Variability in the structure of epiphytic assemblages of the Mediterranean seagrass Posidonia oceanica in relation to depth
Abstract
The aim of the present study was to evaluate whether the variability in the structure of the epiphytic assemblages of leaves and rhizomes of the Mediterranean seagrass Posidonia oceanica differed between depths at a large spatial scale. A hierarchical sampling design was used to compare epiphytic assemblages at two different depths (10 and 20 m) in terms of both species composition and abundance and patterns of spatial variability in the Tuscan Archipelago (North Western Mediterranean Sea, Italy). Results showed significant differences in the structure of assemblages on rhizomes and leaves at different depths. These differences were related to species composition and abundance; differences were not significant for total biomass, total percentage cover and percentage cover of animals and algae. Whereas the higher variability was observed among shoots in all the studied systems, patterns of spatial variability at the other spatial scales investigated differed between the two studied depths. Moreover, in the present study, analogous patterns between depths resulted for both the assemblages of leaves and rhizomes, suggesting that factors that change with depth can be responsible for the spatial variability of both the assemblages (leaves and rhizomes), and operate regardless of the microclimatic conditions and the structure of assemblages.
Problem
Epiphytes play a central role in the ecological function of seagrass ecosystems by contributing greatly to the diversity and primary production of the system, and they constitute an important food resource for many invertebrates and fishes of these habitats (Borowitzka & Lethbridge 1989; Klumpp et al. 1992; Moncreiff et al. 1992; Van Elven et al. 2004). Moreover, epiphytic assemblages are considered useful indicators of disturbances (May 1982; Borum 1985; Frankovich & Fourqurean 1997; Leoni et al. 2006). For these reasons, accumulating knowledge about distribution patterns of epiphytes represents an important goal for ecologists and many studies have focused on this topic (Heijs 1985; Borowitzka et al. 1990; Kendrick & Burt 1997; Reyes et al. 1998; Trautman & Borowitzka 1999; Vanderklift & Lavery 2000; Lavery & Vanderklift 2002; Pardi et al. 2006; Balata et al. 2007; Piazzi et al. 2007). Most of the studies on seagrasses have been carried out in shallow waters (Duarte 1991), so the importance of depth to influence seagrass communities has so far received little consideration by non-European ecologists (Collier et al. 2007). In clear Mediterranean waters, Posidonia oceanica (L.) Delile frequently occurs at depths from 0 to >40 m. In this context, depth may influence the structure of epiphytic assemblages, as for seagrass physiological responses (Olesen et al. 2002). The colonization of epiphytes both on leaves and rhizomes depends on a combination of stochastic events and factors acting in the meadows at various spatial and temporal scales, such as differences in shoot density, water motion, light, temperature, grazing and competition among sessile organisms (Trautman & Borowitzka 1999; Vanderklift & Lavery 2000). Many of these factors are related directly or indirectly to depth, which therefore plays a key role in the distribution of seagrass epiphytes (Cinelli et al. 1984; Boero et al. 1985; Pansini & Pronzato 1985;Casola et al. 1987; Buia et al. 1989; Mazzella et al. 1989; Peirano & Morri 1990; Piazzi et al. 2002; Esposito et al. 2003; Tsirika et al. 2007). However, the studies that have addressed this topic were limited to leaf or rhizome epiphytes, considered only animals or algae, or were carried out on small spatial scales, mainly along depth gradients on individual meadows. Works taking into account both rhizome and leaf habitats, animal and algal components identified at a high taxonomic levels, and performed on multiple spatial scales are necessary to evaluate spatial patterns of the whole system. In fact, leaf and rhizome habitats are characterized by different environmental conditions, as leaves attenuate light and water movement, creating a sheltered habitat on rhizomes (Boudouresque 1974;Borowitzka & Lethbridge 1989; Chimenz et al. 1989; Mazzella et al. 1992; Gacia et al. 1999; Balata et al. 2007); thus, rhizome assemblages could respond differently to depth compared to leaf assemblages. Moreover, studies taking into account wider spatial scales may reduce the influence of stochastic events to determine patterns of spatial variability.
The aim of the present study was to evaluate whether the variability and the overall structure of epiphytic assemblages of both leaves and rhizomes of the Mediterranean seagrass P. oceanica differed among depths at different spatial scales. In particular we tested the following hypotheses: (i) epiphyte assemblages differed between depths in terms of both composition and abundance of species; and (ii) the variability and structure of assemblages of rhizome and leaf respond differently to depth. A hierarchical sampling design was used to compare epiphytic assemblages at two different depths and three different spatial scales in terms of both species composition and abundance.
Material and Methods
Study site
The epiphytic assemblages on leaves and rhizomes of P. oceanica were studied at the depths of 10 and 20 m; these depths were chosen as being above and below, respectively, the depth separating meadows with different characteristics normally recognized for P. oceanica (Mazzella et al. 1989). The study was carried out in three different islands in the North-west Mediterranean Sea; these islands (Capraia, Elba and Pianosa) were randomly chosen among the islands of the Tuscan Archipelago National Park (Tyrrhenian Sea and Ligurian Sea, Italy) and were characterized by low anthropogenic pressure to avoid additional factors that could confound the results (Fig. 1).
Map of the study area, with location of the studied Posidonia oceanica meadows (islands).
Sampling was done during late August, which corresponds to the period in which epiphytic assemblages of P. oceanica reach their maximum development (Panayotidis 1980; Mazzella & Ott 1984). Because the kind of substrate, the distance from rocky reefs and the morphological features of the meadows (e.g. distribution, cover, density, canopy, etc.) can influence the structure of epiphyte assemblages (Van Elven et al. 2004; Giovannetti et al. 2008), we selected, at both depths, meadows colonizing rocky substrate and characterized by comparable values of cover (about 100%), density (density in the range 652 ± 19.5 to 681 ± 33.2 and 583 ± 27.9 to 601 ± 22.7 shoots·m−2, at 10 and 20 m depth, respectively, mean ± SE, n = 5) and canopy (47.09 ± 1.71 to 58.51 ± 1.71 and 32.29 ± 1.19 to 40.77 ± 1.12 cm) assessed during preliminary investigations.
Sampling design and data collection
At each island (location), 10 km apart from each other, three sites (portions of meadow about 600 m2 and 100 m apart) were chosen randomly; two depths were selected, 10 m (shallow) and 20 m (deep); five replicate plots (1 m2 each about 10 m apart) were selected at random in each site at each depth. Within each plot, five randomly chosen shoots were uprooted and preserved in 4% formalin sea water for laboratory observation. The oldest part (the first 10 cm from the tip) of the internal face of the two external leaves of each shoot and the first 10 cm of rhizome were examined under a dissecting microscope. These leaves and portion represent the oldest ones within the shoot (Buia et al. 2004). Only the internal side of leaves was observed because it was considered more colonized than the external side (Casola et al. 1987; Casola & Scardi 1989; Alcoverro et al. 2004). Data from the two leaves within each shoot were averaged so that shoots provided replicates in the analyses (Piazzi et al. 2004; Balata et al. 2007, 2008). Animal and macroalgal organisms were identified to the level of species or genus, and the abundance of each taxon was calculated as the surface covered by the taxon in orthogonal projection relative to the total surface of leaves or rhizomes sampled. Final values were expressed as percentage cover. Moreover, the cumulative percentage cover of total animals and total algae was calculated by summing all the species belonging to each category (Piazzi et al. 2004).
In each plot, five additional shoots were sampled to determine the biomass of epiphytic assemblages. For each shoot, the internal face of the first 10 cm from the tip of the two external leaves was scratched with a razor blade and biomass of epiphytes was evaluated as dry weight after 48 h at 60 °C.
The incidence of grazing was also evaluated as the percentage of bitten leaves on the total leaves of the sampled shoots.
Analysis of data
Multivariate analysis of variance based on permutations (PERMANOVA) was used to test the hypothesis that epiphytes showed different patterns of variation in composition and in abundance of species in relation to depth and spatial scales (Anderson 2001). For both rhizome and leaf epiphytes, the analysis consisted of a four-way model with Depth (shallow versus deep, as fixed factor), Island (three levels, random), Site (three levels, random and nested within Island) and Plot (five levels, random and nested within the interaction Depth × Site(Island), with five replicates for each plot. PERMANOVA was conducted on Bray–Curtis dissimilarity matrix (Bray & Curtis 1957), calculated from untransformed data. To detect whether the potential differences between the assemblages of the two depths were due to differences in species composition or in relative abundances, the same analyses were repeated using presence/absence data. The pairwise test was performed to determine which levels were responsible of significant interactions.
Pseudo-components of variance were calculated for all the spatial scales, separately for each depth both for untransformed and presence/absence data to determine any differences in spatial variability between the two studied assemblages.
The program IndVal (Indicator Value) was used to identify the species most contributing to the multivariate patterns, defined as the most characteristic of each group, being found mostly in a single group or present in the majority of samples belonging to that group (Dufrene & Legendre 1997). In our study the IndVal program was used to determine which species separated the assemblages of different depths.
A two-dimensional nMDS (non-metric multidimensional scaling), based on the centroids for replicate plots, was used for a graphical representation of the data for leaf and rhizome epiphytic assemblages. Distances among centroids were obtained using principal coordinate axes from the original Bray–Curtis matrices (untransformed and based on presence/absence data) for epiphytic assemblages of both leaves and rhizomes.
Differences in biomass, in number of taxa and in percent cover of total animals and of total algae were analysed using univariate analysis of variance (ANOVA). Biomass of epiphytic assemblages of leaves was also analysed. Factors and levels considered in these analyses were the same described for the multivariate analysis. Cochran’s C-test was used before each analysis to check for homogeneity of variance, and data were transformed when necessary. Student–Newman–Keuls (SNK) test was used for a posteriori multiple comparison of means (Underwood 1997).
Results
A total of 135 taxa were identified in the epiphyte assemblages: 51 Macroalgae (33 Rhodophyta, 12 Ochrophyta and 6 Chlorophyta), 38 Bryozoa, 11 Cnidaria (Hydrozoans), 7 Annelida, 8 Tunicata, 8 Porifera and 5 Rhizopoda (Foraminifera). The total number of species was 78 and 85 on leaves, and 107 and 101 on rhizomes, in shallow and deep assemblages, respectively. Seventy-five species were present in both rhizome and leave assemblages.
Leaf assemblages were dominated by encrusting algae belonging to genus Pneophyllum and Hydrolithon; common algal species were also Sphacelaria cirrosa, Dictyota spp. and Laurencia spp. Among animals, the Bryozoa Electra posidoniae and Escharoides coccinea, the Tunicata Botryllus schlosserii, and the Rhizopoda Cyclocibicidus vermiculatus were widely distributed.
Rhizome assemblages were mostly characterized by the Rhodophyta Acrothamnion preissii and Peyssonnelia spp.; other common species were the Bryozoa Scrupocellaria reptans, Aetea lepadiformis, Chilidonia pyriformis, Diastopora patina and Schizobrachiella sanguinea, the Rhizopoda Miniacina miniacea and the Porifera Plakortis simplex.
For leaf assemblages, PERMANOVA analyses performed on untransformed data showed as significant the interactions Depth × Island and Depth × Site. Pairwise tests showed that differences between depths were significant at all the islands and for eight of the nine sites studied. The interaction Depth × Site was also significant in the analysis performed on presence/absence data even though the differences between depths were significant in all the sites (Table 1).
| source | df | no transformation | p/a transformation | ||||
|---|---|---|---|---|---|---|---|
| MS | pseudo-F | P-value | MS | pseudo-F | P-value | ||
| Depth = De | 1 | 100,120.00 | 4.58 | 0.008 | 48,573.00 | 3.55 | 0.018 |
| Island = Is | 2 | 56,627.00 | 5.25 | 0.001 | 71,795.00 | 8.68 | 0.001 |
| Site (Is) = Si(Is) | 6 | 10,793.00 | 4.54 | 0.001 | 8276.10 | 5.90 | 0.001 |
| Plot (De x Si(Is)) | 72 | 2374.40 | 1.98 | 0.001 | 1399.30 | 1.29 | 0.001 |
| De x Is | 2 | 21,860.00 | 2.03 | 0.029 | 13,680.00 | 1.66 | 0.098 |
| De x Si(Is) | 6 | 10,780.00 | 4.53 | 0.001 | 8237.80 | 5.88 | 0.001 |
| Residual | 360 | 1201.20 | 1085.70 | ||||
| Total | 449 | ||||||
| pairwise test | ||
|---|---|---|
| no transformation | p/a transformation | |
| De × Is | Always significant differences between depths | – |
| De × Si | At one site there are no differences between depths | Always significant differences between depths |
- Significant effects are indicated in bold.
For rhizome assemblages, analysis on untransformed data and on presence/absence data showed a significant Depth × Site interaction; pairwise tests showed that differences between depths were significant at all sites in the analysis with presence/absence data and at seven of the nine sites studied with untransformed data (Table 2).
| source | df | no transformation | p/a transformation | ||||
|---|---|---|---|---|---|---|---|
| MS | pseudo-F | P-value | MS | pseudo-F | P-value | ||
| Depth = De | 1 | 37,788.00 | 3.60 | 0.002 | 47,812.00 | 4.19 | 0.007 |
| Island = Is | 2 | 25,442.00 | 1.63 | 0.038 | 41,764.00 | 4.28 | 0.001 |
| Site (Is) = Si(Is) | 6 | 15,621.00 | 4.75 | 0.001 | 9752.70 | 4.83 | 0.001 |
| Plot (De x Si(Is)) | 72 | 3281.10 | 2.32 | 0.001 | 2015.40 | 1.59 | 0.001 |
| De x Is | 2 | 10,509.00 | 0.88 | 0.628 | 11,409.00 | 1.72 | 0.051 |
| De x Si(Is) | 6 | 11,893.00 | 3.62 | 0.001 | 6629.80 | 3.28 | 0.001 |
| Residual | 360 | 1414.60 | 1263.90 | ||||
| Total | 449 | ||||||
| pairwise test | ||
|---|---|---|
| no transformation | p/a transformation | |
| De × Is | – | – |
| De × Si | At two sites there are no differences between depths | Always significant differences between depths |
- Significant effects are indicated in bold.
Variability among plots was significant for rhizome and leaf assemblages in both the analyses (Tables 1 and 2).
nMDS ordinations of leaf epiphytic assemblages showed two groups constituting the shallower plots and the deeper plots; this pattern was less evident in the island of Capraia in the presence/absence transformed data (Fig. 2).
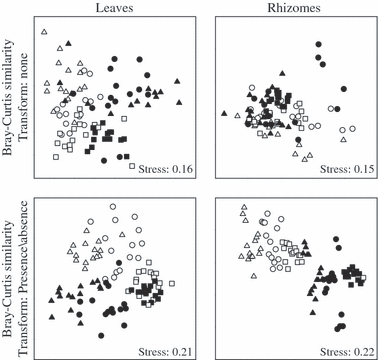
Two-factor non-metric MDS plots of centroids obtained from Bray–Curtis dissimilarities calculated on both untransformed and transformed (presence/absence) data. White, shallow assemblages; black, deep assemblages. Squares, Capraia; circles, Pianosa; up triangles, Elba.
nMDS ordinations of rhizome epiphytic assemblages showed that separation between depths was more evident in the analysis with presence/absence data (Fig. 2).
On leaf assemblages the Rhodophyta Hidrolithon farinosum, the Ochrophyta Cladosiphon irregularis, the Bryozoa Electra posidoniae and Aetea truncata and the Rhizopoda Planorbulina mediterranensis were more abundant in the shallower sites in relation to their cover and/or presence. In contrast, the encrusting Bryozoa Tubulipora spp. and Collarina balzaci and the Rhizopoda Lobatula lobatula and Rosalina brady characterized the deeper sites, as shown by IndVal analyses (Table 3). On the rhizome assemblages the shallow sites were mostly characterized by the Rhizopoda Planorbulina mediterranensis and by the Bryozoa Aetea truncata, Callopora lineata and Fenestrulina malusii; on the contrary, the Rhodophyta Peyssonnelia spp., Laurencia spp. and Womersleyella setacea and the Bryozoa Scrupocellaria reptans and Tubulipora spp. increase from the shallow to the deep sites (Table 3).
| taxa | presence | IndVal results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| leaves | rizhomes | leaves | rizhomes | |||||||
| S | D | S | D | IndVal | S | D | IndVal | S | D | |
| Chlorophyta | ||||||||||
| Cladophora dalmatica Kützing | + | − | + | + | ||||||
| Cladophora prolifera (Roth) Kützing | − | + | + | + | 51.1 | 0/0 | 1/23 | |||
| Flabellia petiolata (Turra) Nizamuddin | − | − | + | + | 43.3 | 0/3 | 4/21 | |||
| Pseudochlorodesmis furcellata (Zanardini) Børgesen | + | + | − | − | ||||||
| Siphonocladus pusillus (C. Agardh ex Kützing) Hauck | + | − | − | − | ||||||
| Valonia macrophysa Kützing | − | − | + | + | ||||||
| Ochrophyta | ||||||||||
| Aglaozonia parvula (Greville) Zanardini | − | − | − | + | 13.3 | 0/0 | 0/6 | |||
| Asperococcus bullosus Lamouroux f. bullosus | + | + | − | − | ||||||
| Cladosiphon irregularis (Sauvageau) Kylin | + | + | − | − | 36.0 | 2/17 | 0/9 | |||
| Dictyota dichotoma (Hudson) J.V. Lamouroux | + | + | + | + | ||||||
| Dictyota fasciola (Roth) J.V. Lamouroux | + | + | + | + | ||||||
| Dictyota linearis (C. Agardh) Greville | + | + | + | + | 53.3 | 1/13 | 6/29 | |||
| Elachista intermedia P. et H. Crouan | + | + | − | − | ||||||
| Giraudia sphacelarioides Derbés et Solier | + | + | − | − | 11.9 | 0/1 | 0/6 | |||
| Halopteris filicina (Grateloup) Kützing | − | + | + | + | 15.5 | 0/0 | 0/7 | 27.1 | 0/2 | 1/13 |
| Sphacelaria cirrosa (Roth) C. Agardh | + | + | + | + | 33.5 | 0/19 | 0/6 | |||
| Sphacelaria plumula Zanardini | + | + | − | − | ||||||
| Zanardinia typus (Nardo) P C. Silva | + | + | − | − | ||||||
| Rhodophyta | ||||||||||
| Acrothamnion preissii (Sonder) Wollaston | + | + | + | + | ||||||
| Antithamnion cruciatum (C. Agardh) Nägeli | − | + | + | + | 20.1 | 0/1 | 0/10 | |||
| Botryocladia boergesenii J. Feldmann | − | − | + | + | 39.9 | 1/13 | 4/24 | |||
| Ceramium codii (Richards) G. Feldmann | + | + | + | + | ||||||
| Ceramium diaphanum (Lighfoot) Roth | + | + | + | + | ||||||
| Ceramium flaccidum (Kützing) Ardissone | + | + | + | − | ||||||
| Ceramium spp. | + | + | + | + | ||||||
| Chondria capillaris (Hudson) Wynne | + | + | + | + | 14.1 | 0/3 | 0/7 | |||
| Corallina mediterranea Ellis & Solander | − | − | + | − | ||||||
| Dipterosiphonia rigens (Schousboe ex C. Agardh) Falkenberg | − | − | + | + | 37.5 | 0/2 | 1/18 | |||
| Erythrotrichia carnea (Dillwyn) J. Agardh | − | + | + | + | ||||||
| Eupogodon planus (C. Agardh) Kützing | − | − | + | + | 37.2 | 0/4 | 1/20 | |||
| Feldannophycus rayssiae (Feldmann et Feldmann-Mazoyer) Augier et Boudouresque | − | + | + | + | 27.2 | 0/8 | 1/18 | |||
| Heterosiphonia crispella (C. Agardh) Wynne | + | + | − | − | ||||||
| Halydiction mirabile Zanardini | + | − | − | − | ||||||
| Hydrolithon farinosum (Lamouroux) Penrose et Chamberlain | + | + | + | + | 68.7 | 25/45 | 12/44 | |||
| Hypoglossum hypoglossoides (Stackhouse) Collins et Harvey | − | + | + | + | 39.9 | 1/13 | 4/24 | |||
| Jania adhaerens J. V. Lamouroux | + | + | + | + | ||||||
| Laurencia chondrioides Børgesen | + | + | + | + | 62.4 | 0/4 | 3/29 | |||
| Laurencia minuta Vandermeulen, Garbary et Guiry subsp. Scammaccae, Furnari et Cormaci, | + | + | + | + | ||||||
| Laurencia spp. | + | − | − | + | ||||||
| Mesophyllum lichenoides (J. Ellis) M. Lemoine | + | + | + | + | ||||||
| Monosporus pedicellatus (Smith) Solier | + | + | + | + | ||||||
| Nitophyllum micropunctatum Funk | + | + | + | + | ||||||
| Peyssonnelia bornetii Boudouresque et Denizot | − | − | + | + | ||||||
| Peyssonnelia dubyi P. et H. Crouan | − | − | + | + | ||||||
| Peyssonnelia rubra (Greville) J. Agardh | − | − | + | + | 54.5 | 14/14 | 54/31 | |||
| Pleonosporium borreri (J.E. Smith) Nägeli | − | − | + | + | ||||||
| Plocamium cartilagineum (Linnaeus) Dixon | − | − | + | − | ||||||
| Pneophyllum coronatum (Rosanoff) Penrose | − | − | + | + | ||||||
| Pneophyllum fragile Kützing | + | + | + | + | ||||||
| Polysiphonia scopulorum Harvey | + | + | + | + | ||||||
| Womersleyella setacea (Hollenberg) R. E. Norris | + | + | + | + | 87.7 | 2/19 | 23/42 | |||
| Wrangelia penicillata (C. Agardh) C. Agardh | + | − | + | − | ||||||
| Rhizopoda | ||||||||||
| Cyclocibicides vermiculatus d’Orbigny | + | + | + | + | ||||||
| Lobatula lobatula Walker et Jacob | + | + | + | + | 66.1 | 0/19 | 0/38 | |||
| Miniacina miniacea Linnaeus | − | − | + | + | 53.6 | 4/33 | 7/38 | |||
| Planorbulina mediterranensis Walker et Jacob | + | + | + | + | 55.7 | 0/33 | 0/22 | 54.7 | 1/39 | 0/36 |
| Rosalina brady Cushman | + | + | + | + | 47.3 | 0/19 | 0/29 | |||
| Porifera | ||||||||||
| Clathrina contorta Minchin, 1905 | − | − | + | + | ||||||
| Dysidea avara (Schmidt, 1862) | − | − | + | + | ||||||
| Haliclona fulva (Topsent, 1893) | − | − | + | + | ||||||
| Ircina variabilis (Schmidt, 1862) | − | − | + | + | ||||||
| Mycale massa (Schmidt, 1862) | − | − | + | + | 26.0 | 0/1 | 0/10 | |||
| Oscarella lobularis (Schmidt, 1862) | − | − | + | + | ||||||
| Plakortis simplex (Schulze, 1880) | − | − | + | + | ||||||
| Sycon raphanus (Schmidt, 1862) | − | − | + | + | ||||||
| Cnidaria | ||||||||||
| Aglaophenia harpago Von Schenk, 1965 | + | + | − | + | 37.8 | 0/0 | 1/17 | |||
| Clytia haemisphaerica (Linnaeus, 1767) | + | + | + | + | ||||||
| Dynamena disticha (Bosch, 1802) | − | − | + | − | ||||||
| Laomedea angulata Hincks, 1861 | − | + | + | + | ||||||
| Obelia geniculata (Linnaeus, 1758) | + | + | + | − | 33.3 | 1/15 | 0/0 | |||
| Orthopyxis asymmetrica Stechow, 1919 | + | + | + | + | 30.2 | 0/17 | 0/5 | 54.4 | 4/36 | 2/25 |
| Paractinia striata (Risso, 1826) | − | + | − | − | ||||||
| Plumularia obliqua Thompson, 1879 | + | + | + | + | 19.9 | 1/9 | 0/1 | |||
| Podocoryne carnea (M. Sars, 1846) | + | + | + | − | ||||||
| Sertularella mediterranea Hartlaud, 1901 | − | − | + | − | ||||||
| Sertularia perpusilla Stechow, 1919 | + | + | + | − | 22.2 | 4/10 | 0/0 | |||
| Annelida | ||||||||||
| Hydroides norvegicus Gunnerus, 1768 | − | − | + | + | ||||||
| Janua pagenstecheri (Quatrefageau, 1865) | + | + | + | + | 34.7 | 0/19 | 0/13 | |||
| Pomatocerus triqueter (Linnaeus, 1767) | − | − | + | + | ||||||
| Serpula vermicularis (Linnaeus, 1767) | − | − | − | + | ||||||
| Spirobranchus polytrema (Philippi, 1844) | − | − | + | + | 33.6 | 1/21 | 0/15 | |||
| Spirorbis marioni Caullery & Mesnil, 1897 | + | + | + | + | ||||||
| Spirorbis spirorbis (Linnaeus, 1758) | + | + | + | + | 27.4 | 0/14 | 0/12 | |||
| Bryozoa | ||||||||||
| Aetea lepadiformis Waters, 1906 | + | + | + | + | ||||||
| Aetea truncata (Landsborough, 1852) | + | + | + | + | 39.6 | 1/26 | 0/14 | 61.9 | 9/41 | 2/27 |
| Amathia lendigera (Linnaeus, 1761) | + | + | − | + | 34.8 | 0/15 | 1/19 | 37.2 | 0/0 | 0/13 |
| Beania hirtissima (Heller, 1867) | + | + | + | + | 28.9 | 2/13 | 0/0 | |||
| Beania magellanica (Busk, 1852) | + | + | + | − | ||||||
| Bowerbankia gracilis (Leidy, 1855) | + | + | + | + | ||||||
| Caberea boryi (Audouin, 1826) | − | + | − | − | ||||||
| Callopora lineata (Linnaeus, 1767) | + | + | + | + | 54.8 | 5/13 | 1/23 | |||
| Carbasea papyrea (Pallas, 1766) | − | − | + | + | ||||||
| Cellaria salicornioides (Audouin, 1826) | − | − | + | + | ||||||
| Cellepora pumicosa (Pallas, 1766) | + | + | + | + | 21.6 | 0/2 | 1/10 | |||
| Chilidonia pyriformis (Bertoloni, 1810) | + | + | + | + | ||||||
| Chorizopora brongniartii (Audouin, 1826) | + | + | + | + | 27.8 | 1/14 | 0/12 | |||
| Collarina balzaci (Audouin, 1826) | + | + | + | + | 40.7 | 0/10 | 1/22 | |||
| Colletosia radiata Moll | + | + | + | + | ||||||
| Crisia denticulata (Lamack, 1816) | + | + | + | + | ||||||
| Cryptosula pallasiana Moll, 1803 | − | − | + | + | ||||||
| Diastopora patina (Lamarck, 1816) | + | − | + | + | ||||||
| Diplosolen obelium (Johnston, 1838) | + | + | + | + | 38.8 | 0/9 | 1/22 | |||
| Disporella ispida (Fleming, 1828) | − | + | − | − | ||||||
| Electra posidoniae Gautier, 1954 | + | + | − | − | 44.2 | 13/28 | 5/25 | |||
| Escharoides coccinea (Abildgaard, 1806) | + | + | + | + | ||||||
| Fenestrulina malusii (Audouin, 1826) | + | + | + | + | 75.2 | 9/41 | 2/27 | |||
| Hippodiplosia foliacea (Ellis et Solander, 1786) | − | − | + | + | ||||||
| Idmonea serpens Bidenkap, 1900 | + | + | + | + | 46.3 | 2/25 | 0/9 | |||
| Lichenopora radiata (Audouin et Savigny, 1826) | + | + | + | + | ||||||
| Margaretta cereoides (Ellis et Solander, 1786) | − | + | + | + | ||||||
| Micropora coriacea (Johnston, 1847) | − | − | + | + | ||||||
| Microporella ciliata (Pallas, 1766) | − | + | + | + | ||||||
| Nolella dilatata (Hincks, 1860) | − | − | − | + | ||||||
| Pherusella tubulosa (Ellis et Solander, 1876) | − | + | + | + | 34.4 | 3/19 | 1/13 | |||
| Reteporella spp. | − | − | + | + | ||||||
| Savignyella lafontii (Audouin, 1826) | + | + | + | − | ||||||
| Schizobrachiella sanguinea (Norman, 1868) | + | + | + | + | ||||||
| Scrupocellaria reptans (Linneaus, 1767) | + | + | + | + | 17.0 | 0/4 | 1/18 | 75.6 | 3/29 | 17/41 |
| Tubulipora liliacea (Pallas, 1766) | + | + | + | + | ||||||
| Tubulipora notomale (Busk, 1875) | + | + | + | + | ||||||
| Tubulipora piumosa Harmer, 1898 | + | + | + | + | 48.5 | 0/19 | 1/30 | 58.1 | 7/41 | 10/44 |
| Tunicata | ||||||||||
| Ascidia mentula O.F. Müller, 1776 | + | + | + | + | ||||||
| Botrylloides leachi (Savigny, 1816) | + | + | + | + | ||||||
| Botryllus schlosseri (Pallas, 1776) | + | + | + | + | 34.1 | 0/11 | 2/17 | |||
| Didemnum fulgens (Milne-Edwards, 1841) | − | − | + | + | ||||||
| Diplosoma listerianum (Milne-Edwards, 1841) | + | + | + | + | ||||||
| Distomus variolosus Gaertner, 1774 | − | + | − | − | ||||||
| Sidnyum turbinatum (Savigny, 1816) | − | − | + | + | ||||||
| Tridemnum cereum (Giard, 1872) | − | − | + | + | ||||||
- S, shallow assemblages; D, deep assemblages.
- Only significant values of t are reported. Numbers refer to cover/presence values.
The components of variance did not show great differences between untransformed and transformed data for either leaf or rhizome assemblages. The highest variability was detected at Shoot level for all analyses. Interesting patterns between the two depths at the levels of Plot and Sites were analogous for both leaf and rhizome assemblages independently of the transformation. In fact, at Plot level shallow assemblages showed higher variability, whereas an opposite trend was observed at Site level. Epiphytic assemblages of leaves showed a higher variability than those of rhizomes at Island level (Fig. 3).
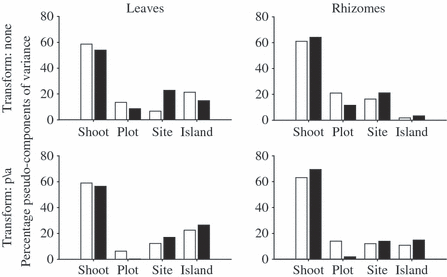
Percentage pseudo-components of variance. White bars, shallow assemblages; black bars, deep assemblages.
The interaction Depth × Site and the variability among plots for the number of species, biomass, the total percent cover and the percent cover of animals and algae in both leaf and rhizome assemblages was significant using ANOVA analyses (Table 4). None of the SNK tests detected a clear disjunction among depths for the studied variables except for percent cover of algae of leaves (SNK SE 0.1088), which showed higher values in shallower meadows, with the exceptions of one site in Elba and one in Capraia (not significant). A significant difference among islands was also detected on leaves and rhizomes for the number of species and the percent cover of algae.
| source | df | leaves | rhizomes | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| biomass | total % cover | no. species | algal % cover | animal % cover | total % cover | no. species | algal % cover | animal % cover | |||||||||||
| F | P-value | F | P-value | F | P-value | F | P-value | F | P-value | F | P-value | F | P-value | F | P-value | F | P-value | ||
| Depth = De | 1 | 0.65 | 0.504 | 15.71 | 0.058 | 0.35 | 0.613 | 22.88 | 0.041 | 0.10 | 0.777 | 1.29 | 0.374 | 0.61 | 0.517 | 7.66 | 0.109 | 2.44 | 0.259 |
| Island = Is | 2 | 1.68 | 0.263 | 1.94 | 0.224 | 31.11 | 0.000 | 23.79 | 0.014 | 0.01 | 0.991 | 0.09 | 0.917 | 39.58 | 0.000 | 0.36 | 0.711 | 0.23 | 0.804 |
| Site (Is) = Si(Is) | 6 | 1.29 | 0.271 | 6.95 | 0.000 | 3.49 | 0.004 | 0.92 | 0.486 | 9.61 | 0.000 | 3.61 | 0.003 | 6.32 | 0.000 | 5.59 | 0.000 | 10.66 | 0.000 |
| Plot(De x Si(Is)) | 72 | 2.25 | 0.000 | 2.96 | 0.000 | 2.31 | 0.000 | 2.77 | 0.000 | 2.70 | 0.000 | 4.54 | 0.000 | 1.63 | 0.002 | 3.99 | 0.000 | 2.98 | 0.000 |
| De x Is | 2 | 1.40 | 0.317 | 0.40 | 0.688 | 1.14 | 0.381 | 0.95 | 0.439 | 2.46 | 0.166 | 0.52 | 0.620 | 16.80 | 0.003 | 0.40 | 0.685 | 2.12 | 0.202 |
| De x Si(Is) | 6 | 6.46 | 0.000 | 8.70 | 0.000 | 16.72 | 0.000 | 2.88 | 0.014 | 7.47 | 0.000 | 3.63 | 0.003 | 2.51 | 0.029 | 3.28 | 0.007 | 2.46 | 0.031 |
| Residual | 360 | ||||||||||||||||||
| Transformation | none | none | Ln (x + 1) | Sqrt (x + 1) | none | none | none | none | Ln (x + 1) | ||||||||||
| Cochran’s test | C | 0.344 (P < 0.01) | 0.065 n.s. | 0.044 n.s. | 0.073 n.s. | 0.063 n.s. | 0.067 n.s. | 0.074 n.s. | 0.054 n.s. | 0.059 n.s. | |||||||||
- Significant values are indicated in bold.
The percentage of bitten leaves was higher in shallow meadows (0.96 ± 0.15 at Capraia; 1.11 ± 0.13 at Elba; 0.96 ± 0.11 at Pianosa; average ± SE, n = 75) than in deep meadows (0.19 ± 0.04 at Capraia; 0.15 ± 0.04 at Elba; 0.23 ± 0.06 at Pianosa; average ± SE, n = 75).
Discussion
This study documents the presence of significant differences in the structure and variability of assemblages of epiphytes of Posidonia oceanica on both rhizomes and leaves at two different depths. These differences were related to species composition and abundance, whereas differences were not significant for total biomass, total percent cover or percent cover of both animals and algae.
Differences between epiphytic assemblages of P. oceanica leaves related to depths have been already observed in small-scale studies (Cinelli et al. 1984; Boero et al. 1985; Casola et al. 1987; Buia et al. 1989; Tsirika et al. 2007). This pattern was evident both with species analyses and with analysis of morphological groups (Mazzella et al. 1989). On the contrary, depth did not appear significant in the structure of the assemblages along the coasts of Sardinia (Italy), where spatial variability of assemblages appeared to be influenced by other factors (Esposito et al. 2003). Results of the present study confirmed that differences between shallow and deep epiphytic assemblages of P. oceanica are generally consistent through different spatial scales (from a few meters up to tens of kilometers) and related to both species composition and abundance. However, no significant differences in the animal/plant ratio were found. In fact, even though on leaves a general pattern indicated a higher abundance of algae in shallow water than in deeper water, this pattern was not confirmed for all the sites. Moreover, although most animals increased their abundance with depth, species such as the Bryozoa E. posidoniae showed higher abundance in the shallow sites, whereas several algae also showed higher percentage cover in the deep sites. This finding disagrees with results of previous investigations that documented a decrease in abundance of algae at increasing depths and an increase in abundance of bryozoans and hydrozoans (Lepoint et al. 1999). On the contrary, the distribution of epiphytes along a depth gradient seems species-specific, as shown in previous investigations (Mazzella et al. 1989).
Shallow sites were mostly colonized by species considered characteristic of P. oceanica leaves, such as the Ochrophyta C. irregularis and the Bryozoa E. posidoniae, whereas in deep sites, different Bryozoa, such as E. coccinea, Chorizopora brongniarti and C. balzaci, were abundant. In such patterns, light plays a strong role, restricting spread of epiphytic brown algae (i.e. C. irregularis) (Mazzella et al. 1989; Dalla Via et al. 1998; Cebrian et al. 1999). In contrast, the tolerance of crustose coralline algae to light-level variability (Figueiredo et al. 2000) allows them to colonize P. oceanica leaves across the entire depth range of the meadow (Mazzella et al. 1989; Dalla Via et al. 1998; Cebrian et al. 1999).
Differences between assemblages may also be related to the influence of biotic factors that vary with depth. In fact, the differences in grazing pressure found between meadows at the two depths could have contributed to the differences in the structure of assemblages. Moreover, the shift in the period of vegetative growth of leaves between deep and shallow meadows (Mazzella & Ott 1984) may influence temporal patterns of epiphyte recruitment (Mazzella et al. 1989).
There have been no significant differences found between depths in rhizome assemblages when studied solely on the abundance of morphological algal groups (Piazzi et al. 2002), whereas the present study, which analysed both algae and animal species, showed significant differences for rhizome assemblages, confirming that the response of epiphytes to depth is species-specific.
Patterns of spatial variability indicated differences at the two studied depths, but analogous patterns between depths for the assemblages of both leaves and rhizomes. In particular, at Plot level, shallow assemblages showed higher variability, whereas an opposite trend was observed at Site level. These results suggest that factors that change with depth, such as light or/and hydrodynamics, can be responsible for the spatial variability of the assemblages of both leaves and rhizomes, and thus operate independently of the microclimatic and local conditions of the two compartments, and on the composition and structure of the assemblages. All the assemblages studied showed the highest variability among shoots, confirming results of previous studies that highlighted a high small-scale variability for epiphyte assemblages of P. oceanica and other seagrasses (Vanderklift & Lavery 2000; Lavery & Vanderklift 2002; Balata et al. 2007; Piazzi et al. 2007). The present study showed that this pattern is consistent for shallow and deep assemblages on both leaves and rhizomes; moreover, analogous results obtained with untransformed data and presence/absence data suggested that this pattern was related to the variability of both species composition and abundance.
Results of present study confirmed differences in the structure of epiphytic assemblages in relation to depth at wide spatial scales (among locations); however, this pattern seems mainly related to the composition and the abundance of species instead of the morphological groups which were found to be the determining factor in previous small-scale investigations (Cinelli et al. 1984; Buia et al. 1989; Mazzella et al. 1989; Tsirika et al. 2007). Another important finding is represented by the different patterns of spatial variability between assemblages in relation to depth, which could be useful to optimize sampling designs in further studies. Finally, this study underlined the importance of high taxonomic resolution in ecological investigations on epiphytic assemblages.
Further manipulative or correlative investigations, taking into account biotic and abiotic variables, may be useful to evaluate the relative importance of the main factors determining the patterns of distribution of epiphytic assemblages.
Acknowledgements
We thank Maria Cristina Gambi, Andrew Irving and two anonymous reviewers for their comments that improved the quality of the manuscript. This study is part of a PhD Dissertation discussed by U.N. at the University of Pisa.




