Eco-physiological repercussions of dietary arachidonic acid in cell membranes of active tissues of the Gray whale
Abstract
The radiation of the mammalian land species that became the baleen whales happened about 27–34 Mya. Mammals require omega 6 fatty acids for reproduction. With this long exposure to the omega 3-rich marine food chain, the Gray whale (Eschrichtius robustus) might be expected to have lost its requirement for omega 6 fatty acids. We report an unexpectedly high content of omega 6 arachidonic acid (ArA) in the Gray whale liver and muscle lipids. This whale migrates 10,000 km from the cold polar, omega 3 oil-rich food chain to that of the breeding lagoons of the tropical waters. The food web of tropical waters is a source of omega 6 fatty acids, which are hardly present in the cold polar food web. We suggest the reason for this longest of migrations from cold to warm waters is to meet the requirement for omega 6 fatty acids for mammalian reproduction and brain growth. This extreme conservation of omega 6 fatty acids in Gray whale biology has critical implications for mammalian biology and especially for whale conservation.
Problem
In a study on Bottlenose dolphins (Tursiops truncatus) we previously reported a surprising quantity of arachidonic acid (ArA) present in tissue lipids of liver, muscle and brain cell membranes (Williams et al. 1987). The aim of that study was to provide background data on Bottlenose dolphin nutrition and ecology. The large amounts of ArA in dolphin cell membranes presented a puzzle, as the marine food chain is claimed to be an omega 3 food web but ArA is an omega 6 fatty acid. We speculated that the reason for this unusual finding was that Bottlenose dolphins feed part of their time in warm water regions, which contain more omega 6 than found in the traditional fish oils from the cold northern waters. It is also possible that significant amounts of arachidonic acid might be made available from preying on squids. However, one tantalizing question remained: was the Bottlenose dolphin’s food selection programme designed to satisfy the mammalian requirement for omega 6 fatty acids for reproduction? Moreover, both docosahexaenoic acid (DHA) and ArA are required for mammalian brain structures (Crawford & Sinclair 1972; Crawford et al. 1976). Marine mammals are noted for their relatively large brain size, which in the Bottlenose dolphin is 1.8 kg (Marino 2002). As neither fatty acid can be synthesized de novo from two carbon units, the dolphin has to obtain both omega 6 and omega 3 in the marine food web.
Of the land mammals that migrated into the marine habitat, the migration of dolphins occurred most recently, around 7–12 Mya, whereas that of baleen whales is one of the oldest, occurring 27–34 Mya (Nikaido et al. 2001). The opportunity arose to study the membrane lipids in the Gray whale to test whether this longer exposure to the omega 3 habitat had resulted in the loss of ArA.
The essential fatty acids occur in nature as two separate omega 6 and omega 3 families. The omega denotes the start of a sequence of two to six methylene interrupted double bonds as numbered from the methyl end. The omega 6 parent, cis, cis-9,12-octadecadienoic acid (linoleic acid C18:2ω6) is stored in seed oils and has been abundant on land since the Cretaceous with the emergence of abundant flowering plants and their protected seeds. Animals insert more double bonds, making the fatty acid more unsaturated, and also extend the chain length. Linoleic acid (C18:2ω6), for example, is converted this way to arachidonic acid, which has 20 carbons and four double bonds (all-cis-5,8,11,14-eicosatetraenoic acid C20:4ω6 ArA). This fatty acid is also converted to eicosanoids, which are important in regulating cell function and are required in mammalian reproduction (Hluwalia et al. 1967; Ogburn et al. 1981; Min & Crawford 2004).
The parent omega 3 fatty acid all-cis-9,12,15-octadecatrienoic acid (α-linolenic acid C18:3ω3) occurs in the green, photosynthetic parts of plants. Without protected seed-bearing flowering plants, the marine food chain is characterized by omega 3 fatty acids, especially the omega 3 superunsaturated fatty acids (SUFA), which are synthesized from the parent fatty acids and bio-magnified through the carnivorous hierarchy. In marine food, the omega 3-SUFA predominate, with docosahexaenoic acid (all cis -4,7,10,13,16,19-docosahexaenoic acid C22:6ω3 – DHA) and eicosapentaenoic acid (5,8,11,14,17-eicosapentaenoic acid C20:5ω3 – EPA) being the most abundant (Sargent et al. 1999). By contrast to the land mammals, fish require omega 3 fatty acids for reproduction (Yu & Sinnhuber 1975).
The migration of land mammals into the sea started some 52 Mya (Gingerich et al. 2001). This long time scale of adaptation of mammals to the marine habitat raises the question ‘are marine mammal lipids adapted to omega 3 fatty acids, like fish, or do they still rely on omega 6 fatty acids?’ The marine mammals, through millions of years of inhabiting the omega 3-rich oceans (Yu & Sinnhuber 1975; Ackman 1989a,b) and having lost many visible characteristics of land mammals, might be expected to have similarly lost biochemical requirements for omega 6 fatty acids and exist in the oceans like the fish, which thrive on omega 3-rich lipids.
The evidence of the high omega 3 SUFA content in the fatty acids in fish oils and marine mammal fats and blubber would seem to uphold the notion of a switch from omega 6 to omega 3. However, the oils and blubber are largely triglycerides. By contrast, active organ lipids are mostly phosphoglycerides with a quite different composition than we reported for the Dolphin (Tursiops truncatus) (Williams et al. 1987).
Baleen whales are inextricably linked with the most productive marine ecosystems in the polar areas (Tynan et al. 2001), with polar food webs dominated by omega 3 SUFA (i.e. EPA and DHA) (Falk-Petersen et al. 2000). During the polar summer the long days result in an abundance of zooplankton, krill, amphipods and fish. The energy derived from the oils of their prey enables the whales to build up their blubber stores prior to reproductive migration. It is claimed that they fast during lactation, using their ability to synthesize high fat, low carbohydrate milk from the blubber during the 5–7-month lactation period (Oftedal 1993). If they fast, one might ask – what is the reason for this longest of migrations of a mammal? Each year the pregnant Gray whales of the Eastern Pacific stock leave their polar feeding grounds in Alaska (Bering and Chukchi seas), where their main prey are the ampeliscid amphipods (Ampelisca macrocephala) that dominate the benthic community of these areas (Highsmith & Coyle 1990). They migrate to three coastal lagoons in Bahia California Sur, Mexico, where calving and breeding occur (Fig. 1). Here we answer the question of whether the Gray whale has adapted to the omega 3-rich food chain of the cold, polar waters and suggest that they do feed in the tropical lagoons and during their migration. Not all species of whales exhibit this long migratory habit. However, we suggest the reason for this long migration of the Gray whale may be to obtain omega 6 fatty acids, assumedly for reproduction, from the warm, tropical waters.
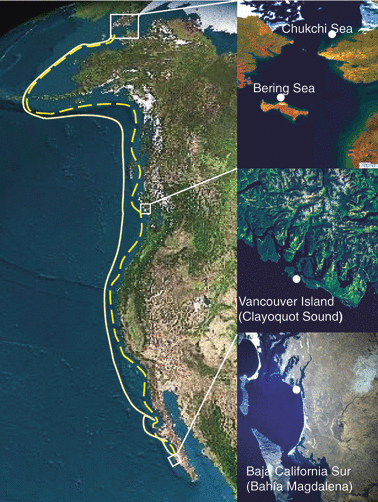
Blue circles indicate the locations where the amphipods detected as prey of this baleen whale were collected. Yellow line represents the southbound migration and the blue dotted line the northbound migration.
Material and Methods
Field sampling
The Bahía Magdalena bay (Fig. 1) was surveyed during the calving season in 2000 to assess the feeding behavior of Gray whales in the breeding grounds. Four benthic samples were taken with an Ekman dredge (6′ × 6′ × 6′) in four areas of recent Gray whale feeding activity. These samples were pooled to average the local variations and then were washed with sea water through a 0.5-mm screen. Samples were kept on ice and frozen after arrival in the laboratory (−20 °C). A set of baleen plates was withdrawn from a stranded 1-year-old Gray whale (8.5 m in length).
At the beginning of the calving season in 2001, Bahía Magdalena was surveyed to collect blubber biopsies from mothers with offspring (n = 10) to provide signature information on the diet from the north feeding grounds. Biopsies were taken with a crossbow and a special fire-cleaned, hollow, stainless-steel biopsy dart (2.5 cm long and 0.6 cm in diameter) and stored in liquid nitrogen.
Gray whale benthic prey were taken from three different latitudes (Vancouver Island, Bering and Chukchi seas) using a Wildco (0.02 m2) and a van Veen (0.1 m2) dredge. Sampling stations were placed at areas of recent Gray whale feeding activity. Organisms were transported on ice after washing with sea water through a 0.5-mm screen and frozen after arrival in the laboratory (−20 °C). At Vancouver Island, Gray whales were observed feeding on benthic amphipods and five replicates were collected. In the Bering Sea, 17 replicates of amphipods were selected randomly from net stations of the Alpha-Helix cruiser of the University of Alaska at Fairbanks, which was designed to evaluate the feeding grounds of Gray whale in the Bering and Chukchi seas.
In the calving season of 2004 we surveyed the three calving lagoons to locate fresh stranded whales and obtain fresh muscle and liver samples from the Gray whale. A Gray whale calf (∼ 4 m in length) that was stranded in Laguna Ojo de Liebre with a fractured caudal fin offered the opportunity to collect these unusual samples. The baleen plates, liver, muscle, blubber and blood samples were isolated using stainless-steel knifes and stored in liquid nitrogen. The objective was to establish whether the cell membrane structural lipids in liver and muscle contained omega 6 arachidonic acid and, if so, the proportion relative to the expected omega 3 EPA and DHA. Although it was possible to obtain blubber samples from the internal tissues of several Gray whales, we aimed to only obtain samples of these tissues from one whale for ethical reasons. The single sample of several muscle sites and liver from this one stranded whale were, from experience with previous comparative studies of Dolphins and many land-based mammals (e.g. Crawford et al. 1976; Williams et al. 1987), sufficient to establish whether the cell membranes of the Gray whales were dominated by omega 3, as in fish, or omega 6, as on land. The data were expressed as a weight percentage of the total fatty acids. A list of tissues sampled and the reasons in the 2006 observations are given in Table 1.
Laboratory techniques
δ13C Determinations
No attempt was made to remove lipids from benthic or dermal skin samples before the stable isotope analysis (SIA). Hence the measured values of δ13C represent the whole benthic specimen and dermal skin compositions. At a point next to the blubber from each whale biopsy, small subsamples were taken transversely across the skin core using a fire-cleaned hollow stainless-steel biopsy tip 0.8 cm long and 0.2 cm in diameter. Each benthic and dermal skin sample was freeze-dried and pulverized in a mortar, then ground again into a homogeneous fine powder. A subsample of 10–20 mg was analysed to determine the δ13C signature (DeNiro & Epstein 1977).
Baleen plates
The consistency of stable carbon isotope ratios (δ13C) in the transverse section of a baleen plate was tested at ∼ 1 cm from the base, following the strategy described by Schell & Saupe (1993). Both tests showed a consistent pattern to that reported for Balaena mysticetus (see Caraveo-Patiño & Soto 2005). Using a scalpel, scissors and tweezers (previously washed with 30% HCl) four samples were obtained across the plate, at intervals of about 1 cm. Each sample was placed in a clean vial and washed with 30% HCl, rinsed with distilled water, freeze-dried and pulverized in an agate mortar. Samples were then ground into homogeneous fine powder from which a subsample of 10–20 mg was analysed to determine the δ13C signature. Isotope patterns of transverse ridges allowed a visual match between adjacent plates (Williams et al. 1987). Hence, such paired samples were those that had been deposited at the same time. We examined δ13C signatures between two adjacent baleen plates from the same jaw (at 26 and 27 cm). These plates were aligned side-by-side and six subsamples (10–20 mg) were taken from equivalent locations along each plate.
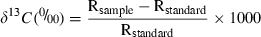
The stable isotope ratio for an animal’s tissue (δtissue) is directly related to that of its diet (δdiet) as δtissue = δdiet + Δdt (Thompson 1996; Tynan et al. 2001). Therefore, we attempted to corroborate that the δ13C of the start and the end of the baleen (δtissue) reflected the Gray whale diet at Bahía Magdalena consumed in winter and the diet from Alaska in summer (Fig. 2). In the equation we used the same trophic enrichment factor (Δdt) of the dermal skin and the value of the δ13C of the baleen (δtissue). We had previously calculated the Δdt value with the signatures of dermal skin (i.e. most recently grown) and the value of the diet in Alaska and Bahía Magdalena (δdiet). We assumed that the baleen and skin tissues of the Gray whale would show similar carbon isotopic fractionation to those corroborated in seals (Falk-Petersen et al. 2000). We expected that the values obtained directly from the benthic fauna (Bahía Magdalena and Alaska) would be similar to those values inferred by the equation. The aim was to test whether the 13C/12C ratio of the polar region persisted through to the warm waters of the reproduction lagoons. As the 13C/12C ratio in the tropical waters is different to that of the cold polar regions, a stable ratio throughout the migration would confirm the absence of feeding in the reproduction lagoons.
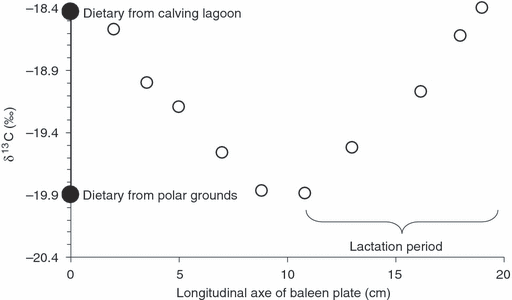
The δ13C values corresponding to the period of formation of baleen plate of a 1-year-old whale at various points calculated using the same trophic enrichment factor (Δdt = 2‰) established previously by Caraveo-Patiño & Soto (2005) for Gray whales (white circles). Black circles are the values of dietary sources.
PUFA profiles from blubber, skin biopsies and prey
At a point next to the blubber from each whale biopsy, a subsample was taken transversely across the biopsy using a fire-cleaned stainless-steel scalpel. Each blubber subsample of ∼ 10 mg was homogenized in a glass homogenizer and filtered using glass microfibre filters (GF/C) after extraction of fatty acids.
Total lipids from benthic fauna and from biopsies were homogenized and extracted using the method of Folch et al. (1957) (Oftedal 1993). The polar lipid fractions of phosphoglycerides were separated from the total lipids by thin-layer chromatography (TLC) on pre-coated silica gel 60 plates (Merck KgaA, Germany) using a developing solvent system of chloroform/methanol/methylamine (65:35:15, by vol.) with 0.01% butylated hydroxytoluene (BHT) as an antioxidant. Similarly, the neutral lipid fractions of triglycerides (TG) and cholesterol esters (CE) were separated on the pre-coated TLC plates by using a solvent system of petroleum spirit/diethyl ether/formic acid/methanol (85:15:2.5:1, by vol.) with 0.01% BHT. The different phosphoglyceride and neutral lipid bands were visualized by spraying the developed plates with a methanolic solution of 2,7-dichlorofluorescein (0.01% wt/vol), and identification using authentic standards.
Heptadecanoic acid (C17:0) was used as an internal standard in the lipid fractions for the quantitative analysis. Fatty acid methyl esters (FAMEs) were prepared using the lipid fraction reacted with 4 ml of 15% acetyl chloride–methanol solution in a sealed vial at 70 °C for 3 h. FAMEs were separated using gas–liquid chromatography (HRGC MEGA 2 Series; Fisons Instruments, Milan, Italy) fitted with a BP 20 capillary column (30 m × 0.32 mm ID, 0.25 μm film; SGE Ltd, UK. Hydrogen was used as a carrier gas. The temperature of the injector, oven and detector was 240, 195 and 260 °C, respectively. The FAMEs were identified by comparison of retention times with authentic standards and interpretation of equivalent chain length values. Peak areas were integrated and normalized using computer software (ezchrom 6.6 Chromatography Data System; Scientific Software, Inc., San Ramon, CA, USA).
Fatty acids were identified by comparing mass spectra obtained against records from the library of the National Institute of Standards and Technology and retention times from various commercial standards. Principal fatty acid content was expressed as the percentage of total fatty acid methyl ester (% FAME). The content of principal fatty acids was expressed as a weight percentage of the total fatty acids, and total amounts were calculated from the external standard.
Results
Amphipods (mainly Ericthonius brasiliensis) appeared to be the main prey of the whales, together with a small fraction of isopods. The δ13C value of the Gray whale’s prey in Bahía Magdalena was −18.4 ‰ ± 0.2 ‰. Isotopic ratio results using the dermal skin values showed that the dietary enrichment from prey in Bahía Magdalena corresponded to 1.9 ± 0.1‰ (n = 7; average ± SD, n = number of replicates), whereas previously published results for prey in Alaska showed an enrichment of 3 ± 0.1‰ (n = 7) (Fig. 2). The proportion of 13C in the food web varies from the Arctic to the Equator. If the Whales did not feed during the migration, or in the lagoons then the 13C data along the growing baleen plates should be uniform longitudonally. That is in Figure 2 the data should be represented by a straight line. However, the isotope ratios we obtained changed across the migration route and back again. Moreover, the change in isotopic composition along the longitudinal axis of the baleen plate of the young Gray whale was equivalent to the difference between amphipod prey from Alaska and from Bahía Magdalena (i.e. 1.5%). These data suggest feeding activity along the route and in both polar regions and calving lagoons (Fig. 2) and further confirm feeding behaviour in Bahía Magdalena.
Our Gray whale observations were based on 23 sightings during February, March and April 2006. Underwater observations and photographic recordings showed four Gray whales with general feeding behaviour. Previous underwater photography added further support for this behaviour, as reported by Caraveo-Patiño & Soto (2005).
The blubber EPA/ArA ratio was reduced in the breeding lagoons as compared to the polar regions, again consistent with a change in feeding pattern (Fig. 3). EPA and DHA dominated the total PUFA content (18% and 7%, respectively) of blubber samples, whereas ArA was relatively scarce (1.5%) (Table 2). However, the membrane lipids from liver and muscle tell a different story (Table 2). The compositional data of Gray whale active tissues, as in the dolphin Tursiops truncatus, is more consistent with an omega 6 environment (Fig. 4).
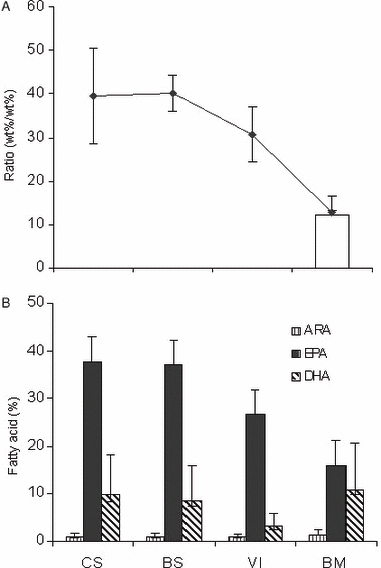
(A) Fatty acid ratios EPA/ArA (♦ and vertical lines, mean ± SD) in the total lipids of the amphipod prey of Gray whale from different latitudes and in the blubber from gray whale mothers (n = 10) collected in the southern-most calving lagoon (white bar). (B) Relative amounts of principal long chain highly unsaturated fatty acids (wt%, mean ± SD) in total lipids of prey along the migratory corridor and from the southern-most calving lagoon, i.e. Bahía Magdalena. CS, Chukchi Sea; BS, Bering Sea; VI, Clayoquot Sound in Vancouver Island; BM, is Bahía Magdalena. The proportion of omega 3 fatty acids declines from polar regions to the warm water of Bahia. The sampling in the Bahia region was done out at sea. It is likely from other published data that the amphipods in the river mouths would be different and if so more likely to have a higher ArA content, as suggested by the work of Lahdes et al. (2000). More data on the bivalves and amphipods from the polar and Bahia regions are needed in view of the data available on fish from tropical waters, which contain substantially more ArA.
| Fatty Acid | Blubber (n = 10) | Liver (n = 2) | Muscle (n = 4) | |||||
|---|---|---|---|---|---|---|---|---|
| Total Fat | PC | PE | TG | CE | PC | PE | TG | |
| 16:0 | 36.17 ± 5.7 | 19.03 | 8.58 | 17.52 | 9.06 | 19.29 ± 0.58 | 2.55 ± 0.74 | 21.03 ± 1.96 |
| 16:1ω7 | 8.24 ± 2.09 | 8.63 | 2.14 | 3.80 | 9.66 | 5.77 ± 0.26 | 1.85 ± 0.65 | 4.31 ± 0.47 |
| 18:0 | 7.28 ± 5.69 | 12.05 | 26.94 | 11.35 | 8.28 | 10.08 ± 0.26 | 11.14 ± 1.08 | 11.46 ± 1.11 |
| 18:1ω9 | 15.0 ± 4.2 | 15.7 | 5.83 | 15.1 | 28.5 | 26.5 ± 0.99 | 10.4 ± 1.12 | 23.6 ± 5.84 |
| 18:2ω6 | 1.8 ± 0.4 | 0.39 | 0.22 | 0.44 | 0.37 | 0.39 ± 0.02 | 0.17 ± 0.06 | 2.62 ± 1.66 |
| 18:3ω3 | nd | 0.02 | 0.02 | 0.12 | 0.02 | 0.01 ± 0.02 | 0.02 ± 0.02 | 0.41 ± 0.31 |
| 20:4ω6 | 1.5 ± 0.3 | 5.76 | 16.93 | 7.32 | 3.63 | 4.72 ± 0.20 | 10.1 ± 0.49 | 2.68 ± 2.26 |
| 20:5ω3 | 18.1 ± 7.9 | 2.71 | 5.52 | 2.9 | 1.65 | 7.47 ± 0.37 | 15.4 ± 0.33 | 1.83 ± 0.87 |
| 22:6ω3 | 7.0 ± 2.8 | 6.44 | 8.07 | 2.07 | 1.7 | 1.18 ± 0.03 | 3.49 ± 0.31 | 1.11 ± 0.67 |
| ArA/EPA | 0.08 ± 0.04 | 2.1 | 3.0 | 2.5 | 2.2 | 0.67 ± 0.05 | 0.70 ± 0.06 | 1.46 ± 0.98 |
| ArA/DHA | 0.21 ± 0.11 | 0.89 | 2.1 | 3.5 | 2.1 | 4.00 ± 0.01 | 2.89 ± 0.05 | 2.41 ± 2.11 |
| EPA/ArA | 12.3 ± 4.3 | 0.48 | 0.33 | 0.40 | 0.45 | 1.49 ± 0.43 | 1.43 ± 0.62 | 0.68 ± 0.38 |
- The blubber contains only small amounts of ArA, unlike the liver and muscle. Assumedly the relative abundance of EPA and DHA in the marine food chain and the scarcity of ArA, except in warm waters, means that the whale is selectively conserving its ArA content in cell membrane structure and function whilst enjoying the luxury of depositing the abundant ω3 fatty acids in blubber, which would help provide appropriate insulation and fluidity in very cold waters. However, the conservation of ArA over 40–50 million years, through the delibration migration to warm waters for reproduction has interesting implications for evolutionary biology and conservation.
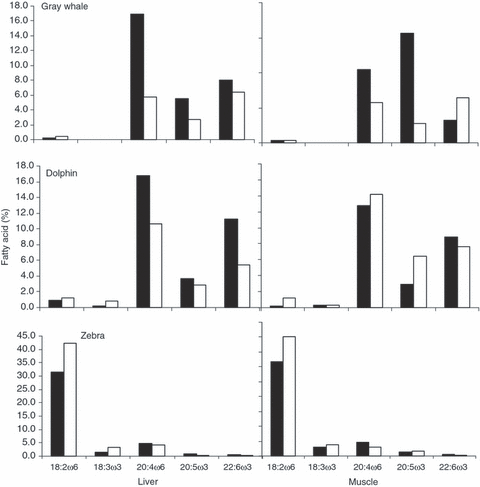
Mean proportions (wt %) of principal cell membrane ω3 and ω6 PUFA in phosphatidylethanolamine (PE, white bars) and phosphatidylcholine (PC, black bars) of the liver and muscle from (A) Gray whale, (B) dolphin and (C) zebra.
The liver is the main site of metabolism in mammals in which essential fatty acids derived from the diet via the small gut and portal system are sorted and incorporated into appropriate fractions. Lipids are sorted for distribution to cell membranes, transport systems or fat stores. Table 1 presents data on the lipid fractions from the liver of the Gray whale. ArA is the richest SUFA in all fractions of the liver lipids and is present in higher proportions than DHA, which answers the question of whether the Gray whale has abandoned its omega 6 EFA requirements in exchange for an omega 3 marine system. In the inner cell membrane fraction (phosphatidylethanolamine, PE) ArA was 17%, compared to only 8% for DHA or 5% for EPA. However, in the phosphatidylcholine (PC) fraction the proportion of DHA and ArA was similar. The proportion of ArA in cholesterol esters and triglycerides was two to three times greater than DHA.
| tissue | reason |
|---|---|
| Blubber | To assess the composition of the storage fats that are residual after essential fats have been selected for membrane growth, maintenance and repair. |
| Liver | The liver is the place where the dietary lipids are integrated into different lipid fractions for use by other biological systems. |
| Muscle | Represents the large bulk of cell membrane lipids. |
| Baleen | Used for isotope ratios to determine if the ratio was the same over its length to confirm that there was no feeding at different latitudes. Feeding during the migration would be reflected by a change in the ratio consistent with the differences in isotopes in the food web at different latitudes. |
| Lung & blood | These tissues were collected for future analysis but no data are reported here. |
| Lipid fractions | Separation into phospholipid fractions (PC – choline external membrane and PE – ethanolamine, inner cell membrane), cholesterol ester and triglycerides was done because the phospholipids represent the membranes of active organs, the cholesterol esters, transport and the triglycerides are mainly for storage and energy. The compositions of these fractions are completely different, reflecting their different functions. |
| Amphipods | Were obtained from Vancouver, Bering Sea and Bahia Magdalena regions to confirm their fatty acid dominance of omega-3 fatty acids and the latter to test whether they were a source of ArA in Bahia Magdalena. More work needs to be done to establish the ArA sources in the warm water food web. |
The results of the muscle data in Table 2 are essentially similar to those in the liver but with EPA present in larger proportions. In the triglycerides the proportions of ArA, for example in blubber, were much lower than in the active muscle cell membranes, but even so they were twice that of EPA or DHA.
A collection of data on some land mammals and fish fatty acids from warm and cold waters is also presented in Table 2. There is a paucity of data on warm water prey of the Gray whale such as the bivalves and crustacea but the fish from the warm waters can only be deriving their ArA from the warm water food web. This data justifies a detailed investigation of the warm water food web of the breeding lagoons.
In Fig. 4 and Table 4 we re-state our analysis of the dolphin and zebra liver and muscle for comparison with that of the Gray whale. The phosphatidylethanolamine (PE, mainly inner cell membrane), as in the dolphin, was dominated by ArA, representing 16.9% and 16.8%, respectively (Fig. 4). The lipids of land-based zebra were especially rich in omega 6 linoleic acid. The PUFA lipid composition in Gray whale liver phosphatidylcholine (PC) was characterized by both by ArA and DHA (5.8 and 6.4%, respectively). Dolphin liver PC had more ArA (14.2%) (Fig. 4).
| Dolphin muscle | Dolphin liver | Giraffe muscle | Zebra muscle | |||||
|---|---|---|---|---|---|---|---|---|
| PC (n = 14) | PE (n = 2) | PC (n = 14) | PE (n = 4) | PC (n = 6) | PE (n = 6) | PC (n = 6) | PE (n = 6) | |
| 18:2ω6 | 1.2 ± 0.12 | 0.23 | 1.2 ± 0.13 | 0.94 | 24 ± 2.1 | 11 ± 1.8 | 40 ± 3.6 | 32 ± 3.4 |
| 18:3ω3 | 0.3 ± 0.12 | 0.32 | 0.8 ± 0.3 | 0.8 | 3.5 ± 0.35 | 2.7 ± 0.3 | 3.7 ± 0.65 | 2.9 ± 0.27 |
| 20:3ω6 | 0.4 ± 0.15 | 0.14 | 0.6 ± 0.071 | 0.54 | 2.7 ± 0.23 | 1.3 ± 0.22 | 2.1 ± 0.15 | 1.9 ± 0.16 |
| 20:4ω6 | 14 ± 1.1 | 13 | 11 ± 1.2 | 17 | 7.4 ± 0.9 | 13 ± 1.1 | 3.0 ± 0.4 | 4.5 ± 0.39 |
| 22:4ω6 | 0.88 ± 0.9 | 1.6 | 2.1 ± 0.4 | 2.6 | 0.6 ± 0.03 | 3.8 ± 0.18 | 0.1 ± 0.02 | 1.1 ± 0.08 |
| 20:5ω3 | 6.4 ± 0.9 | 2.9 | 2.9 ± 0.3 | 3.7 | 1.4 ± 0.14 | 0.75 ± 0.04 | 1.5 ± 0.2 | 1.3 ± 0.09 |
| 22:5ω3 | 1.2 ± 0.12 | 0.26 | 1.4 ± 0.2 | 3.6 | 4.5 ± 0.51 | 7.8 ± 0.66 | 1.2 ± 0.22 | 2.3 ± 0.21 |
| 22:6ω3 | 7.6 ± 0.6 | 8.8 | 5.4 ± 0.9 | 11 | 0.25 ± 0.02 | 1.3 ± 0.02 | 0.23 ± 0.02 | 0.58 ± 0.03 |
| ω6/ω3 | 1.1 | 1.1 | 1.4 | 1.2 | 3.7 | 2.4 | 6.9 | 5.6 |
ArA was surprisingly prominent in all active tissue lipids of the Gray whale, a finding unpredicted by examination of the blubber.
The fatty acid composition of the fauna identified as carbon sources for Gray whales in both the northern grounds and the southern-most breeding lagoon, plus other points along the migration corridor, showed that the EPA content from Alaska (Chukchi and Bering seas) had the highest average (37.7% and 37%, respectively) and that from breeding grounds had the lowest (16%). The ArA presence was relatively scarce (∼ 1%) and very similar between the different species preyed upon by the Gray whale (Table 3). As the maternal Gray whales proceed from the north to the calving lagoon, the EPA/ArA ratio in their prey falls from 39.5 to 12.7 (Table 4). The EPA/ArA ratio from the arctic fauna was significantly higher than the ratio of the outer blubber of the Gray whale mothers (i.e. the layer situated just under the epidermis), whereas the EPA/ArA ratio of the prey from the calving lagoon was very similar to that in the outer blubber (Table 5).
| wild salmon (Alaska) | Sea trout (Scotland) | Cod muscle | Cod liver oil | A Chukchi sea | A Bering sea | A Bahía Magdalena | |
|---|---|---|---|---|---|---|---|
| 18:2ω6 | 1.2 | 3.4 | 0.6 | 0.9 | 0.61 | 1.2 | 1.6 |
| 18:3ω3 | 0.87 | 0.93 | 0.5 | 0.8 | 0.27 | 0.3 | 0.84 |
| 20:3ω6 | 0.16 | 0.16 | 0.3 | 0.7 | – | – | – |
| 20:4ω6 | 0.52 | 0.4 | 1.4 | 0.8 | 1.0 | 0.9 | 1.3 |
| 22:4ω6 | 0.07 | n.d. | 0.01 | 0.3 | – | – | – |
| 20:5ω3 | 8.1 | 4.4 | 1.7 | 9.6 | 38 | 37 | 16 |
| 22:5ω3 | 3.1 | 2.2 | 2.3 | 2.0 | – | – | – |
| 22:6ω3 | 14 | 11 | 47 | 8.0 | 9.9 | 8.5 | 11 |
- The Amphipode lipids actually contain little ArA and the species studied may not be the main source of ArA in the warm water regions (see Lahdes et al. 2000), although the much lower level of EPA would make what little ArA is present more biologically available. Note the high concentrations of ArA in the tropical fish from fresh water and sea. The dolphin choline phosphoglycerides have higher concentrations of ArA than either of the land mammals of similar body weights. By contrast, the land mammal membrane lipids are rich in linoleic acid. Note the relative paucity of DHA in the lipids of the land mammals where the docosapentaenoic acid (ω3) accumulates instead. It is interesting that the land plant precursors of ArA and DHA exceed by far the proportions of these products, reflecting the rate limitations in their synthesis (data from this study).
| Turkana Tilapia | Turkana Perch | Epinephelus sp. Dar-es-Salaam | Bonito (Sarda sp.) Dar-es-Salaam | Buffalo, Syncerus caffer | Giraffe | Pig, Phacochoerus aethiopicus | |
|---|---|---|---|---|---|---|---|
| 18:2ω6 | 2.2 | 1.8 | 1.3 | 0.8 | 15 | 22 | 32 |
| 18:3ω3 | 0.9 | 0.8 | 0.8 | 0.6 | 5.1 | 4.7 | 4.9 |
| 20:3ω6 | 2.4 | 1.9 | 3.7 | 1.2 | 1.8 | 2.7 | 2.9 |
| 20:4ω6 | 8.4 | 7.7 | 16 | 17 | 7.3 | 8.7 | 6.8 |
| 22:4ω6 | 2.8 | 3.5 | 4.2 | 2.1 | 1.8 | 2.2 | 2.7 |
| 20:5ω3 | 2.8 | 1.8 | 4.5 | 4.0 | 3.6 | 2.2 | 3.4 |
| 22:5ω3 | 3.3 | 3.8 | 2.6 | 1.4 | 5.1 | 4.7 | 4.8 |
| 22:6ω3 | 16 | 18 | 20 | 16 | 0.8 | 0.6 | 1.1 |
| ω6/ω3 | 0.71 | 0.61 | 0.91 | 0.95 | 1.8 | 2.9 | 3.1 |
Discussion
The adult length of baleen plates of the Eastern Pacific stock Gray whale calves is reached during the lactation period (Sumich 2001). Hence we expected the carbon source that laid down the keratinous tissues of the baleen plates of all 1-year-old calves to reflect the carbon sources of the main prey from the summer feeding grounds in the arctic on the assumption that there was no feeding in transit or in the breeding lagoons. The stable carbon isotope values at various points along the longitudinal axis of a baleen plate from a stranded 1-year-old suggests that the supposition that Gray whales mothers do not feed during the lactation period is wrong (Fig. 2) (Caraveo-Patiño & Soto 2005).
The total fatty acid content in blubber biopsies of Gray whale mothers revealed that the omega 3 fatty acids clearly dominated the PUFA content. In contrast, the lipid composition of fresh samples of muscle and liver from several sites of a calf contained substantial quantities of long chain omega 6 fatty acids, especially ArA. This difference most likely reflects the selective uptake of ArA for cell membranes and the general requirement for omega 6 fatty acids in mammals (Marcel et al. 1968). We cannot prove that the whale uses the omega 6 fatty acids for reproduction but as this is a common requirement in land mammals, we believe that this is highly likely to be true.
As the arctic pack ice retreats, exposing the sea to continuous sunlight, the Gray whale would make use of the tremendous seasonal abundance of omega 3-rich food. The Gray whale is mainly a bottom feeder, sucking small invertebrates and crustaceans from the sand and mud. Mating is seasonal, occurring in November–December, although some may not conceive ‘until in the winter assembly area or even on the northward spring migration’ (Jones & Swartz 2000).
At first the high levels of ArA in the structural tissues of marine mammal species seems puzzling. Ackman (1982, 1989a,b) pioneered the analysis of marine faunal lipids working from Halifax, Nova Scotia, mostly on cold water species in which the omega 3 fatty acids dominated. This dataset has left people with the impression that the marine system only provides omega 3 fatty acids. However, in Brazilian Amazonian and Australian warm water fish, significant amounts of omega 6 fatty acids, especially ArA, have been reported sufficient to increase the ArA blood levels when consumed by humans (Sinclair et al. 1983). ArA also occurs in seaweed, Chrondrus crispus, boreal freshwater fishes (Agren et al. 1987) and fish obtained from low latitudes (Lahdes et al. 2000). This suggests an important source related to fresh water, coastal or shallow water origins. The omega 6 content of the food web increases with increasing temperature (Hazel 1979; Lahdes et al. 2000); the higher the temperature of the water, the richer amphipod fatty acids are in ArA. Up to 9.3% ArA has been reported in the phospholipids at 15 °C compared to 3.6% at 8 °C (Lahdes et al. 2000). Notably, conception is linked to the sojourn of these whales in the warm water where the ArA would be available. At arctic temperatures the proportion of ArA we recorded was little more than 1% of the fatty acids. Similar data on ArA enrichment has been reported for seals (Käkelä & Hyvärinen 1998). More detailed analyses of the food webs in the tropical regions and indeed polar regions are needed to better identify the sources of ArA and also to assess differences in co-existing micronutrients which are relevant to the utilization of the SUFA and mammalian physiology.
As we have clear visual and stable isotope evidence of feeding, it is plausible that the reason for the long migration and the Gray whales’ choice of breeding grounds in warm waters is to obtain the omega 6 fatty acids that are reasonably abundant in the warm water food web. This biological conservation of ArA by the Gray whale over the best part of 50 million years raises two interesting questions. First, it questions the commonly held view and current enthusiasm for the exclusivity of omega 3 fatty acids for human health. Secondly, it questions the popular claim of the hazardous nature of ArA, which contributed to the design of COX2 inhibitors and their consequent problems.
From our experience of Gray whale behaviour and the data presented here and previously (Caraveo-Patiño & Soto 2005), it seems that in the winter the migrating whales feed mostly at the mouth of rivers or estuaries. This conclusion has important implications for conservation. Like Gray whales, many other baleen whale species breed and calve in coastal habitats, or feed along shelf edges or areas of ocean upwelling. The remarkably high proportion of ArA in the membrane implies that the omega 6 requirement is likely to be common for marine mammals. Unfortunately, coastal areas are also the focus of much anthropogenic activity such as mineral extraction, dredging and shipping. The deliberate seeking of the estuarine and lagoon food web exposes these species to pollution. Examples of mortality amongst marine mammals have been reported along the Californian coast and have been referred to as ‘sentinels’, analogous to the canaries the miners used to warn them of unhealthy air (Gulland 1999; Scholin et al. 2000). The fact that the Gray whale is seeking nutrients that are likely essentials to its reproduction along this coast and in the breeding lagoons of Bahia Magdalena just to the south, adds a new dimension to conservation concerns.
Conclusions
The Gray whale has conserved its use of omega 6 ArA in cell membranes despite a 50–30 million year exposure to the marine omega 3-rich environment. It is likely that this serves the mammalian requirement for omega 6 fatty acids for reproduction as well as for the brain.
The δ13C values of dietary sources across the migration route, consistent with underwater photography, imply that the Gray whale may feed en route and certainly in the tropical breeding lagoons.
It is likely that one reason for the extensive migration of the Gray whale is to obtain ArA for reproduction, as ArA is scarce in the polar food web but more abundant in the warm water of the tropical breeding lagoons. However, caution is needed with these limited findings and this conclusion can only be a guide to further work.
As estuarine products that contain omega 6 are potentially contaminated with the products of human pollution, the utilization of omega 6-containing food for reproduction in the breeding lagoons is likely to be important to Gray whale conservation and relevant to the conservation of other marine mammals, which will similarly be seeking omega 6-rich food resources in warm, coastal regions.
Acknowledgements
J. Caraveo-Patiño would like to mention very special thanks for all people who gave valuable logistic and financial support during our field work, the staff of La Reserva de la Biosfera EI Vizcaíno, Exportadora de Sal S.A. de C.V. Guerrero Negro, Ecoturismo Kuyima, the oceanographic Ship R/V Alpha Helix, CONABIO and CIBNOR. Particularly to Susan J. Chivers, Dawn Goley, Alicia Mallo, Misty Niemeyer, Shannon Fitzgerald, Dave Duffus, Heather Patterson, Sue More, Raymond C. Highsmith, Ken Coyle, Steven Hastings, Patrick Hahn and family, Saravia Family.




