Opposite diel patterns of nitrogen fixation associated with salt marsh plant species (Spartina foliosa and Salicornia virginica) in southern California
Abstract
In marine wetlands, nitrogen fixation is a potentially important nutrient source for nitrogen-limited primary producers, but interactions between nitrogen fixers and different vascular plant species are not fully understood. Nitrogen fixation activity was compared in sediments vegetated by three plant species, Spartina foliosa, Salicornia virginica, and Salicornia bigelovii in the Kendall Frost Reserve salt marsh in Mission Bay (CA). This study addressed the effects of plant type, day and night conditions, and sediment depths on nitrogen fixation. Higher rates of nitrogen fixation were associated with S. foliosa than with either of the two Salicornia spp., which are known to compete more effectively than Spartina for exogenous nitrogen in the salt marsh environment. Rates of nitrogen fixation, determined by acetylene reduction, in sediments vegetated by S. virginica were low during the day (7.7 ± 1.2 μmol C2H4 m−2 h−1) but averaged 13 ± 6.6 μmol C2H4 m−2 h−1 at night, with particularly high rates in samples from locations with visible cyanobacterial mats. The opposite diel pattern was found for sediments containing S. foliosa plants, in which average daytime and nighttime rates of nitrogen fixation were 62 ± 23 and 21 ± 15 μmol C2H4 m−2 h−1, respectively. For S. foliosa, nitrogenase activity of rinsed roots and different sediment sections (0–1, or 4–5 cm depths) were measured. Although nitrogen fixation rates in vegetated sediment samples were substantial, all but one of rinsed S. foliosa root samples (n = 12) and subsurface sediments at 4–5 cm depths failed to show nitrogen fixation activity after 2 h, suggesting that the most active nitrogen fixers in these systems likely reside in surface sediments. Further, nitrogenase activity in shaded and unshaded S. foliosa samples did not differ, suggesting that nitrogen fixers may not rapidly respond to changes in plant photosynthetic activity. Average nitrogen fixation rates in S. foliosa-vegetated samples from the Mission Bay salt marsh were on the same order as those of highly productive Atlantic coast marshes, and this microbially-mediated nitrogen source may be similarly substantial in other Mediterranean wetlands. Sediment abiotic variables seem to exert greater control upon nitrogen fixation activity than the effects of particular plant species. Nonetheless, dominant plant species may differ substantially in their reliance on nitrogen fixation as a nutrient source, with potentially important consequences for wetland conservation and restoration.
Problem
Nitrogen is known to be a limiting nutrient for primary producers in many marine wetlands (Valiela & Teal 1974; Covin & Zedler 1988; Boyer & Zedler 1999; Tyler et al. 2003) although bacteria are possibly limited by phosphorus (Sundareshwar et al. 2003). In vegetated wetlands such as salt marshes, the conversion of dinitrogen gas into biologically available nitrogen by bacteria, via nitrogen fixation, may be an important control not just on primary productivity but also on the health of habitat-forming vascular plants. For instance, Spartina alterniflora (Atlantic cordgrass), a dominant plant of Atlantic coast marshes, has a tight, mutualistic relationship with nitrogen-fixing bacteria in its rhizospheres. The microbes respond rapidly to changes in plant photosynthetic activity and consume labile organic substrates produced by the cordgrass in exchange for rapid, direct provision of fixed nitrogen (Boyle & Patriquin 1981; Whiting et al. 1986).
Although much is known about interactions between nitrogen fixers and S. alterniflora (Boyle & Patriquin 1981; Whiting et al. 1986; Piceno & Lovell 2000), less is known about associations of nitrogen fixers with other vascular plant species that dominate coastal regions worldwide, such as those in salt marshes of Mediterranean climates.
Several wetlands worldwide experience Mediterranean climates, including those on the coasts of the Mediterranean sea, central Chile, south Africa, southwestern Australia, and the western US, which are characterized by dry summers that can be stressful for vascular plants. Mediterranean salt marshes constitute a major category of wetlands in which nitrogen fixation has been understudied but is likely to be significant. Most Mediterranean marshes experience relatively rare rainfall events (coastal mean in southern California = 25 cm year−1, Langis et al. 1991). As a consequence, rain rarely washes nutrients into wetlands from surrounding watersheds (Langis et al. 1991). Further, the hypersalinity, characteristic of Mediterranean marsh sediments, may increase nitrogen demands by vascular plants, as nitrogen-containing compounds such as proline and glycinebetaine are thought to be used in osmotic regulation by halophytes (Stewart & Lee 1974; Cavalieri & Huang 1979).
In salt marshes of southern California, nitrogen fixation is likely to be a significant ecosystem function. Southern California marshes are much smaller than their Atlantic coast counterparts, partly due to extensive urban development (Schoenherr 1992), so any watershed-based nutrients are thought to pass quickly through the wetlands (Langis et al. 1991). Experimental nitrogen additions suggest that vascular plant productivity is nitrogen limited including that of the dominant plant at low marsh zones on the Pacific coast, Spartina foliosa, and of Salicornia virginica and Salicornia bigelovii which comprise most of the upper marsh zone in the Kendall Frost Reserve (McCray 2001). More specifically, nitrogen has been found to limit the height of S. foliosa plants, which directly impacts the ability of these plants to provide adequate nesting habitat for the endangered Clapper rail (Boyer & Zedler 1999). Nitrogen fixers have been isolated from roots of S. virginica (Bagwell et al. 2001) and S. bigelovii (Rueda-Puente et al. 2003) and shown to be physiologically distinct from those of other salt marsh plants (Bagwell et al. 2001), although in situ rates of nitrogen fixation associated with this species have not been reported. The abundance of cyanobacteria in southern Californian salt marshes has been noted (Zedler 1980), further suggesting that these sites have high nitrogen fixation potential. Research is needed to characterize interactions between nitrogen fixers and vascular plant species of southern California because nitrogen dynamics are known to regulate competition between S. foliosa and Salicornia species (Covin & Zedler 1988; Boyer & Zedler 1999; McCray 2001). Therefore, nitrogen fixation may be an important factor affecting interspecific plant interactions. Further, studies of nitrogen fixation associated with plant species other than S. alterniflora can test the prevalence of mutualistic interactions between vascular plants and nitrogen-transforming microbes.
Many studies have examined nitrogen fixation activity associated with only one species of salt marsh plant and have not compared the influence of different plant species on this microbial function. However, one pioneering study revealed moderate nitrogenase activities associated with excised roots of 33 plant species from 13 elevational zones of a Nova Scotian salt marsh (Patriquin & Keddy 1977). Another study found higher nitrogen fixation in low marsh dominated by S. alterniflora than in high marsh zones where S. patens and Distichlis spicata grew, although rates supported by each species were not specifically compared (Valiela & Teal 1979). A more recent study found that distinctions between physiological profiles of nitrogen fixers associated with three different plant species (Spartina patens, S. alterniflora, Juncus roemerianus) were greater than those of microbes isolated from different habitats occupied by one species, S. patens (Bergholz et al. 2001). This research suggested that, under some circumstances, plant species can have stronger influences on nitrogen fixers than environmental factors (Bergholz et al. 2001). More work is needed to determine how salt marsh plant species differ in their interactions with nitrogen fixers and should not only include studies in Mediterranean environments but also more detailed assessment of temporal dynamics of nitrogen fixation.
In addition to the influence of plant species, nitrogen fixers are affected by dramatic environmental changes associated with shifting day and night conditions. Previous salt marsh research has indicated that nitrogen fixation activity in sediments of S. alterniflora marshes can display dramatic diel patterns, with peak times of activity varying seasonally and with dominant cyanobacterial species (Currin 1996). Diel or other temporal patterns of nitrogen fixation have not been described in such detail in Mediterranean or southern Californian marshes, although one study documents seasonal variation in two S. foliosa marshes in San Diego Bay (Langis et al. 1991). A basic characterization of diel patterns of nitrogen fixation is important for accurate estimates of the magnitudes of nitrogen fixation. Also, diel patterns of nitrogen fixation associated with various plant species may help elucidate the nature of plant–microbe interactions, as nitrogen fixers intimately associated with vascular plants likely display the highest nitrogen fixation rates during the daytime, when plant photosynthesis occurs, rather than at night (Whiting et al. 1986).
The nature of plant–microbe interactions can also be characterized by experimental determination of microhabitats in which nitrogen fixers are the most active. Specific sites of nitrogen fixation among microhabitats of salt marshes have best been identified in wetlands dominated by S. alterniflora. They include plant rhizospheres (roots and surrounding sediments) (Teal et al. 1979; McClung et al. 1983), dead plant culms and shoots (Newell et al. 1992; Currin & Paerl 1998; Moisander et al. 2005) as well as surface sediments (Whitney et al. 1975; Patriquin & Keddy 1977; Currin 1996; Piehler et al. 1998). Few investigations have attempted to detail the nature of interactions of S. foliosa and nitrogen-fixing microbes (Langis et al. 1991) although this species is likely to harbor nitrogen fixers in the same microhabitats as its close relative, S. alterniflora.
The purpose of this research was to compare nitrogen fixation activity in sediments vegetated by three plant species in the Kendall Frost Reserve salt marsh, Mission Bay (CA) and to characterize diel (day versus night) patterns of nitrogen fixation for S. foliosa- and S. virginica-vegetated sediments. I tested the null hypotheses that (a) daytime nitrogen fixation rates do not differ in rhizospheres of S. foliosa, S. virginica, and S. bigelovii and (b) daytime nitrogen fixation rates in S. foliosa- and S. virginica-vegetated sediments do not differ from nighttime rates (April– May 2005). As oxygen concentrations can affect nitrogen fixation rates, an experiment in May 2005 with S. virginica addressed the null hypothesis that aerobic, anaerobic, and microaerobic treatments in the headspace of flasks did not affect nitrogen fixation rates of S. virginica-vegetated sediments during the day or night.
In experiments with S. foliosa, which was predicted to support higher rates of root-associated nitrogen fixation compared to Salicornia species, major sites of microbial activity in S. foliosa samples were characterized by: (i) comparing rates in different sediment depth intervals, (ii) assaying rinsed roots, and (iii) testing effects of plant shading on nitrogen fixation. The null hypotheses addressed by these experiments (in April 2005) were (a) nitrogen fixation rates do not vary between different sediment depth intervals (0–1, 4–5 cm) of S. foliosa-vegetated cores, (b) nitrogen fixation activity does not differ between rinsed S. foliosa roots and S. foliosa-vegetated sediment cores and (c) shading of S. foliosa shoots does not affect nitrogen fixation activity in rhizosphere sediments. For S. virginica, an experiment in August 2005 tested simply whether leaf and stem surfaces were as important sites of nitrogen fixation as rhizospheres by comparing epiphytic rates of nitrogen fixation on S. virginica to those of S. virginica-vegetated sediments during the day and night.
Study Area
This research took place within the University of California Kendall Frost Reserve in Mission Bay, San Diego, California (32°47′35′ N, 117°13′00′′ W). This reserve consists of 16 acres of salt marsh dominated by S. virginica and S. bigelovii in upper elevations and S. foliosa at low elevations. This reserve represents the last vestiges of a wetland that once spanned more than half the bay, prior to its transformation in the late 1940s to a recreational water park (City of San Diego, Parks and Recreation). Low S. foliosa elevations of the salt marsh fall within the boundaries of the adjacent Northern Wildlife Preserve of the City of San Diego. The vegetation of the Kendall Frost Reserve and Northern Wildlife Preserve collectively provide nesting habitat for several bird species including the federally endangered Clapper rail (Rallus longirostris levipes) and the state endangered Belding Savannah Sparrow (Passerculus sandwinchensis beldingi).
Material and Methods
Nitrogen fixation in Spartina foliosa marsh
To characterize nitrogen fixation rates, 12 experimental blocks (10 m × 5 m) were established parallel to the shoreline in the Spartina foliosa zone on the border of the Kendall Frost Marsh and Northern Wildlife Preserve. In April (2005), triplicate vegetated cores (approximately 2 cm diameter, 6 cm deep), centered around and containing the culms and attached roots of live S. foliosa plants, were taken from random positions within each of the 12 blocks for determination of nitrogen fixation (acetylene reduction) rates. One additional unvegetated core of the same dimensions, not centered around a plant culm, was also extracted within 0.25 m of the triplicate vegetated cores from blocks one to six for nitrogen fixation assays of surface and sub-surface sediment sections. These cores were sealed, and processed in situ for determination of acetylene reduction rates as described below. On the same date, 12 S. foliosa samples were collected in the evening (just prior to sunset) and assayed in situ just after sunset. These samples were processed in the same manner as samples used in daytime assays except the flasks were not wrapped in foil.
From each block, one sediment core (4.8 cm diameter, 6 cm deep) was extracted in April (2005) for determination of sediment grain size and organic matter content. These cores were kept on ice while being transported to the laboratory and were then frozen at −17 °C. A sediment core (2 cm diameter, 6 cm deep) was also extracted from each block, sealed in a 50 ml centrifuge tube, and stored on ice until processing for porewater nutrient measurements (described below). Plant density was determined by counting the number of S. foliosa stems in a small quadrat (0.25 m × 0.25 m) randomly positioned within each block. The average plant height in each quadrat was also determined by measuring heights of up to 10 S. foliosa plants. The heights and biomass of individual S. foliosa plants collected in the sediment cores that were used for measurement of nitrogen fixation rates were also recorded. Following completion of acetylene reduction assays, plant biomass was separated into above- and below-ground components by clipping the plant stem at the top of the sediment core. Both fractions were then rinsed with water on a 0.5-mm sieve to remove the attached sediment, dried for 48 h at 60 °C, and weighed (following methods employed by Boyer et al. 2001; Howard & Rafferty 2006; Saunders et al. 2006). Sections of live S. foliosa shoots were also removed from each sample and stored at −17 °C until they could be processed for tissue nitrogen (N) content analyses.
Acetylene reduction assays
Intact plant samples (sediment cores containing plant roots to a depth of 6 cm, with attached culm, stem and shoots) were extruded into 125 ml flasks. Plant stems and shoots protruded from the flasks while roots and attached sediments were sealed inside using rubber stoppers and gas-tight tape. During assays, the flasks were wrapped in aluminum foil (to prevent artificial temperature increases upon exposure to sun). Into the headspace of each flask, 15 ml of acetylene gas was subsequently injected. Sediment cores used in acetylene reduction assays did not exceed 2 cm diameter, so acetylene and headspace gas could diffuse to their interior within the time period of the assay. All assay flasks were incubated in situ by being placed into a tub of water, which was replenished hourly to maintain temperatures below 25 °C, and were positioned on the sediment in the upper marsh of Kendall Frost Reserve. Subsampling of the headspace in each flask was conducted upon initiation of the assay (injection of acetylene), and after 2 and 3.5 h by withdrawing 2.5 ml of gas from the flask and storing it in N2-flushed Vacutainers (Becton-Dickinson). These samples were analysed on an FID-equipped gas chromatograph in peak height mode under conditions described by Capone & Montoya (2001). Although S. foliosa samples produced detectable acetylene reduction activity after only 2 h, Salicornia samples did not show activity until 3.5 h. For studies involving Salicornia-associated acetylene reduction activity, these later subsamples (at 3.5 h) were used for analyses in this study.
Spartina foliosa manipulations (shading, rinsed roots, and sediment sectioning)
The triplicate S. foliosa samples collected in April (2005) were used to address: (i) whether nitrogen fixation rates differed for samples of vegetated sediments versus rinsed S. foliosa roots and (ii) whether nitrogen fixation rates were affected by shading of plant shoots. One of each set of triplicate samples was exposed to sunlight during an in situ assay. For comparison to the first sample (containing an S. foliosa plant and sediment), the second sample of each block was rinsed free of sediment prior to having its roots sealed in the 125 ml flask and was used to measure the rates of acetylene reduction directly associated with plant roots. To compare the acetylene reduction rates in sediments vegetated by light-exposed plants with those in sediments vegetated by shaded plants, the third sample in each group was left intact but shaded by being placed in a large wire cone (approximately 4 feet high) that had been wrapped in shade cloth to achieve approximately 95% light reduction. For all triplicate samples, the attached plant stem and shoots protruded from the assay flasks without being severed from the roots. The shoots of plants used in such rinsed treatments were exposed to sunlight during the assay, but the roots were in foil-wrapped flasks. All assay flasks employed in this study (containing roots and sediment) were wrapped in foil so that nearly no light reached sediments regardless of the light treatment applied to S. foliosa plants, but plant shoots and leaves rose out of the flasks so that light levels reaching the plants could be manipulated independently of those affecting sediments within the flasks.
To compare the nitrogen fixation rates in surface versus subsurface sediment depths, the additional vegetated cores sampled in April (one from each block) were sectioned into 0–1 and 4–5 cm depth intervals, and the sediment (but not plant material) from each section was sealed in a 50-ml flask and processed similar to the procedure for the 125-ml flasks. Sediment sections were not shaded during acetylene reduction assays.
Nitrogen fixation in Salicornia spp. marsh
To measure nitrogen fixation activity in Salicornia-vegetated sediments, a total of eight blocks (10 m × 5 m) were interspersed (separated by approximately 20 m) throughout the Kendall Frost Reserve at elevations in which Salicornia virginica and Salicornia bigelovii occur (1.7–1.8 m above MLLW). These species co-occur throughout much of the salt marsh, and both species were present in each block. In May 2005, an experiment was conducted in order to: (i) compare rates of nitrogen fixation in intact cores of S. virginica during daytime and nighttime in situ incubations and (ii) to test how nitrogen fixation rates varied under different conditions of oxygen exposure. For this experiment, triplicate vegetated sediment cores (approximately 2 cm diameter, 6 cm deep) were collected from each of four experimental blocks during the morning and again in the evening. Each of the triplicate samples received a different headspace oxygen concentration (aerobic, anaerobic, and anaerobic with 5 ml of air). For anaerobic assays, the headspace inside these flasks was flushed for 2 min with nitrogen gas prior to initiation of the acetylene reduction assay (described above). Anaerobic samples that had an additional 5 ml of air injected in the flasks prior to assay initiation were used to determine nitrogen fixation rates under microaerobic conditions. Acetylene reduction assays were then conducted as described above.
In a subsequent experiment (May 2005) designed to compare nitrogen fixation rates in sediments vegetated by different Salicornia species, paired samples of the two Salicornia spp. were extracted from each of eight blocks. These samples were assayed in situ (as described above) during daytime incubations with exposure to natural light levels and were aerobic. For each of these samples, individual plant heights were recorded. During August (2005), sediment cores containing S. virginica plants were sampled along with S. virginica shoots (clipped free of roots) from each of the eight blocks, for comparison of acetylene reduction rates associated with sedimentary (rhizospheric) and epiphytic microbes in daytime and nighttime assays. Plant shoots (without roots) were assayed in the same manner as vegetated sediments (described above), except plant shoots were entirely enclosed within the sealed flasks. All samples assayed in August were aerobic.
In each Salicornia block, the percent cover by each plant species was determined within randomly positioned quadrats (0.5 m × 0.5 m). Sediment cores were also taken for determination of porewater ammonium (3.5 cm2 × 6 cm), and analyses of sediment grain size and bulk organic matter content (18.02 cm2 × 6 cm) and were processed according to the methods described below. In August 2005, above- and below-ground biomasses of S. virginica samples used in acetylene reduction assays were measured by separating these portions at the surface of sediment cores in which they were collected and determining the weights of the plant matter dried overnight at 60 °C.
Laboratory analyses
Plant shoots were frozen at −17 °C until they could be processed for tissue nitrogen content. To remove epiphytes, shoots were rubbed with 5% hydrochloric acid and rinsed with distilled water prior to drying overnight at 60 °C. Samples were then ground to a fine powder and analysed for carbon and nitrogen content (by % weight) using a CHN elemental analyzer (Costech 4010, 0.5% precision). Porewater was extracted from sediment cores (2 cm diameter, 6 cm deep) via centrifugation on all sampling dates. Porewater was filtered with 0.45 μm filters (Acrodisc) and ammonium concentration was determined by colorimetric techniques following Solorzano (1969). Porewater nitrite levels were also measured following the protocol of Strickland & Parsons (1968). To determine the percentage of combustible organic matter content in sediments, sediment cores were homogenized and passed through a 2-mm sieve to remove large plant material. These sediments were combusted at 300 °C for at least 4 h and organic matter content was calculated by mass difference. Grain size percentages (% sand, % clay) were determined, using a homogenized subsample of these sediments, from the dry mass of sediments separated by a 63-μm sieve following digestion with hydrogen peroxide to remove organic matter.
Statistical analyses
Nitrogen fixation rates associated with different plant species, shading treatments, and sediment intervals were compared using t-tests or one-way ANOVAs. Comparisons of nitrogen fixation rates for samples collected from the same experimental block were conducted using paired t-tests. Percentage composition data (organic matter, grain size, tissue nitrogen and carbon data) were arcsine-square root transformed prior to analyses. In cases where data did not meet assumptions of normality even after transformation, non-parametric tests, including the Wilcoxon test, were used to compare means. Relationships between nitrogen fixation rates and plant or environmental parameters were analysed and described, where appropriate, using linear regression models. All statistical analyses were performed with JMP 4.0 software.
Results
Nitrogen fixation rates associated with different plant species
Average daytime rates of nitrogen fixation measured in samples with Spartina foliosa in April were more than twice those detected in samples with Salicornia bigelovii or Salicornia virginica taken in early May (Fig. 1a). S. virginica-vegetated sediments were also assayed in August, with daytime nitrogen fixation rates averaging 26 ± 8.4 μmol C2H4 m−2 h−1, but these activity rates were not significantly different from those determined in May (paired t-test, t7 = 0.48, P = 0.32). In nighttime assays, average rates of nitrogen fixation in S. foliosa and S. virginica-vegetated sediments did not differ (Fig. 1B and 1C, Wilcoxon, z = 0.69, P = 0.46).
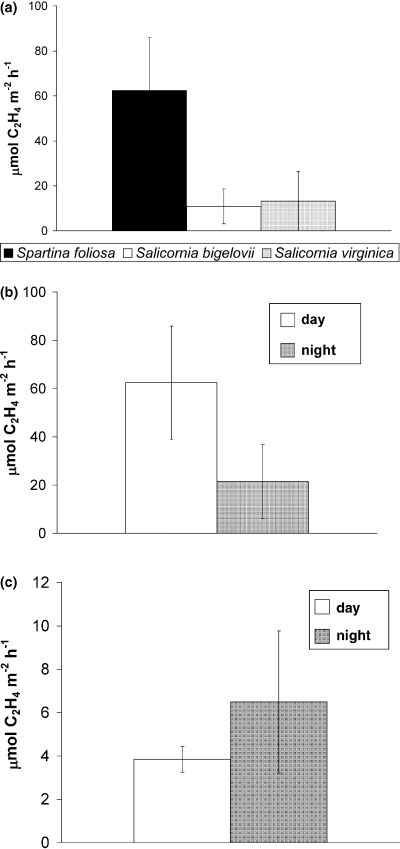
(a) Average daytime nitrogen fixation (acetylene reduction) rates in aerobic sediments vegetated by Spartina foliosa (April 2005), Salicornia virginica (May 2005), and Salicornia bigelovii (May 2005) in Kendall Frost for 2 hour assays with exposure to full sunlight (Kruskal Wallace, χ2 =6.88, P = 0.03) Error bars represent one standard error; (b) Nitrogen fixation (acetylene reduction) rates (and one standard error) of S. foliosa-vegetated sediments during the day and at night, based on 2-h assays (paired t-test, t11 = 1.28, P = 0.11); (c) Nitrogen fixation (acetylene reduction) rates measured in S. virginica samples in May 2005 during daytime and nighttime assays after 3.5 h (paired t-test, t10 = 6.51, P < 0.01) Error bars represent one standard error.
In a daytime comparison of nitrogen fixation rates in sediments vegetated by S. virginica and S. bigelovii, which occupy a common elevation in Kendall Frost, no differences between nitrogen fixation rates were found (paired t-test, t14 = 0.436, P = 0.67).
Diel patterns of nitrogen fixation
Samples of S. foliosa-vegetated sediments that were collected and assayed during the daytime exhibited average rates of nitrogen fixation (62 ± 23 μmol C2H4 m−2 h−1) that were more than twice those of samples collected in the evening and assayed at night (21 ± 15 μmol C2H4 m−2 h−1, Fig. 1b). These differences however were not statistically significant (paired t-test, t11 = 1.28, P =0.11), likely due to the high variability among nitrogen fixation rates within each treatment.
Among S. virginica-vegetated samples collected in May, nitrogen fixation rates were higher during the night than during the day based upon measurements taken at 3.5 h (paired t-test, t10 = 6.51, P < 0.01, Fig. 1c). Average daytime nitrogen fixation rates were 3.8 ± 0.5 μmol C2H4 m−2 h−1 while nighttime rates were 6.5 ± 3.3 μmol C2H4 m−2 h−1. As one nighttime sample showed a very high nitrogen fixation activity (38 μmol C2H4 m−2 h−1), there was large variance among the samples assayed at night compared with those assayed during the day (Fig. 1c). This diel pattern was opposite to that observed in samples of S. foliosa (Fig. 1b). Nitrogen fixation rates in S. virginica-vegetated sediments did not differ across a gradient of three oxygen treatments during either day (F2,8 = 1.56, P = 0.27) or night assays (F2,10 = 0.67, P = 0.54). A few months later (in August), no differences were found between daytime (26 ± 8.4 μmol C2H4 m−2 h−1) and nighttime (21 ± 7.1 μmol C2H4 m−2 h−1) nitrogen fixation rates measured in intact S. virginica-vegetated cores (P = 0.88) or between daytime (4.8 ± 1.6 nmol C2H4 g−1 h−1) or nighttime (5.4 ± 2.0 nmol C2H4 g−1 h−1) epiphyte samples (plant shoots only) (P = 0.89).
Characterizing sites of nitrogen fixation
Nitrogen fixation activity (equivalent to 1.4 ± 0.63 μmol C2H4 m−2 h−1) was detected only in the top (0–1 cm) sections of S. foliosa-vegetated sediment cores and not in the deeper (4–5 cm) sections, although variability was high, such that differences between these intervals were not significant (P = 0.32).
Nitrogen fixation rates of intact S. foliosa-vegetated sediment cores were significantly higher than those of plants with rinsed roots regardless of whether plants with sediments were assayed in light (Wilcoxon test, z = −1.98, P = 0.04) or shade (z = 2.41, P = 0.01). The highest nitrogen fixation activity rate in S. foliosa-vegetated sediment was 282 μmol C2H4 m−2 h−1. Twelve samples of rinsed roots were assayed, but only one produced detectable nitrogen fixation activity (119 μmol C2H4 m−2 h−1).
In August, nitrogen fixation rates in S. virginica-vegetated sediment cores did not exceed those of S. virginica epiphytes during either daytime (P = 0.79) or nighttime assays (P = 0.56), when the data were normalized to plant biomass. Nitrogen fixation rates for intact cores were equivalent to an average of 4.2 ± 1.3 nmol C2H4 g−1 h−1 and 4.8 ± 1.9 nmol C2H4 g−1 h−1 during the day and night, respectively, while those for epiphytes alone were 4.8 ± 1.6 C2H4 g−1 h−1 (day) and 5.4 ± 2.0 C2H4 g−1 h−1 (night).
No significant effect of S. foliosa plant shading on nitrogen fixation rates in vegetated sediments was observed after 2 hours (P = 0.99). Average nitrogen fixation rates (with standard error) in sediment cores containing shaded plants were 68 ± 27 μmol C2H4 m−2 h−1 and those in cores with S. foliosa plants exposed to full sunlight were 62 ± 23 μmol C2H4 m−2 h−1.
Relationships between nitrogen fixation, plant parameters, and abiotic factors
Spartina foliosa
Nitrogen fixation rates did not vary with S. foliosa height of individual plants (P = 0.91) or average heights within quadrats (P = 0.14), total plant biomass (P =0.55), leaf tissue N content (P = 0.41), or plant density (P = 0.58). Of six plant samples randomly selected for tissue N content analyses, highest nitrogen fixation rates were found in samples with intermediate values of leaf N content. Nitrogen fixation rates were also not clearly related to porewater ammonium values, although the concentrations (5–37 μm) measured in all S. foliosa blocks of Kendall Frost were well below reported inhibition thresholds (Carpenter et al. 1978; Teal et al. 1979).
Salicornia spp.
Nitrogen fixation rates of sediment samples containing S. bigelovii were positively related to porewater nitrite levels (r2 = 0.89, P < 0.01) and to the percentage of mud in sediments (r2 = 0.79, P = 0.04). There was a negative trend between the percentage of nitrogen in S. virginica shoots and porewater ammonium concentrations (r2 =0.54, P = 0.06). No such relationships were found for samples of S. bigelovii
During August, nitrogen fixation rates were positively related to below-ground biomass of S. virginica plants (Fig. 2). On this date, there was no relationship between nitrogen fixation rates in S. virginica-vegetated sediments and porewater ammonium levels (r2 = 0.44, P = 0.15). The percentage of tissue nitrogen in S. virginica shoots was not related to either nitrogen fixation rates or porewater ammonium. There was a strong negative relationship in August between nitrogen fixation (acetylene reduction) rates and S. virginica height in daytime assays (Fig. 3a), but this relationship was not found in samples assayed at night. A non-linear relationship, with lowest nitrogen fixation rates occurring in samples with nearly the highest above-ground S. virginica biomass, also existed (Fig. 3b, see Discussion).
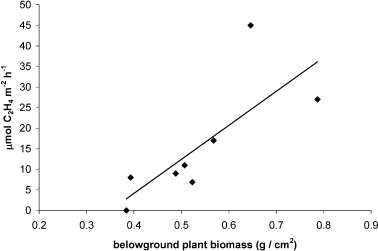
The relationship between nitrogen fixation (acetylene reduction) rates and belowground biomass of Salicornia virginica in August 2005 (r2 = 0.58, P = 0.03).
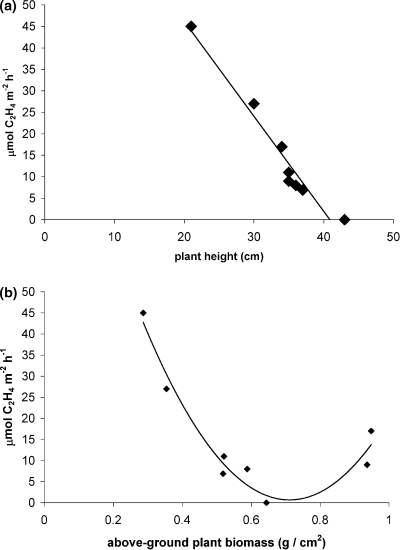
(a) The relationship between nitrogen fixation (acetylene reduction) rates and Salicornia virginica plant height in August (r2 =0.95, P < 0.01); (b) The relationship between above-ground biomass of S. virginica and nitrogen fixation rates in August (P < 0.01, r2 =0.95).
Discussion
Nitrogen fixation in Spartina foliosa and Salicornia zones
Nitrogen fixation rates in the Kendall Frost Reserve have been shown to be substantial but dynamic in space and time (Fig. 1). In sediments vegetated by Spartina foliosa and Salicornia virginica, rates as high as 282 μmol C2H4 m−2 h−1 (11 g N m−2 year−1) and 76 μmol C2H4 m−2 h−1 (3.1 g N m−2 year−1) respectively were observed, although the average rates were lower (Fig. 1). Notable differences were found between rates associated with different plant species (Fig. 1a) as well as between day and night rates (Fig. 1b and 1c), although oxygen levels did not affect the magnitude of nitrogen fixation activities. The results of this study suggest that the performance of nitrogen fixation is partitioned both in space and time, such that higher daytime rates may occur in S. foliosa-vegetated zones of the salt marsh, while nighttime activities may be greater in Salicornia spp. zones. Further, these opposite diel patterns of nitrogen fixation indicate that the nature and extent of interactions between Spartina or Salicornia and nitrogen fixers may differ substantially.
This is the first study to suggest that S. foliosa, which has been considered an inferior competitor for exogenous nutrients compared to Salicornia bigelovii (Boyer & Zedler 1999), may rely more heavily on nitrogen fixation as a nutrient source than Salicornia species. Salicornia plants, on the other hand, may rely more on exogenous, recycled nutrients as suggested by both the lower daytime nitrogen fixation rates than those associated with Spartina plants (Fig. 1a) and the negative trend between S. virginica shoot tissue nitrogen content and porewater ammonium (discussed above), which was consistent with uptake of porewater nutrients by that species.
Nitrogen fixation rates may vary between sediments vegetated by different plant species due to edaphic factors that vary between elevational zones occupied by each plant species. These factors include porewater nutrient concentrations, salinity or evaporation rates and temperature. Alternatively, particular plant species differentially affect nitrogen fixers in salt marsh sediments, either via production of distinct oxygenation patterns or exudation of specific organic substances. In this study, the two Salicornia species that occupied a common zone did not differ in nitrogen fixation rates (Fig. 1a), so environmental factors rather than plant-species effects may have driven patterns in nitrogen fixation. In particular, porewater ammonium concentrations were well below the inhibitory thresholds (Carpenter et al. 1978; Teal et al. 1979) in Spartina zones where daytime nitrogen fixation rates were the highest (Fig. 1a), but were possibly inhibitory in Salicornia zones where concentrations were significantly higher (F3,29 = 12.98, P < 0.01) and exceeded 100 μm.
Diel patterns of nitrogen fixation
The opposite diel patterns of nitrogen fixation observed in S. foliosa- and S. virginica-vegetated sediments suggest that the active nitrogen fixers interacting with these plant species differ substantially. Different types of nitrogen fixers are thought to be more active during the day than at night in salt marsh sediments (Currin 1996). High daytime nitrogen fixation rates measured in S. foliosa-vegetated sediments (Fig. 1a and b) are possibly performed by autotrophic cyanobacteria with oxygen tolerance or heterocysts or by plant-associated heterotrophic bacteria residing in plant rhizospheres that benefit from plant-derived photosynthetic products (i.e. labile carbon). As daytime nitrogen fixation rates associated with S. foliosa were greater than those for Salicornia spp. (Fig. 1a), there may be some degree of stimulation as a result of the specific influence of Spartina on microbes, but nitrogen fixation could also be higher as a result of favorable abiotic conditions, as discussed above. High nighttime activity associated with Salicornia (Fig. 1c) may be attributed to non-heterocystous cyanobacteria that can be dominant seasonally among microphytobenthic communities (Currin 1996). A seasonal change in active nitrogen fixers could also explain the lack of a strong diel pattern in nitrogen fixation of S. virginica-vegetated sediments during August compared with that found in May. Active nitrogen fixers were not identified in the present study but can be characterized via reverse-transcripted PCR targeting nifH genes (Brown et al. 2003).
Nonetheless, the result that Salicornia- and Spartina-vegetated sediments showed opposite temporal patterns of nitrogen fixation activity suggests that different plant species can engage quite distinct nitrogen-fixing microbial communities.
Microhabitats for nitrogen fixation (sediments, epiphytes, roots)
Surface sediments as well as plant surfaces were active sites of nitrogen fixation. In sectioned cores from S. foliosa zones, nitrogen fixation was found only in surface sediments, while none was found in 4–5 cm depths. Nitrogen fixation activity was detected for only one rinsed S. foliosa roots and was less than half the rate of the most active sediments on the same date. These results suggest that nitrogen fixers associated with this species may not reside within plant tissues, as with other Spartina species (Spartina alterniflora: Whiting et al. 1986; Gandy & Yoch 1988; S. maritima: Nielsen et al. 2001), but rather in surrounding sediments. However, disruption of biogeochemical gradients in rhizosphere sediments may have affected the activity of nitrogen fixers in subsurface sediment intervals and on rinsed roots, underestimating the rates of nitrogen fixation in these samples. Nonetheless, the failure of shading S. foliosa plants to affect nitrogen fixation activities in sediments is consistent with the greatest activity being due to epibenthic nitrogen fixers rather than those in plant rhizospheres. In contrast, rapid responses of nitrogen fixers to changes in the plants’ photosynthetic status have been observed for S. alterniflora (Whiting et al. 1986). The photosynthetic status of S. foliosa samples was not quantified in this study. While rhizospheric bacteria have not been entirely ruled out as important players, these results contribute evidence for the ability of surface-dwelling microbes to dominate nitrogen fixation activity in the immediate vicinity of a Spartina plant (as in Jones 1974).
In measurements of epiphytic nitrogen fixation rates associated with S. virginica, the only plant species for which such measurements were made, the rates were comparable with those in intact sediment cores. In Atlantic coast marshes, epiphytic rates were similarly found to be substantial but were only approximately half the nitrogen fixation rates found in S. alterniflora rhizospheres (Currin & Paerl 1998). Epiphytic nitrogen fixation is not considered to be a direct source of nutrients to plants, although it can be of substantial importance as a nitrogen source in wetland food webs (Currin & Paerl 1998).
Nitrogen fixation and plant parameters
The positive relationship between belowground biomass of S. virginica and nitrogen fixation rates in August (Fig. 2) was consistent with that found in San Diego Bay in S. foliosa-vegetated cores (Zalejko 1989) as well as other wetland studies (Hanson 1983; McGlathery et al. 1998; Welsh 2000) and indicates that nitrogen fixation may be stimulated by plant roots (i.e.via oxygenation of rhizospheres or release of labile carbon). Few other strong relationships were found between plant parameters and nitrogen fixation in this study, possibly because data were collected at only one site and several plant properties did not vary substantially across salt marsh zones. Nitrogen fixation rates, on the other hand, were highly variable and possibly governed by patchy but highly active cyanobacteria on sediment surfaces rather than in rhizospheres, confounding relationships between rhizospheric nitrogen fixation rates and plant parameters.
The negative relationship between nitrogen fixation rates and S. virginica height (Fig. 3a) and the decline in nitrogen fixation rates with increasing plant biomass (the left portion of Fig. 3b) in August was likely due to positive correlation of these two parameters with environmental factors that inhibited nitrogen fixation. In fact, porewater ammonium concentrations showed positive trends with both S. virginica height (r2 = 0.57, P = 0.08) and above- ground biomass (r2 = 0.52, P =0.10) while a negative trend was found between ammonium and nitrogen fixation (r2 = 0.44, P = 0.15). Although previous reports of relationships between S. virginica and nitrogen fixation are not known, positive relationships between S. virginica characteristics (biomass, number of branches, branch tissue nitrogen concentration) and sediment nutrient levels have been reported previously (Boyer et al. 2001). Contrary to the case with plant height, samples with the highest above-ground biomass showed a secondary increase in nitrogen fixation that is consistent with plant stimulation of nitrogen fixation found in several other studies (Hanson 1977, 1983; Boyle & Patriquin 1981). While plants are often reported to stimulate nitrogen fixation via release of organic exudates from the roots, above-ground plant biomass may also benefit nitrogen-fixing communities via shading and retention of vital moisture in arid Mediterranean marshes.
Significance of nitrogen fixation in southern Californian marshes
Annual average rates of nitrogen fixation in S. foliosa habitat found in the present study (equivalent to 5.1 ± 1.9 g N m−2 year−1) are of the same order as those reported for a mature S. alterniflora marsh on the Atlantic coast (6.1 ± 0.5 g N m−2 year−1, Tyler et al. 2003) but are higher than those reported for S. foliosa in San Diego Bay, the only other known nitrogen fixation data for southern California marshes, by more than an order of magnitude (Langis et al. 1991). Several methodological differences might contribute to the disparities between the results of this study and those of Langis et al. (1991). Specifically, this study employed shallower sediment cores but also used intact plant samples while Langis et al. (1991) clipped plants at the sediment surface. Langis et al. also incubated samples at relatively low light levels (10.5 μmol m−2 s−1) rather than in natural sunlight, as done in this study at Kendall Frost Marsh. These nitrogen fixation measurements have also been taken in a different bay than those of Langis et al. and therefore many site-specific factors could differ between these locations.
Using literature-based estimates of the aboveground net primary productivity for S. foliosa in a nearby salt marsh (Winfield 1980), along with the average tissue nitrogen content of S. foliosa shoots obtained in this study, one may estimate that nitrogen fixation rates can meet between 36% and 92% of annual nitrogen demands. This estimate is based upon the average daytime and average nighttime fixation rates for S. foliosa-vegetated sediments in this study, which had large standard errors because of high variability among nitrogen fixation rates. Similar calculations for S. virginica-vegetated sediments suggest that nitrogen fixation could provide between 32% and over 100% of nitrogen demanded by the plant, although the majority of this would be fulfilled by nighttime activity, and is likely uncoupled with plant nitrogen demands. These calculations do not include the nitrogen fixation performed by epiphytes or that in sediments deeper than 6 cm and therefore underestimate the nitrogen fixation associated with both plant species. As productivity of salt marsh plants in southern California exhibits high interannual variability (Zedler et al. 1992), assessments of the significance of nitrogen fixation rates in this study could be improved if derived from more recent or contemporaneous productivity measurements. Nitrogen fixation rates may also show seasonal variability, which might not be accurately reflected in nitrogen fixation rates calculated from one or two sampling dates and were not addressed in this study. The actual contribution of nitrogen fixation to plant nitrogen demands will depend on several factors including the extent to which fixed nitrogen is actually being assimilated by the plant. Reliance of vascular plant species on fixed nitrogen has not been demonstrated in this study but can be determined using 15N2 enrichment experiments to trace fixed nitrogen into plant tissues (Jones 1974).
Nonetheless, this calculation shows that the magnitude of nitrogen fixation is high enough that it could be an important nutrient source to primary producers and consumers in southern California and in Mediterranean marshes in general. In southern California, where the need for effective salt marsh restoration is especially pertinent to the survival of endangered bird and plant species, nitrogen fixation may warrant consideration as an important function affecting the development of a healthy ecosystem. As Spartina and Salicornia species are common in marine wetlands worldwide (i.e. US Atlantic and Gulf coasts, Mediterranean, South African coasts), the different ways that these plants engage nitrogen-fixing microbes require further study to advance understanding of nutrient dynamics in wetlands globally. Further study of interactions between habitat-forming plants and the microbial communities with which they coexist can improve ecosystem-based views of wetland health and function, enabling greater understanding of the ramifications of biodiversity loss and degradation that increasingly threaten coastal habitats.
Conclusions
Nitrogen fixation activity associated with vascular plants of Kendall Frost Marsh Reserve was high but dynamic across marsh elevations and time. Sediments vegetated by two dominant plants, Spartina foliosa and Salicornia virginica, showed opposite diel patterns, with higher daytime nitrogen fixation rates found in S. foliosa-vegetated sediments than in those vegetated by S. virginica. Nitrogen fixation activity was the highest in S. foliosa-vegetated sediments, although little activity was found in rinsed S. foliosa roots. Surface sediments also supported greater rates of nitrogen fixation than subsurface (4–5 cm) intervals, suggesting that patchy but active epibenthic cyanobacteria may be major nitrogen fixers in this system. Nitrogen fixation by sedimentary bacteria could provide substantial portions of S. foliosa nitrogen demands, although S. virginica is less likely to benefit as greatly from this nutrient source as it does from exogenous nitrogen, with implications for restoration and conservation strategies for these habitat-forming wetland plant species.
Acknowledgements
This work was performed at the University of California Natural Reserve System, Kendall Frost, and supported by a Mildred E. Mathias Graduate Student Research Grant from the University of California Natural Reserve System. Support for research expenses has also come from the Michael M. Mullin Memorial Fund, the U.C. Marine Council Grant (UCMARINE 32114), and the Graduate Department of Scripps Institution of Oceanography. The author's stipend has been provided by the National Science Foundation (Graduate Research Fellowship) and the Alliance for Graduate Education in the Professoriate (Minority Access to Science Engineering and Mathematics). Dr Carolyn Currin and Dr Lisa A. Levin provided important guidance regarding research directions. Doug Capone and Tanya Cane helped to provide training acetlyene reduction techniques. The author also thanks Isabelle Kay for coordinating access to the Kendall Frost Reserve. Numerous undergraduate students at U.C. San Diego provided valuable assistance with field work including Maria del Carmen Rivero, Laura Mendoza, Katie Dayton, Tanya Perez, Ethan Hua, Howard Hsiung, and Isaac Paerlman. Dr Lihini Aluwihare provided use of the FID-equipped gas chromatograph and assistance with acetylene reduction analyses. The author also thanks Tracy Washington and Maria del Carmen Rivero for their help with laboratory analyses, and Guillermo Mendoza provided valuable assistance with manuscript formatting. Finally, the author is grateful to two anonymous reviewers who contributed useful feedback for this publication.
Salicornia virginica has recently been renamed Sarcocornia pacifica but is referred to as the former throughout this paper.




