Current trends of space occupation by encrusting excavating sponges on Colombian coral reefs
Abstract
Some sponges of the genus Cliona (Porifera, Hadromerida, Clionidae) simultaneously excavate and encrust calcareous substratum, competing aggressively for illuminated space with corals and other organisms. To interpret current trends of reef space occupation, the patterns of distribution and size of three Caribbean species were examined at San Andrés Island and Islas del Rosario in Colombia. While Cliona aprica was ubiquitous, C. caribbaea (= C. langae) preferred deep and protected reef zones, and C. tenuis shallow and wave-exposed settings. In contrast to the effect on other excavating sponges, chronic exposure to raw sewage did not significantly increase the abundance of the studied sponges. Substratum occupation/availability ratios showed a positive tendency of the sponges toward certain coral skeletons, and a negative or neutral tendency toward calcareous rock, indicating that establishment may be easier on clean, recently dead coral than on older, heavily incrusted substratum. High relief generally limits sponge size to that of the illuminated portions of the substratum. A generally lower proportion of small individuals than of larger ones indicates currently low recruitment rates and low subsequent mortality. Successful events of higher recruitment seem to have occurred for C. tenuis. These are related to the massive acroporid coral die-off in the early 1980s and to asexual dispersion during storms, resulting in a current 10% substratum cover. Reefs with high coral mortality were and/or are thus more susceptible to colonization and subsequent space occupation by these sponges, although relief may prevent space monopolization.
Problem
Sponges are a significant component of coral reefs, their bottom cover and density often being similar to or higher than those of stony corals (Rützler 1978; Zea 1993). Among the various morpho-functional types of reef sponges, those that excavate the calcium carbonate reef framework play a key role in the balance between reef accretion and erosion (reviews in Wilkinson 1983; Glynn 1997). Additionally, excavating sponges that encrust the substratum are also strong reef space competitors, aggressively undermining and displacing live coral tissue (Rützler 1975, 2002; López-Victoria et al. 2003). Indeed, during the last three decades, the cover of these sponges has increased considerably, especially at zones suffering massive coral tissue die-off from disease, bleaching and other causes (Cortés et al. 1984; Glynn 1997; Williams et al. 1999; Rützler 2002). This has led some authors to catalog them as a contemporary threat to coral reefs (Antonius & Ballesteros 1998; Williams et al. 1999). The increase of these and other excavating sponges has also been related to other factors which are detrimental to reef corals but are favorable to sponges, such as organic pollution or temperature increase (Rose & Risk 1985; Holmes 1997, 2000; Rützler 2002).
Cliona aprica Pang, 1973, C. caribbaea Carter, 1882 (= C. langae Pang, 1973) and C. tenuisZea & Weil, 2003, subjects of this study, are three of the encrusting excavating Caribbean sponges whose recent increase in space occupation and resulting coral tissue mortality have been reported (Glynn 1997; Rützler 2002; López-Victoria et al. 2003; Zea & Weil 2003; López-Victoria & Zea 2004). These sponges spread laterally, forming a shallow depression in the substratum, ca. 1–2 cm deep and from a few centimeters to several meters wide, covered extensively or completely with dark brown to brown-black tissue; vertical excavation of galleries and chambers, filled with dark yellow tissue, is limited to the upper 1–2 cm layer of the substratum. They have associated intracellular zooxanthellae and preferentially inhabit well-illuminated substratum (Zea & Weil 2003). Their undermining process involves sending out excavating tissue filaments that erode the skeletal support of the coral polyps (Schönberg & Wilkinson 2001; López-Victoria et al. 2003), which retract or are sloughed off; the sponge then advances over and below the freed substratum (López-Victoria et al. 2003). To contribute to the understanding of the processes that have led to current levels of reef space occupation by these three species, we evaluated their response in terms of density, cover and size, substratum availability, and the presence and abundance of live corals and other bottom components. Rates of lateral spread against corals and other neighbors and the factors that control them have been (López-Victoria et al. 2003; López-Victoria & Zea 2004) and will be published elsewhere.
Material and Methods
1. Studied sponges
Cliona aprica occurs as fields of closely spaced, small (up to 4 mm in diameter), dark brown to black inhalant and exhalant (oscular) papillae; these may fuse extensively, forming an incomplete thin crust which may extend sideward up to about 50 cm in diameter. Oscules are up to 1.9 mm in diameter, encircled sometimes by a grayish rim. C. caribbaea Carter, 1882 (junior synonym C. langae Pang, 1973) covers completely the excavated substratum with a thicker (up to 2 mm) amber brown or gray brown tissue. Size may reach up to 1 m in diameter. Oscules are up to 2.3 mm in diameter, scattered and conspicuous, usually with a creamy rim. Cliona tenuisZea & Weil, 2003 encrusts the entire excavated substratum with a thin veneer of rather transparent brown tissue, with yellowish, greenish, reddish or orange shades, through which the underlying excavated carbonate structures are usually visible. Diameters may reach 3–5 m. Oscules are small and inconspicuous, up to 1.4 mm in diameter. For detailed descriptions and synonymy and for the general ecology of the three species see Zea & Weil (2003) and López-Victoria et al. (2003).
2. Study areas
The study was carried out at San Andrés Island and at Islas del Rosario Archipelago, Colombia (Fig. 1). San Andrés is an oceanic island located in the SW Caribbean and surrounded by an extensive calcareous platform with barrier, terrace and lagoonal reefs (Díaz et al. 1995). Rosario is a complex of low islands and cays located directly on the Colombian continental shelf, surrounded by fringing, terrace and lagoonal reefs (Díaz et al. 2000; Cendales et al. 2002).
Map of the study area with sampling stations. At San Andrés: BB, Bajo Bonito (patch reef at the base of the shallow leeward terrace, 12–15 m in depth); AN, off the raw sewage outlet (leeward deep terrace, 12 m); WP, deep Wildlife (leeward deep terrace, 12–15 m); WS, shallow Wildlife (leeward shallow terrace, 4–6 m). At Islas del Rosario: MY, Majayura, CF, Canal del Francés (both part of the windward fringing reef, 4–6 m).
3. Data gathering and analyses
To establish the general distribution and habitat preference of these sponges, an overall qualitative survey of the studied areas was carried out, whereby sponge presence and abundance was recorded, in relation to the degree of reef development and coral health status. Quantitative density and cover data of the sponges and other benthic components were obtained at six stations, four at San Andrés (for C. aprica and C. caribbaea) and two at Islas del Rosario (for C. tenuis) (Fig. 1). Cliona tenuis also occurs at San Andrés, but is restricted to the windward side, where prevailing strong surge prevented quantifications. Cliona aprica and C. caribbaea are scarce at Islas del Rosario and were thus not quantified there. As we intended to evaluate the response of these sponges to the surrounding coralline environment, the stations were chosen for their relatively high sponge abundances. San Andrés stations were located on the two major topographical features of the leeward platform, the shallow (2–9 m) and the deep (10–20) m terraces, two gently sloping, hard-ground shelves separated by a sand channel. The shallow terrace consists of low-relief pavement interspersed with small (usually <50 cm diameter) coral heads; the deep terrace has a profuse development of massive corals, its outer edge sloping down to the island drop off (see Díaz et al. 2000). Shallow Wildlife station (WS, 4–9 m in depth) was located on the shallow leeward terrace. Deep Wildlife (WP) and Aguas Negras (AN) stations were both located on the deep terrace (10–17 m in depth), but AN, being located off the major sewage outlet of the island, has a lower relief and sessile animal cover than WP and is dominated by calcareous rock (dead coral) covered by turf algae and fine sediments. Bajo Bonito (BB, 9–12 m in depth) station is a mound-shaped patch reef of large (up to 2–3 m diameter) coral heads located at the base of the shallow terrace. The two stations in Islas del Rosario were located on the windward, north-facing fringing reef, at the 3–6 m deep elkhorn coral Acropora palmata (now dead) reef zone. In both stations – Canal del Francés (CF) and Majayura (MY) – the bottom is covered by still-standing and fragmented, dead A. palmata, interspersed with large (up to 2–4 m diameter) massive corals.
Sponge density and frequency of occurrence on the various coral skeletons and substrata were recorded in two to three band transects (20 × 2 m) at each station. A 20-m tape was deployed on the bottom, and data were obtained by swimming over a 1-m area on each side of the tape, using a 1-m-long rod as reference. Every substratum not occupied by macroinvertebrates or macroalgae, in which the original builder organism could not be identified (such as terrace rocky pavement or old dead coral, both heavily incrusted by turf and/or crustose algae), was combined in a single category of ‘calcareous rock’. Maximum diameter of every sponge individual in the band was measured to the nearest centimeter with a tape measure attached to the reference rod. Size distribution histograms were drawn for the following size ranges: 0–5 cm in diameter (small), 6–15 cm (mid-size), 16–45 cm (large), and >45 cm (very large); in general, growth is uniform in all directions, with the exception of very large individuals, which may have several separated fronts of growth (Acker & Risk 1985). Cover of sponges, stony corals (live and dead), sand-rubble, other invertebrates, trash, and calcareous rock were estimated by measuring the distance of the tape that intercepted each component, and calculated as percent area relative to the total tape length.
The patterns of substratum (coral species or calcareous rock) utilization by the studied sponges were analyzed by calculating, for each station (all transects combined), the ratio of percent sponge individuals occupying a substratum to the percent availability (cover) of that substratum. This occupation/availability ratio indicates the tendency a sponge has towards a substratum. If the ratio is >1, the substratum is occupied in a proportion greater than its availability; if <1, it is occupied in a proportion lower than its availability; if the value = 1, it is occupied in the same proportion of its availability. The ratio is brought about by processes at larval settlement (choice, avoidance, or inability to settle) along with differential survival after settlement, and was used to infer substratum effects in distribution. For C. aprica and C. caribbaea at San Andrés, using each station as replicate (all transects combined), a Student's t-test (two tails) was employed to determine if the occupation/availability ratio was significantly different from 1, with the null hypothesis of mean ratio = 1, and the alternative hypothesis of mean ratio≠ 1. The test was carried out only when the sponge–substratum combination was present in three or more stations. Hence, data for C. tenuis, obtained only at two Islas del Rosario stations, could not be tested.
Results
1. Distribution, cover and density
Overall, the presence and abundance of the studied excavating sponges was related to depth and degree of wave exposure, and varied according to the geographical location. At San Andrés, C. aprica was found in almost all reef zones and at practically all reef depths. In those sites where hard bottom space seemed limiting, either because of the high cover of other invertebrates (e.g. corals, sponges) or because there were many macroalgae or trapped sediments in the light-exposed substratum, this species grew in semicryptic spaces and in the papillated growth stage. In contrast, in places where the availability of well-lit calcareous rock (as defined in ‘Material and Methods’) was greater, this species was rather conspicuous; here, it was encrusting and larger, growing as extensively fused papillae. At Islas del Rosario this species was less conspicuous, found only papillated and in shallow and mid-depth (down to 12 m) lagoonal environments in rubble and in the base of branching live corals. At San Andrés, C. caribbaea was found restricted to depths below 5–6 m in the leeward side and below 12–15 m in the windward side; it always inhabited well-lit surfaces of the substratum, with a slight tendency to be more abundant in sites with greater amounts of calcareous rock. At Islas del Rosario this species was rare, restricted to deep (>20 m) leeward reef zones and growing only in the papillated stage. Cliona tenuis was present and very conspicuous as large encrusting sheets in shallow (<6–8 m), well-lit surfaces of windward settings of both San Andrés and Islas del Rosario. In the latter area, it was restricted to the pavement and the dead A. palmata thickets and rubble of the northern, windward fringing reefs and fore-reef terraces. Within these reefs, wherever massive (live) or foliose (live or dead) corals were dominant, C. tenuis was less abundant. In leeward settings, it occurred only in the shallow (2–4 m) rocky shore of San Andrés.
Despite the local differences in predominant growth stage and occurrence in semicryptic versus exposed substratum, throughout the four quantitative stations at San Andrés, the cover (0.5–2.0%) and density (0.4–0.7 indiv.·m−2) of C. aprica were of about the same magnitude, with no particular trend in relation to reef zone or degree of reef development or health status (Tables 1 and 2). In contrast, C. caribbaea had a lower density and cover than C. aprica, with the largest (although still low) density (0.3 indiv.·m−2) and cover (0.3%) at the deep terrace station located off the raw sewage outlet (AN) where the reef is strongly impacted. At Islas del Rosario stations, the C. tenuis cover varied between 7.6% and 9.5%, while its density varied between 1.3 and 1.8 indiv.·m−2; the station with the greatest C. tenuis abundance (CF) had the highest cover of calcareous rock (mostly dead branches of the coral A. palmata) and the lowest cover of live coral and other organisms (mostly zoanthids and macroalgae) (Tables 1 and 2).
| station | cover | ||||||
|---|---|---|---|---|---|---|---|
| cro | lco | snd | other | Cliona aprica | Cliona caribbaea | Cliona tenuis | |
| San Andrés | |||||||
| WP (n = 3) | 44.2 ± 2.5 | 29.0 ± 3.1 | 22.7 ± 2.0 | 3.6 ± 0.3 | 0.5 ± 0.3 | + | n.p. |
| WS (n = 3) | 70.2 ± 6.0 | 20.9 ± 1.4 | 5.5 ± 5.2 | 1.7 ± 0.9 | 1.7 ± 1.0 | + | n.p. |
| BB(n = 3) | 56.7 ± 4.1 | 36.7 ± 5.3 | 3.2 ± 0.7 | 1.4 ± 1.1 | 2.0 ± 0.7 | + | n.p. |
| AN(n = 2) | 76.8 ± 1.3 | 2.1 ± 1.1 | 14.1 ± 0.4 | 5.2 ± 1.0 | 1.5 ± 1.3 | 0.3 ± 0.02 | n.p. |
| I. del Rosario | |||||||
| CF(n = 3) | 68.9 ± 3.0 | 13.5 ± 2.6 | 2.3 ± 2.3 | 6.1 ± 2.5 | n.p. | n.p. | 9.5 ± 3.5 |
| MY (n = 3) | 45.7 ± 4.9 | 17.3 ± 1.7 | 6.5 ± 1.3 | 22.8 ± 5.0 | n.p. | n.p. | 7.6 ± 2.3 |
- Station codes are those of Fig. 1.
- cro, calcareous rock (pavement and dead coral strongly incrusted by crustose coralline and turf algae); lco, live coral; snd, sand and rubble; other, invertebrates, frondose algae, trash; +, present on station but not on transect; n.p., not present.
| station | density (indiv.·m−2) | ||
|---|---|---|---|
| Cliona aprica | Cliona caribbaea | Cliona tenuis | |
| San Andrés | |||
| WP (n = 3) | 0.65 ± 0.14 | 0.06 ± 0.02 | n.p. |
| WS (n = 3) | 0.53 ± 0.04 | 0.01 ± 0.01 | n.p. |
| BB(n = 3) | 0.55 ± 0.12 | 0.01 ± 0.01 | n.p. |
| AN(n = 2) | 0.43 ± 0.08 | 0.30 ± 0.10 | n.p. |
| I. del Rosario | |||
| CF(n = 3) | n.p. | n.p. | 1.79 ± 0.67 |
| MY (n = 3) | n.p. | n.p. | 1.33 ± 0.33 |
- Station codes are those of Fig. 1.
- n.p., not present.
2. Size frequency distribution
At deeper San Andrés stations (WP, AN, BB), regardless of degree of reef development or health status, C. aprica occurred in a greater number of mid-size (6–15 cm) and sometimes large (16–45 cm) than of small (0–5 cm) and very large (>45 cm) individuals (Fig. 2). The same held true for C. caribbaea at the station off the raw sewage outlet (AN; the other stations had too few individuals to express a pattern). On the shallow terrace (WS), C. aprica had a similar number of small, mid-size and large and a few very large individuals (there was a single, very large individual of C. caribbaea at this station). Most individuals of C. aprica at this station were growing on coral heads, and the few very large ones usually occurred directly on the terrace flat pavement.
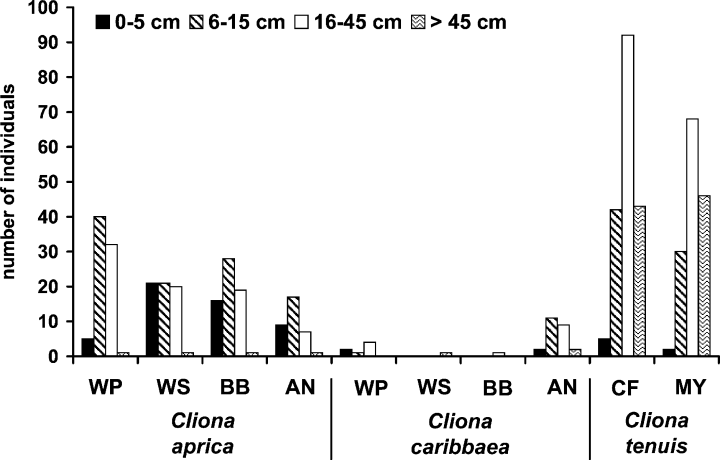
Size-frequency distribution for the three studied sponges. Size is maximum diameter. Size intervals are small (0–5 cm), mid-size (6–15 cm), large (16–45 cm) and very large (>45 cm). For station codes see Fig. 1.
The size frequency of C. tenuis at both Islas del Rosario stations differed from the other two sponge species at San Andrés, with a dominance of large individuals and an important proportion of very large ones. Despite slight differences in the proportion of bottom components between the two studied stations, the sponge size distributions were similar (Fig. 2).
3. Substratum utilization
At San Andrés, C. aprica significantly tended to occupy Siderastrea siderea as substratum in a greater proportion than its availability (Table 3; ratio = 4.76, P = 0.0006, Student's t-test, null hypothesis of ratio = 1, n = 3 stations). The same sponge species had a borderline tendency (ratio = 0.6, P = 0.048, n = 4 stations) to occupy calcareous rock in a lower proportion than its availability. In contrast, C. caribbaea was found in calcareous rock in the same proportion as its availability (ratio = 1.1, P = 0.31, n = 4 stations). Several other sponge–substratum combinations exhibited ratios consistently >1, but they were not significantly different from 1 due to the low number of replicates. Such was the case for C. aprica and Agaricia agaricites (mean ratio = 9.0, P = 0.28, n = 3), and for the same sponge and Montastraea annularis (ratio = 2.4, P = 0.26, n = 3). The remaining sponge species-substratum type combinations (of a total of 26) were not statistically tested because they occurred in only one to two stations.
| substratum (skeleton) | Cliona aprica | Cliona caribbaea | ||||
|---|---|---|---|---|---|---|
| n | ratio | P | n | ratio | P | |
| Agaricia agaricites | 3 | 9.0 ± 4.7 (2.5–19.8) | 0.28 | n.p. | ||
| Montastraea annularis | 3 | 2.4 ± 0.9 (1.4–4.3) | 0.26 | n.p. | ||
| Porites astreoides | 3 | 2.0 ± 0.6 (0.8–2.9) | 0.25 | + | ||
| Siderastrea siderea | 3 | 4.8 ± 0.3 (4.2–5.3) | 0.007 | n.p. | ||
| calcareous rock | 4 | 0.6 ± 0.1 (0.4–1.0) | 0.048 | 4 | 1.1 ± 0.1 (0.9–1.4) | 0.32 |
- Values are mean ± 1 SE (minimum-maximum) from n stations where the sponge–substratum combination was quantified (only those with n = 3 are included). P values are the probability of a two-tailed Student's t-test under the null hypothesis of ratio = 1. Sponge–substratum combinations in bold had a ratio significantly different from 1 (P < 0.05).
- +, sponge–substratum combination present on transects, but on the 3 stations; n.p., sponge–substratum combination not present on transects.
Discussion
Wherever the studied encrusting and excavating sponges occur, their local abundance seems to be controlled, among other things, by the availability of substratum to settle and grow, i.e. well-lit calcareous rock (dead coral, pavement) not overgrown by other macroorganisms. This appears to be a general trend for zooxanthellate excavating sponges (see review in Wilkinson 1983). The abundance of C. aprica, however, may be less dependent on overall substratum availability: what varies is the small-scale localization (cryptic versus light-exposed) and the type of growth (papillated versus encrusting).
In some reef areas there has been an increasing abundance of certain clionid sponges near sources of domestic waste (Rose & Risk 1985; Holmes 1997, 2000; Rützler 2002). Although values were not very high, C. caribbaea had its greatest densities and cover in the station off the San Andrés’ raw sewage outlet, in correlation with the greater amount of available calcareous rock (mostly dead coral covered by turf algae and fine sediments). The chronic effect of raw sewage has produced extensive coral death at this site (see Díaz et al. 1995); here, total excavating sponge cover is of the same magnitude as the live coral cover (around 2%). The feeding habit of sponges, which rely heavily on suspended particulate organic matter (e.g.Simpson 1984; Pile 1999), would favor sponges over corals (see also Zea 1994; Parra-Velandia & Zea 2003; Valderrama & Zea 2003, and references therein). Nonetheless, the cover and density of encrusting excavating sponges at this site was lower than at other studied sites in which coral mortality was also important, although not as high. Perhaps these sponges rely more heavily on the translocation of photosynthates from their endosymbiotic zooxanthellae (e.g.Rützler 1990; Rosell & Uriz 1992), and the turbidity associated with sites close to sources of runoff limits photosynthesis. Additionally, the studied sponges spread laterally at lower rates in calcareous rock heavily incrusted by crustose and turf algae than against live coral tissue (López-Victoria 2003). This type of substratum, when heavily silted as is the case off the raw sewage outlet at San Andrés, may also inhibit larval recruitment. Cliona caribbaea can apparently better cope with these stressful circumstances than C. aprica. This is reflected in its tendency to occupy heavily incrusted calcareous rock in the same proportion as its availability (occupation/availability ratio of 1.1), as opposed to the tendency to avoid it by C. aprica (occupation/availability ratio of 0.6, borderline significance).
The high cover and abundance of C. tenuis at the fore reef of the northern fringing reefs of Islas del Rosario parallel the findings of other studies in which its increased abundance was related to high levels of coral mortality (Cortés et al. 1984; Glynn 1997; Williams et al. 1999; Rützler 2002). Islas del Rosario suffered massive die-offs of acroporid corals during the early 1980s as a consequence of white band disease and other adverse and circumstantial conditions (see Werding & Sánchez 1979; Ramírez 1986; Garzón-Ferreira & Kielman 1993). Today, the dead A. palmata on the fore front of the windward fringing reefs is widely covered by C. tenuis. A similarly high cover of this species was also reported by Williams et al. (1999, as C. langae, 10.8%, versus 7.6–9.5% at Islas del Rosario) in Puerto Rico, where there was also a massive die-off of A. palmata, probably from similar causes and around the same time. Another case was put forward by Rützler (2002, as C. caribbaea), who reported cover values of 1.8–2.3% at the back of the Belizean barrier reef in 1997; these values increased to 2.7–6.8% after one year, coinciding with coral mortality associated with stress and diseases when temperatures were high.
From the few cases in which substratum occupation/availability ratios could be calculated and statistically tested, there appears to exist a positive tendency, or bias, towards the occupation of certain coral skeletons (C. aprica) and a negative (C. aprica, borderline significance) or neutral (C. caribbaea) tendency towards occupying calcareous rock. For these sponges, this ratio could be a measure of substratum suitability, i.e. the combined quality that a substratum presents for its larvae to attach, and for the sponge to then excavate and extend laterally. The settlement and subsequent attachment of these sponges is little known (although see Mariani et al. 2000). Further growth may then depend on the nature of the underlying substratum and of the obstacles put forward by live neighbors (Schönberg 2002; López-Victoria 2003). Our occupation/availability ratios, however, indicate that it is more difficult for the studied sponges to settle and/or to grow in calcareous rock, a heavily incrusted and modified substratum, than in corals. Since corals are generally able to prevent direct settlement of other organisms onto their live tissue (e.g. a Cliona, see McKenna 1997; macroalgae, see Díaz-Pulido & McCook 2002), we can assume that sponges currently living in live coral colonies (in many cases completely surrounded by live tissue; personal observation by the authors) must have settled preferentially on rather clean, recently dead tissue areas. This assumption is supported by the fact that some of these sponges tend to be more abundant in areas of recent or ongoing important coral mortalities. In addition, the lateral growth rates of encrusting excavating sponges in calcareous rock are slower than against live coral tissue, and the latter in turn slower than in clean coral skeletons (Schönberg & Wilkinson 2001; Schönberg 2002; López-Victoria 2003).
Maximum size of the studied sponges was limited by the availability of well-lit substratum. In situations of high relief, these sponges spread laterally up to a limit given by the size of the top of the substratum elevations, after which they thicken their tissue instead (see López-Victoria et al. 2003). In contrast, in those reefs or terraces with low relief and with a large cover of calcareous rock unoccupied or only partially occupied by macroalgae or macroinvertebrates, the sponges were generally much larger. An exception to this pattern was the station off the raw sewage outlet at San Andrés (AN), where the sponges were not very large, despite an intermediate to low relief and minimal cover of coral and other macroorganisms. In this case, as mentioned above, turbidity may limit growth, aided perhaps by a smothering effect from silt at the sponge–turf algal border. Another exception was the shallow Wildlife station, a flat terrace with intermediate to low relief, where size in C. aprica was evenly distributed among small, mid-size and large individuals. Perhaps lateral growth is limited here because most individuals of this species are growing on small corals (<50 cm, mostly Siderastrea siderea). The border between flat pavement and coral heads is usually overhanging, making it difficult for the sponges to spread up or down and grow further. Indeed, in shallow windward settings at San Andrés, with a greater amount of pavement, this sponge sometimes reaches very large sizes (authors’ personal observation).
Given that size is not related to age, it is difficult to infer patterns of recruitment and growth from size frequency distributions. The generally low abundance of small versus larger individuals, however, indicates that current recruitment rates to the small size class are relatively low. Moreover, the predominance of larger individuals may also indicate that once a sponge has established, survival is high and subsequent growth goes generally unimpeded until the sponge runs out of suitable substratum. In fact, 180 marked individuals of the three studied sponges seldom lost tissue or died during 13 months of follow up (López-Victoria 2003). Two scenarios, which need to be explored by further research, may be producing these size distributions. In the first – under constantly low recruitment rates, low subsequent mortality and with maximum size limited by available substratum – new individuals will continually be added to the upper size classes as they grow. Alternatively – under currently low but sporadically high recruitment rates, with maximum size also limited by available substratum and perhaps some mortality of large size classes – the larger individuals would be those that survived the high recruitment events (see also Yoshioka 1998). The latter may be the case for C. tenuis at Islas del Rosario because the time of initiation of the local population is known to have occurred after the massive die-off of Acropora palmata in the early 1980s (the studied sponges were virtually absent before; S. Zea, personal observation). Also, about 25% or perhaps more of the individuals now living in massive corals apparently colonized them by dispersion from sponge-colonized dead coral branches moved about during storms (López-Victoria & Zea 2004), in a process of asexual recruitment. Thus, the current high density and cover of C. tenuis, and the dominance of large and an important proportion of very large individuals, can be ascribed to one or several episodes of above-average recruitment, which occurred on the reef space newly freed after the massive death of acroporid corals or after storms.
From all the above, it seems apparent that reefs which have had high coral mortalities, or are experiencing lower but steady coral tissue death, were or continue to be more susceptible to colonization and subsequent increase in space occupation by the studied sponges. Complete reef space monopolization, however, seems unlikely because relief limits sponge spread.
Conclusions
Local abundance of Cliona aprica, C. caribbaea and C. tenuis on Colombian Caribbean reefs depends overall on the availability of well-lit substratum not colonized by corals and other macroorganisms. In contrast to other cases, these sponges did not greatly increase their abundance in reefs subjected to chronic organic pollution. The increase in abundance of C. tenuis in Colombian Caribbean fore reefs followed the massive die-off of acroporid corals in the early 1980s. Substratum occupation/availability ratios indicate that establishment may be more difficult on older substratum than on recently dead coral. Maximum size is generally limited by the extent of the illuminated substratum available for lateral growth. Size frequency distributions point towards currently low recruitment rates and low subsequent mortality. However, haphazard events of higher recruitment, apparently related to massive coral die-off or asexual dispersion during storms, seem to have occurred for C. tenuis. Coral mortality thus makes reefs more susceptible to colonization by these sponges, although relief may prevent space monopolization.
Acknowledgements
This study was supported by the Colombian Science Fund (COLCIENCIAS, grant 110109-10387), with matching funds from INVEMAR (Biodiversity and Marine Ecosystems – BEM Program) and Universidad Nacional de Colombia (National Research Directorship – DINAIN). Logistic support at Islas del Rosario was provided by R. Vieira's CEINER Oceanarium. We are grateful for help during field work to A. Chaves-Fonnegra, F. Parra-Velandia, L. Banda, B. Ossa, N. Guevara and J. M. Medrano. E. Weil (University of Puerto Rico) introduced us to the significance of these sponges and accompanied our initial efforts. The map was made by D. Rozo at INVEMAR's GIS–Lab. Contribution 870 of INVEMAR and 249 of CECIMAR and the Graduate Program in Marine Biology of the Universidad Nacional de Colombia, Faculty of Sciences.




