, 1987; Tomascik 1991). We believe that a 10% and 30% change in the variables studied on these reefs should set off an alarm and prompt a response to degradation, respectively, given the protected status of these relatively pristine studied reefs and the demonstrated widespread degradation of coral reefs in the Caribbean (Buddemeier et al. 2003).
Furthermore, a possible impact is likely to affect not only the variables simulated in this study but also the variability of the system. Warwick & Clarke (1993) noted that, in a variety of environmental impact studies at the community level, the variability among samples from impacted areas was much greater than that from reference sites. Thus, the level of replication estimated for the community level variables of Table 3a may be the minimum required. On the other hand, impact assessment studies on the growth of hard corals at the population level indicate species-dependent change in variance (e.g.Tomascik & Sander 1985; Meesters et al. 2001). Thus, the level of replication estimated for the growth-related, population level variables of Table 3b may be either relaxed or conservative depending on the sensitivity/tolerance of the hard coral species studied to the specific stressor.
A priori power analysis indicated that among the univariate indices examined, coral colony size (maximum diameter in centimetres) and density along with the geometric mean of log-transformed size-frequency distributions of M. annularis, Siderastrea spp., Agaricia spp. and P. asteroides were practically the most sensitive indices in detecting even small changes on benthos. Power analysis does not add to an understanding of nature itself but it does allow some quality control to be exercised over inferences derived from environmental studies. Strengthened inferences give more reliable information about interactions in nature upon which to base management decisions (Fairweather 1991).
The present study provided baseline data on the benthic composition of coral reefs from ‘before’ impact, representative, ‘control’ sites and on its natural spatial variability. We applied a simple, yet robust, method for calculating required sample size for specified effect sizes and power; and evaluated the relative sensitivity of a number of benthic indicators to detect anticipated changes on coral reefs of the TCIs. Thus, it may optimize the spatial component of an environmental impact assessment program, which, when coupled with (currently lacking) water quality data within a required BACI type of sampling design (Green 1979; Underwood 1992; Benedetti-Cecchi 2001), will be able to confidently assert the presence (or absence) of biologically/ecologically/environmentally significant human-induced change on heterogeneous coral reef communities. The results of such a program, in turn, may guide adaptable development decisions, and thus ensure ecological and socio-economic sustainability for the local communities of the TCIs.
Acknowledgements
We thank The School for Field Studies, Center for Marine Resource Studies, TCI, for educational, financial, and logistical support throughout this study. Figure 1 was originally drawn and generously offered by Dr J. Claydon. We thank Prof. L. Benedetti-Cecchi and an anonymous reviewer for astute comments on this work.




