Geographical differentiation of Aphanius dispar (Teleostei: Cyprinodontidae) from Southern Iran
Contributing authors: Tanja Schulz-Mirbach ([email protected]), Hamid Reza Esmaeili ([email protected]) and Bettina Reichenbacher ([email protected])
Abstract
The Arabian cyprinodontid Aphanius dispar (Rüppell, 1829) is known to show considerable morphological variation. It has remained unknown, however, whether this variation is a result of environmental differences or allopatric divergence owing to geographical isolation. In this study, 11 populations of A. dispar from three geographically separated basins were analysed, that is, the Makran Basin (I, one river system), the Hormuzgan Basin (II, five rivers and three hot springs) and the Helleh Basin (III, two hot springs) in southern Iran. Statistical analyses do not indicate significant differences between the fishes from river and hot spring habitats (T-test, p < 0.05), which is also supported by the Canonical Discriminant Analysis (CDA). Nevertheless, morphometric and meristic characters of the fishes, as well as otolith morphology and morphometry, demonstrate that six phenotypic characters discriminate the A. dispar populations of the three basins, that is, (1) predorsal distance (Prdd.SL), (2) head length (HL.SL), (3) pelvic fin length (Lplf.SL), (4) number of pelvic fin rays, as well as relative length of both the (5) medial part and (6) rostrum of the otolith. In addition, these characters display a consistent pattern of variation, thus providing support for the assumption that the phenotypically different A. dispar populations are a result of geographical isolation and not related to environmental differences. It is likely that the geological history of the drainage systems caused isolation event(s) that may date back to the Pleistocene (1.8 million years before present). The high phenotypic differences might suggest that the A. dispar populations from the three studied basins represent separate subspecies or even species.
Introduction
Most cyprinodontid species tolerate a wide range of temperature and salinity regimes, and their small size permits viable populations to persist in restricted habitats (e.g., Wildekamp 1993). These abilities also allow the translocation of cyprinodontids via drainage shifting and stream capture and promote survival of relict populations (Echelle and Echelle 1992). Cyprinodontids therefore represent particularly useful organisms for the study of microevolutionary processes in vertebrates (e.g. Villwock 1976; Tigano et al. 2006; Rocco et al. 2007).
The genus Aphanius is the only representative of the cyprinodontids in the Old World and is currently distributed along the coast of the ancient Tethys Sea (Kosswig 1967; Villwock 1999). Its distribution area includes both coastal (brackish) and landlocked (freshwater to euryhaline) water bodies in the Mediterranean and Persian Gulf areas as far as Iran and Pakistan (Wildekamp 1993). The greatest species diversity appears in the Near East, especially in Anatolia and Iran (Wildekamp et al. 1999; Coad 2000; Hrbek and Meyer 2003; Hrbek et al. 2006). Additional not yet described species may occur in land-locked sites and remote areas (Coad 2000, 2009; Coad and Abdoli 2000; Reichenbacher et al. 2009a).
Among the species of Aphanius, the Arabian killifish Aphanius dispar (Rüppell, 1829) is the most widespread taxon. The native distribution area of this sexually dimorphic species ranges from the Red Sea and Persian Gulf to the Indian Ocean and southern coastline of Pakistan (Wildekamp 1993). In Iran, A. dispar is distributed in several oligohaline and euryhaline inland water bodies such as hot springs and endorheic drainage systems, but it also occurs in estuarine rivers (Krupp 1983; Abdoli 2000; Teimori 2006; Coad 2011).
Wildekamp (1993) provided a comprehensive survey of the numerous populations of Aphanius dispar and pointed out that distinct differences with regard to colour pattern, size of dorsal and anal fins, number of gill rakers and lateral line series exist among the many isolated populations. However, only a few of the numerous A. dispar populations have been investigated in detail to date. Hrbek and Meyer (2003) used mt-DNA data of five A. dispar populations to distinguish between two major A. dispar clades, that is, the Persian Gulf and the Red Sea clade. Moreover, the mtDNA data strongly argue against monophyly of A. dispar because the Red Sea clade also includes A. dispar richardsoni (Boulenger, 1907), while the Persian Gulf clade also includes A. ginaonis Holly, 1929. As a result, Hrbek and Meyer suggested that A. dispar does not constitute a species in terms of the phylogenetic species concept. Reichenbacher et al. (2009a) studied otolith morphology in eight A. dispar populations belonging to the Persian Gulf clade and found distinct otolith changes in the populations from long-term isolated freshwater habitats far inland. These authors hypothesized that otolith morphology is primarily genetically determined and that considerable genetic diversification resulting from allopatric divergence may be present among A. dispar within the Persian Gulf clade.
It is obvious from these studies that a reliable phylogenetic species concept is not available for Aphanius dispar and therefore its taxonomic status is not well understood. Either mt-DNA or otoliths have been used to define differences among populations, whereas a more integrative approach is lacking. The aim of this study is to contribute to the understanding of the phenotypic variation among A. dispar and provide data for the discussion of the question ‘what is Aphanius dispar?’. We studied a large data set of phenotypic characters from non-isolated as well as from isolated A. dispar populations and used the results to discuss the possible causes of phenotypic variation. Our investigation of the determinants of phenotypic characters is also essential for palaeontologists to more accurately determine teleost species diversity in the fossil record (cf. Nolf 1985, 1995; Patterson et al. 1993; Schwarzhans 2010).
Materials and methods
Study area and sampling
The study area is located in southern Iran, where three isolated drainage basins are inhabited by Aphanius dispar (Fig. 1). They include (from East to West) the Makran Basin (Basin I), discharging into the Gulf of Oman, the Hormuzgan Basin (Basin II), discharging into the Strait of Hormuz, and the Helleh Basin (Basin III), discharging into the Persian Gulf. We selected eight populations from the Hormuzgan Basin for the study of the non-isolated populations, and one respectively two populations from the Makran Basin and Helleh Basin to compare the characters among isolated populations.
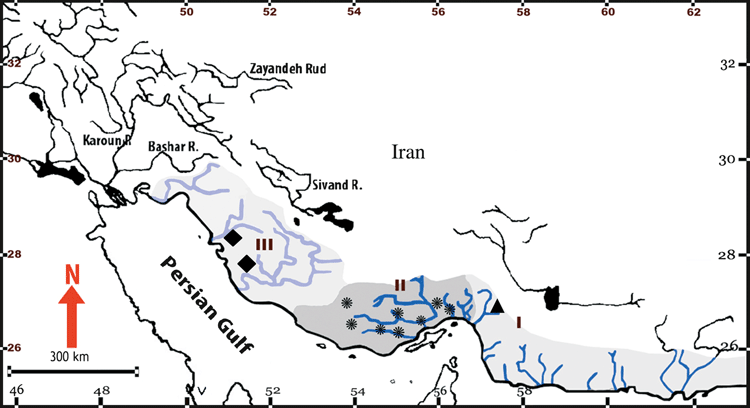
Geographical overview of the basins and sampling sites. I = Makran Basin, II = Hormuzgan Basin and III = Helleh Basin
Table 1 indicates the positions of the sample sites, the number of collected fishes (163 in total) and the type of the habitat. Specimens were caught from large populations, comprising more than several hundred specimens, using a hand net. To avoid shrinkage, the specimens were firstly transferred to ethanol 5% for 10 min and then preserved in ethanol 96% (we do not use formalin because it corrodes the otoliths and thus destroys their morphological features). Only adult specimens (identifiable by the typical colour patterns of males and females) with standard lengths ranging from 25 to 45 mm were collected to avoid ontogenetic effects.
| Sampling sites | Codes | Type of habitat | Basin | GPS Co-ordinates | Altitude (m) | N (♀/♂) | SL of populations (mean ± SD) |
|---|---|---|---|---|---|---|---|
| Rudan | RU | River-brackish water | I | E 57°,15′,14.5˝N 27°,28′,24.4˝ | 210 | 42 (20/22) | 28.5 ± 3.45 |
| Hassan Langi | HL | River-brackish water | II | E 56°,28′,10.2˝N 27°,19′,37.6˝ | 48 | 10 (5/5) | 30.6 ± 3.40 |
| Kol | KO | River-brackish water | II | E 55°,45′,31.2˝N 27°,07′,40.3˝ | 22 | 10 (5/5) | 28.6 ± 3.70 |
| Shur | SH | River-brackish water | II | E 54°,36′,30.5˝N 27°,16′,13.2˝ | 406 | 10 (5/5) | 27.7 ± 2.80 |
| Gotab | GO | River-brackish water | II | E 54°,15′,46.1˝N 27°,08′,39.8˝ | 330 | 10 (5/5) | 29.4 ± 3.35 |
| Kukherd | KU | River-brackish water | II | E 54°,29′,13.1˝N 27°,04′,28.7˝ | 273 | 10 (5/5) | 33.5 ± 3.50 |
| Khurgu | KH | Hot spring | II | E 56°,28′,08.2˝N 27°,31′,34.1˝ | 170 | 10 (5/5) | 31.9 ± 3.80 |
| Faryab | FA | Hot spring | II | E 54°,16′,28.0˝N 27°,25′,16.2˝ | 490 | 10 (5/5) | 29.6 ± 5.30 |
| Howba | HO | Hot spring | II | E 53°,53′,58.4˝N 27°,57′,30.5˝ | 640 | 10 (5/5) | 32.0 ± 4.00 |
| Dalaki | DA | Hot spring | III | E 51°,16′,35.4˝N 29°,24′,07.9˝ | 87 | 10 (5/5) | 33.0 ± 2.45 |
| Mirahmad | MI | Hot spring | III | E 51°,16′,50.9˝N 28°,47′,56.4˝ | 54 | 31 (16/15) | 28.9 ± 4.40 |
- Basin I = Makran, II = Hormuzgan, III = Helleh. SD = standard deviation. The river habitats are located within estuarine systems.
Analysis of fish specimens
Based on the morphometry introduced in Holcik (1989), 14 morphometric parameters were measured using a vernier caliper adjusted to the nearest 0.5 mm (Fig. 2a). They describe the following characters: total length (TL), standard length (SL), predorsal distance (Prdd), postdorsal distance (Podd), preanal distance (Prad), preorbital distance (Prod), length of caudal peduncle (Lcaup), maximum body depth (Maxbd), head length (HL), length of dorsal fin (Ldf), depth of dorsal fin (Ddf), length of anal fin (Laf), length of pectoral fin (Lpcf) and length of pelvic fin (Lplf). According to Lahnsteiner and Jagsch (2005), the values of selected measured morphometric parameters (=Mmp) were standardized to eliminate size effects. Standardization was achieved by using the standard length (SL) (Mmp/SL*100), the preanal distance (Prad) (Mmp/prad*100), the head length (HL) (Mmp/HL*100) and the length of the pectoral fin (Lpcf) (Mmp/Lpcf*100) (Table 2). In total, 27 variables were calculated from the measurements and served as input for the statistical analyses.
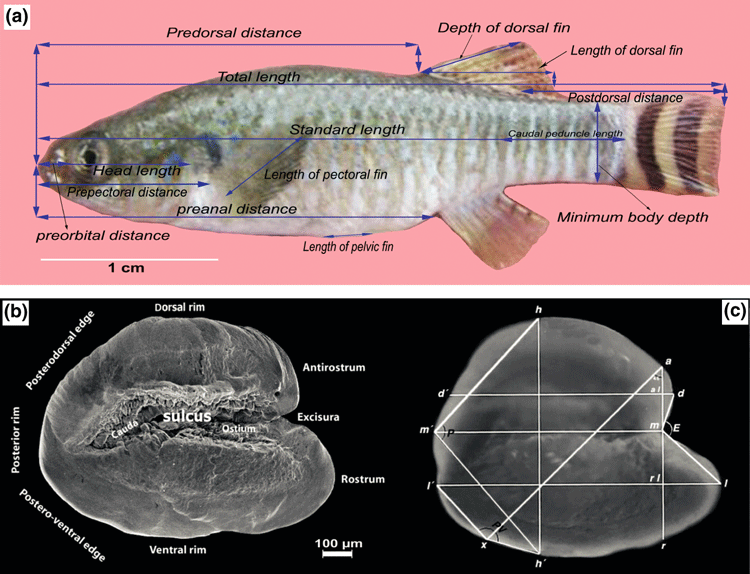
(a) Aphanius dispar (♂) from southern Iran with the linear measurements used in this study. (b) Left otoliths of A. dispar from Khurgu hot spring, Southern Iran showing the terminology of otolith characters according to Nolf (1985), (c) linear measurements and angles according to Reichenbacher et al. (2007), E = excisura angle, P = posterior angle, PV = posteroventral angle, l–l’ = length, h–h’ = height, m–m’ = mdial length, rl–l = rostrum length, r–m = rostrum height, al–d = antirostrum length, m–a = antirostrum height, d–d’ = dorsal length
| Morphometric characters | Abbreviation |
|---|---|
| Total length/Standard length | TL.SL |
| Predorsal distance/Standard length | Prdd.SL |
| Predorsal distance/Head length | Prdd.HL |
| Predorsal distance/Preanal distance | Prdd.Prad |
| Postdorsal distance/Standard length | Podd.SL |
| Postdorsal distance/Preanal distance | Podd.Prad |
| Preanal distance/Standard length | Prad.SL |
| Preorbital distance/Standard length | Prod.SL |
| Preorbital distance/Head length | Prod.HL |
| Preorbital distance/Length of pectoral fin | Prod.Lpcf |
| Length of caudal peduncle/Standard length | Lcaup.SL |
| Length of caudal peduncle/Preanal distance | Lcaup.Prad |
| Maximum body depth/Standard length | Maxb.SL |
| Head length/Standard length | HL.SL |
| Head length/Preanal distance | HL.Prad |
| Head length/Length of pectoral fin | HL.Lpcf |
| Length of dorsal fin/Standard length | Ldf.SL |
| Length of dorsal fin/Head length | Ldf.HL |
| Length of dorsal fin/Length of pectoral fin | Ldf.Lpcf |
| Length of pectoral fin/Standard length | Lpcf.SL |
| Length of pectoral fin/Head length | Lpcf.HL |
| Length of pectoral fin/Preanal distance | Lpcf.Prad |
| Length of pelvic fin/Standard length | Lplf.SL |
| Length of pelvic fin/Head length | Lplf.HL |
| Length of pelvic fin/Preanal distance | Lplf.Prad |
| Length of pelvic fin/Length of pectoral fin | Lplf.Lpcf |
| Depth of dorsal fin/Standard length | Ddf.SL |
In addition, seven meristic characters were counted under a stereomicroscope. They include the number of the gill rakers of the first branchial arch (GR), lateral line series scales (LL), caudal peduncle scales (CPS), pectoral (PCFR), pelvic (PLFR), dorsal (DFR) and anal (AFR) fin rays. The number of rays include only the branched rays.
Analysis of otoliths
Preparation
Ten skulls per population were opened ventrally and right and left otoliths were removed. Otoliths were cleaned from tissue remains with 1% potassium hydroxide solution for 6 h and rinsed in distilled water for 12 h.
All fish specimens are preserved now in ethanol 70% plus glycerine, and otoliths are kept dry in plastic boxes. The material is deposited in the collection of the Biology Department at the Shiraz University, Iran (CBSU11-65, fish specimens; CBSU11-65, otoliths), and in the collection of the Zoological Museum of Shahid Bahonar University of Kerman, Iran (ZM-SBUK66-162, fish specimens; ZM-SBUK66-162, otoliths).
Morphology
Otolith morphology was studied with a stereo-microscope. In addition, three representative otoliths from every population were studied by SEM (Fig. 2a). SEM images were captured with a LEO 1430 VP at the Zoological State Collection Munich (ZSM).
Morphometry
Left otoliths were positioned [with their lateral (outer) face down] on plasticine, and digital images were taken with the Leica Image Access Software (IMAGIC 1000, Imagic Bildverarbeitung AG, Glattbrugg, Switzerland) via a Leica camera (DFC 295) connected to a PC. Based on the method introduced in Reichenbacher et al. (2007), eight linear distances and three angles were measured for every left otolith (Fig. 2b). Linear measurements were standardized as a function of length and height of otolith, respectively, and then, together with the values of the angles, used for statistical analyses. Some of the otoliths from the Makran Basin showed abnormalities in their morphology and therefore were removed from the otolith data set. To have an otolith data set with similar number of specimens, we randomly reduced the number of otoliths from the other two basins.
Statistical analyses
The statistical analyses were carried out using PASW 18.00 (SPSS Inc, 2010) and PAST (Palaeontological Statistics, version 1.81 (Hammer et al. 2001). Univariate analysis of variance (anova, with Duncan’s post hoc test, p < 0.05) was used to test the significance of phenotypic differences among populations and also between the sexes within populations. The Canonical discriminant analysis (CDA) was used for multivariate analyses to show the classification success of the groups. To find the discriminatory importance of each variable (i.e. the values of each variable that contributed most to the separation of the groups) across all discriminant functions, the mean discriminant coefficients were calculated by using the following equation (Backhaus et al. 2006):
Mean discriminant coefficient bj = ∑ |bjk|*EAK (k = 1, k=….)
bjk, standardized discriminant function coefficients for variable j with respect to discriminant function k, EAk, proportion of eigenvalue of discriminant function k of the discriminant.
Furthermore, a dendrogram was constructed based on the Euclidean distance as a measure of dissimilarity. The ‘between groups linkage method’ was used as the clustering algorithm to show the phenotypic relationships between isolated populations.
Results
Variation within populations
In our study of within-population variation, we analysed male and female individuals from each population together because otherwise the specimen numbers would have been too low (see Table 3). The variation of every morphometric, meristic and otolith character within a population was assessed by the comparison of standard deviations (Table 3) and SEM photos (3, 4), respectively. The results reveal 24 morphometric characters (of 27), all seven meristic characters and five (of 10) otolith characters can show high variation of standard deviation within a given population (Table 3). In the following, we consider the populations from the three basins with regard to (1) the characters that show clear within-population variability and (2) the degree of variation that is visible within an individual population (see also Table 3).
| Characters | I | II | III | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RU | HL | KH* | KO | SH | GO | KU | FA* | HO* | DA* | MI* | ||
| N = 42 | N = 10 | N = 10 | N = 10 | N = 10 | N = 10 | N = 10 | N = 10 | N = 10 | N = 10 | N = 31 | ||
| (A) Variation of the morphometric characters of the fishes | ||||||||||||
| TL.SL | Min | 112.0 | 115.9 | 115.9 | 118.6 | 115.9 | 117.0 | 116.2 | 113.0 | 113.4 | 111.0 | 109.0 |
| Mean | 115.5 ± 2.2 | 118.7 ± 1.7 | 119.3 ± 2.1 | 120.7 ± 2.2 | 118.1 ± 1.7 | 119.4 ± 1.8 | 119.7 ± 1.8 | 116.2 ± 2.1 | 117.7 ± 6.2 | 118.2 ± 2.1 | 119.6 ± 6.5 | |
| Max | 120.5 | 121.3 | 122.7 | 125.6 | 121.1 | 122.8 | 123.2 | 119.5 | 122.4 | 121.5 | 123.5 | |
| Prdd.SL | Min | 60.9 | 62.9 | 62.5 | 61.4 | 62.6 | 62.8 | 62.3 | 60.56 | 59.3 | 59.7 | 58.7 |
| Mean | 63.7 ± 1.5 | 66.2 ± 2.14 | 66.2 ± 2.14 | 65.5 ± 2.6 | 65.1 ± 1.5 | 65.4 ± 1.9 | 65.66 ± 1.6 | 63.3 ± 6.8 | 61.4 ± 1.4 | 63.0 ± 2.1 | 63.1 ± 2.4 | |
| Max | 66.7 | 69.1 | 69.1 | 69.6 | 67.8 | 68.4 | 67.7 | 66.3 | 64.5 | 67.6 | 67.9 | |
| Prdd.HL | Min | 200.0 | 202.0 | 205.0 | 189.1 | 189.5 | 198.2 | 206.0 | 212.0 | 198.3 | 183.0 | 171.0 |
| Mean | 227.1 ± 8.4 | 222.8 ± 16.1 | 219.0 ± 7.6 | 216.0 ± 13.7 | 211.6 ± 9.3 | 211.1 ± 8.9 | 216.1 ± 7.0 | 225.3 ± 7.4 | 208.2 ± 7.6 | 196.1 ± 6.1 | 203.0 ± 10.7 | |
| Max | 142.0 | 257.0 | 227.0 | 234.0 | 223.1 | 221.0 | 226.2 | 235.8 | 221.0 | 207.0 | 203.0 | |
| Prdd.Prad | Min | 87.3 | 93.75 | 93.8 | 89.01 | 93.5 | 93.5 | 93.7 | 94.0 | 85.0 | 90.6 | 84.3 |
| Mean | 92.2 ± 2.9 | 98.65 ± 3.4 | 98.3 ± 7.5 | 97.03 ± 4.8 | 96.3 ± 2.3 | 96.0 ± 5.0 | 98.4 ± 2.4 | 96.5 ± 1.8 | 91.4 ± 2.9 | 96.6 ± 3.6 | 89.4 ± 4.3 | |
| Max | 99.0 | 104.0 | 102.0 | 103.9 | 101.4 | 99.6 | 101.5 | 100.0 | 93.2 | 101.4 | 100.9 | |
| Podd.SL | Min | 43.1 | 41.1 | 40.0 | 42.3 | 40.3 | 44.1 | 44.3 | 41.7 | 28.0 | 40.2 | 42.5 |
| Mean | 48.5 ± 2.1 | 43.9 ± 2.1 | 43.8 ± 2.1 | 46.0 ± 2.2 | 42.9 ± 1.5 | 46.1 ± 7.9 | 46.8 ± 1.6 | 47.1 ± 4.9 | 43.6 ± 6.1 | 44.5 ± 2.5 | 49.5 ± 4.2 | |
| Max | 52.2 | 49.4 | 46.6 | 50.4 | 45.8 | 47.2 | 49.7 | 56.0 | 47.2 | 49.7 | 65.9 | |
| Podd.Prad | Min | 64.2 | 59.3 | 58.0 | 62.3 | 58.6 | 64.7 | 65.6 | 61.6 | 42.0 | 58.5 | 60.4 |
| Mean | 70.26 ± 2.7 | 65.6 ± 4.7 | 65.15 ± 4.2 | 68.01 ± 3.1 | 64.0 ± 2.5 | 67.6 ± 2.7 | 70.2 ± 3.7 | 71.7 ± 7.1 | 64.1 ± 9.1 | 68.4 ± 6.6 | 70.2 ± 3.1 | |
| Max | 75.6 | 75.1 | 72.1 | 73.4 | 67.1 | 72.1 | 77.5 | 88.4 | 71.8 | 74.6 | 94.6 | |
| Prad.SL | Min | 63.5 | 63.9 | 64.6 | 64.8 | 65.5 | 64.2 | 63.1 | 62.9 | 65.8 | 61.1 | 66.5 |
| Mean | 69.1 ± 2.3 | 67.1 ± 2.5 | 67.4 ± 6.9 | 67.6 ± 5.5 | 67.5 ± 6.5 | 68.2 ± 2.6 | 66.7 ± 1.4 | 65.6 ± 1.9 | 68.1 ± 2.1 | 65.2 ± 2.3 | 70.5 ± 2.3 | |
| Max | 73.1 | 72.6 | 70.0 | 71.1 | 69.8 | 72.0 | 67.8 | 67.5 | 72.5 | 68.6 | 75.4 | |
| Prod.SL | Min | 6.4 | 8.5 | 8.2 | 7.9 | 7.8 | 9.0 | 9.4 | 7.7 | 7.5 | 9.1 | 7.8 |
| Mean | 7.8 ± 1.1 | 10.1 ± 1.1 | 9.3 ± 0.5 | 9.6 ± 0.9 | 9.7 ± 0.9 | 10.0 ± 0.7 | 10.7 ± 0.8 | 8.8 ± 6.4 | 9.3 ± 1.3 | 10.3 ± 1.4 | 11.0 ± 1.5 | |
| Max | 10.7 | 12.4 | 10.1 | 11.4 | 11.2 | 11.5 | 12.2 | 12.3 | 11.3 | 12.8 | 14.5 | |
| Prod.HL | Min | 23.4 | 30.6 | 28.0 | 27.9 | 23.7 | 28.5 | 32.7 | 27.4 | 25.5 | 28.2 | 27.4 |
| Mean | 28.1 ± 3.8 | 33.9 ± 7.9 | 30.8 ± 1.9 | 31.7 ± 2.1 | 31.4 ± 1.4 | 32.2 ± 2.1 | 35.2 ± 2.9 | 31.1 ± 1.9 | 31.4 ± 2.1 | 32.1 ± 4.0 | 32.8 ± 6.0 | |
| Max | 37.8 | 38.6 | 34.1 | 35.1 | 36.3 | 36.9 | 38.9 | 42.1 | 36.4 | 38.7 | 42.3 | |
| Prod.Lpcf | Min | 32.8 | 43.4 | 41.9 | 41.7 | 36.8 | 45.0 | 47.3 | 39.2 | 41.1 | 47.4 | 29.3 |
| Mean | 41.5 ± 7.2 | 53.1 ± 8.9 | 50.5 ± 5.5 | 51.6 ± 1.3 | 50.5 ± 3.1 | 52.2 ± 7.6 | 56.1 ± 7.2 | 48.6 ± 8.2 | 49.9 ± 6.6 | 56.5 ± 5.3 | 43.3 ± 15.2 | |
| Max | 64.1 | 74.4 | 58.7 | 61.1 | 61.9 | 72.3 | 71.4 | 68.9 | 59.5 | 66.1 | 77.4 | |
| Lcaup.SL | Min | 18.5 | 20.8 | 19.2 | 21.8 | 24.2 | 22.0 | 21.3 | 22.0 | 22.3 | 23.0 | 20.1 |
| Mean | 22.3 ± 2.4 | 24.3 ± 2.2 | 22.9 ± 1.8 | 23.7 ± 5.6 | 25.5 ± 5.8 | 24.2 ± 1.8 | 24.3 ± 6.4 | 25.5 ± 2.6 | 24.9 ± 6.2 | 26.5 ± 2.0 | 22.6 ± 1.4 | |
| Max | 27.7 | 27.1 | 25.0 | 27.5 | 26.5 | 27.2 | 26.1 | 29.1 | 25.9 | 28.7 | 25.4 | |
| Lcaup.Prad | Min | 25.4 | 29.7 | 29.5 | 32.4 | 36.4 | 30.5 | 31.5 | 32.6 | 33.9 | 34.6 | 26.7 |
| Mean | 32.4 ± 4.6 | 36.3 ± 4.1 | 34.1 ± 3.1 | 35.1 ± 3.0 | 37.7 ± 6.3 | 35.6 ± 3.7 | 36.5 ± 2.5 | 39.1 ± 4.9 | 36.4 ± 1.9 | 40.8 ± 7.1 | 32.1 ± 7.5 | |
| Max | 43.1 | 40.9 | 38.7 | 42.2 | 40.4 | 40.9 | 41.3 | 46.3 | 38.6 | 46.4 | 36.7 | |
| Maxb.SL | Min | 20.7 | 19.9 | 20.1 | 20.6 | 21.1 | 21.6 | 21.6 | 21.4 | 22.5 | 23.7 | 26.6 |
| Mean | 22.8 ± 1.4 | 21.9 ± 1.2 | 21.7 ± 0.9 | 22.3 ± 1.3 | 22.2 ± 0.9 | 23.5 ± 1.2 | 23.3 ± 5.4 | 23.0 ± 7.1 | 24.1 ± 1.2 | 25.4 ± 1.5 | 27.8 ± 1.9 | |
| Max | 27.4 | 23.7 | 21.3 | 24.4 | 23.8 | 25.2 | 25.7 | 24.7 | 26.9 | 29.4 | 31.9 | |
| HL.SL | Min | 26.1 | 25.9 | 29.1 | 28.2 | 29.9 | 28.9 | 28.9 | 26.4 | 27.5 | 30.2 | 28.0 |
| Mean | 28.1 ± 1.1 | 29.8 ± 2.2 | 30.2 ± 0.9 | 30.3 ± 1.5 | 30.8 ± 0.9 | 31.1 ± 1.3 | 30.4 ± 1.1 | 28.1 ± 1.2 | 29.5 ± 1.1 | 32.1 ± 5.9 | 31.1 ± 1.4 | |
| Max | 31.1 | 33.4 | 32.5 | 32.6 | 33.1 | 34.1 | 32.8 | 29.9 | 30.9 | 33.2 | 34.6 | |
| HL.Prad | Min | 37.8 | 37.0 | 42.7 | 42.2 | 42.9 | 42.9 | 44.0 | 40.0 | 39.7 | 45.5 | 40.3 |
| Mean | 40.6 ± 1.6 | 44.1 ± 3.9 | 44.9 ± 1.7 | 44.8 ± 2.1 | 45.6 ± 1.9 | 45.5 ± 2.5 | 45.6 ± 1.3 | 42.8 ± 6.4 | 43.3 ± 2.1 | 49.3 ± 2.4 | 44.1 ± 2.5 | |
| Max | 46.1 | 50.3 | 47.1 | 48.1 | 50.1 | 48.8 | 48.7 | 45.1 | 46.3 | 53.3 | 49.2 | |
| HL.Lpcf | Min | 127.1 | 140.0 | 148.5 | 142.5 | 147.1 | 144.1 | 139.4 | 141.5 | 131.9 | 152.9 | 121.8 |
| Mean | 147.0 ± 10.3 | 157.0 ± 28.3 | 163.9 ± 9.5 | 163.0 ± 17.7 | 160.0 ± 17 | 162.0 ± 16.2 | 159.0 ± 19.3 | 156.0 ± 12.8 | 160.0 ± 26.6 | 177.0 ± 17.1 | 156.0 ± 16.7 | |
| Max | 176.4 | 235.4 | 181.6 | 197.5 | 204.7 | 195.7 | 195.9 | 179.5 | 226.2 | 212.0 | 194.1 | |
| Ldf.SL | Min | 9.2 | 7.4 | 9.9 | 9.9 | 10.4 | 10.1 | 10.7 | 9.3 | 9.7 | 7.9 | 8.6 |
| Mean | 10.9 ± 1.1 | 12.5 ± 2.1 | 11.8 ± 1.3 | 11.8 ± 5.1 | 13.8 ± 2.7 | 13.0 ± 1.5 | 12.2 ± 1.0 | 11.8 ± 1.4 | 11.3 ± 1.0 | 11.6 ± 1.8 | 11.4 ± 1.3 | |
| Max | 13.2 | 8.8 | 13.7 | 13.4 | 19.2 | 15.8 | 14.3 | 13.7 | 12.6 | 13.5 | 14.3 | |
| Ldf.HL | Min | 31.6 | 29.1 | 32.7 | 35.0 | 34.9 | 35.1 | 34.9 | 32.4 | 32.7 | 24.5 | 28.3 |
| Mean | 39.0 ± 4.4 | 42.1 ± 7.4 | 38.8 ± 3.8 | 39.1 ± 2.5 | 44.8 ± 8.2 | 41.8 ± 3.9 | 40.3 ± 4.2 | 42.0 ± 5.6 | 38.5 ± 4.1 | 36.1 ± 7.3 | 50.0 ± 12.7 | |
| Max | 50.0 | 50.8 | 45.1 | 42.6 | 62.6 | 47.1 | 48.9 | 52.0 | 45.2 | 41.8 | 71.9 | |
| Ldf.Lpcf | Min | 47.4 | 40.8 | 51.9 | 54.4 | 58.1 | 53.0 | 54.3 | 48.3 | 47.8 | 52.0 | 47.6 |
| Mean | 57.3 ± 5.0 | 65.9 ± 14.1 | 63.7 ± 7.1 | 63.6 ± 6.6 | 71.3 ± 10.4 | 67.8 ± 10.1 | 63.9 ± 8.4 | 65.5 ± 10.5 | 61.7 ± 11.8 | 63.6 ± 8.2 | 57.1 ± 6.1 | |
| Max | 68.6 | 91.4 | 70.9 | 75.6 | 93.7 | 82.1 | 82.3 | 83.3 | 88.1 | 77.7 | 71.9 | |
| Lpcf.SL | Min | 10.6 | 12.1 | 16.7 | 15.9 | 14.6 | 15.9 | 15.6 | 15.2 | 12.7 | 15.2 | 16.1 |
| Mean | 19.1 ± 1.1 | 19.4 ± 3.0 | 18.5 ± 1.8 | 18.7 ± 1.9 | 19.3 ± 1.9 | 19.3 ± 1.8 | 19.3 ± 2.3 | 18.1 ± 1.8 | 18.8 ± 2.6 | 18.2 ± 1.9 | 20.1 ± 1.9 | |
| Max | 21.1 | 22.4 | 20.0 | 22.8 | 21.3 | 22.1 | 22.51 | 21.1 | 21.3 | 21.5 | 24.4 | |
| Lpcf.HL | Min | 56.7 | 42.5 | 55.2 | 50.6 | 48.8 | 51.1 | 51.1 | 55.7 | 44.2 | 47.2 | 51.5 |
| Mean | 68.0 ± 4.7 | 65.0 ± 8.4 | 61.2 ± 6.5 | 61.9 ± 4.3 | 62.7 ± 5.6 | 62.3 ± 5.8 | 63.6 ± 3.0 | 64.5 ± 8.2 | 63.6 ± 3.6 | 56.7 ± 5.4 | 64.6 ± 4.2 | |
| Max | 78.6 | 71.4 | 67.3 | 70.2 | 67.9 | 69.4 | 71.7 | 70.7 | 75.8 | 65.4 | 82.1 | |
| Lpcf.Prad | Min | 24.4 | 18.2 | 24.5 | 23.1 | 20.9 | 23.6 | 23.1 | 23.8 | 12.9 | 22.7 | 22.0 |
| Mean | 27.6 ± 1.6 | 28.9 ± 8.7 | 27.5 ± 1.9 | 27.7 ± 3.2 | 28.6 ± 3.2 | 28.3 ± 2.9 | 29.1 ± 3.5 | 27.7 ± 2.7 | 27.6 ± 4.1 | 28.1 ± 3.2 | 28.4 ± 2.8 | |
| Max | 32.1 | 33.7 | 30.0 | 33.7 | 32.3 | 33.9 | 33.4 | 31.52 | 19.0 | 33.1 | 34.6 | |
| Lplf.SL | Min | 9.2 | 9.1 | 7.9 | 8.0 | 7.8 | 4.4 | 6.8 | 8.1 | 9.0 | 8.0 | 7.0 |
| Mean | 10.7 ± 1.1 | 10.3 ± 0.9 | 9.3 ± 1.3 | 9.9 ± 1.3 | 9.0 ± 1.1 | 9.0 ± 1.8 | 9.3 ± 1.5 | 9.9 ± 1.2 | 10.6 ± 5.2 | 10.3 ± 1.5 | 11.7 ± 1.9 | |
| Max | 15.3 | 11.9 | 11.9 | 12.8 | 10.9 | 11.0 | 11.2 | 12.4 | 13.1 | 12.5 | 14.9 | |
| Lplf.HL | Min | 32.5 | 31.1 | 24.2 | 25.8 | 26.1 | 13.1 | 22.9 | 27.9 | 30.4 | 26.4 | 23.0 |
| Mean | 38.1 ± 4.8 | 34.5 ± 2.6 | 30.7 ± 4.5 | 32.6 ± 3.5 | 29.2 ± 3.4 | 29.1 ± 6.2 | 30.8 ± 5.2 | 35.43 ± 4.6 | 36.1 ± 5.1 | 32.0 ± 4.4 | 37.9 ± 3.5 | |
| Max | 58.9 | 37.5 | 38.3 | 39.5 | 35.9 | 35.2 | 38.4 | 42.7 | 47.4 | 38.3 | 50.0 | |
| Lplf.Prad | Min | 13.2 | 13.5 | 11.2 | 11.9 | 11.7 | 6.2 | 10.1 | 11.8 | 13.5 | 12.1 | 9.6 |
| Mean | 15.5 ± 2.1 | 15.3 ± 1.3 | 13.8 ± 6.2 | 14.6 ± 1.9 | 13.3 ± 1.5 | 13.2 ± 2.7 | 14.1 ± 2.5 | 15.2 ± 2.0 | 15.6 ± 1.9 | 15.8 ± 2.9 | 16.7 ± 2.7 | |
| Max | 24.1 | 18.0 | 17.6 | 19.0 | 15.8 | 15.3 | 17.8 | 18.4 | 19.8 | 20.4 | 21.2 | |
| Lplf.Lpcf | Min | 48.0 | 46.6 | 41.3 | 43.3 | 38.5 | 23.2 | 41.3 | 41.7 | 46.2 | 45.9 | 43.4 |
| Mean | 56.1 ± 6.0 | 54.0 ± 7.8 | 50.2 ± 7.1 | 52.9 ± 5.2 | 46.8 ± 6.2 | 46.8 ± 10.3 | 48.3 ± 5.3 | 55.1 ± 7.7 | 57.6 ± 9.9 | 56.4 ± 6.8 | 58.6 ± 7.6 | |
| Max | 77.8 | 47.4 | 59.7 | 61.0 | 56.1 | 63.8 | 56.2 | 65.9 | 77.4 | 70.5 | 72.7 | |
| Ddf.SL | Min | 14.4 | 14.5 | 14.2 | 15.5 | 15.5 | 15.0 | 15.1 | 13.8 | 16.1 | 21.6 | 12.9 |
| Mean | 15.5 ± 1.9 | 17.1 ± 2.1 | 16.6 ± 5.3 | 17.3 ± 1.4 | 17.5 ± 1.7 | 19.0 ± 2.5 | 18.5 ± 2.9 | 15.9 ± 4.5 | 20.8 ± 4.3 | 16.6 ± 2.7 | 19.1 ± 3.2 | |
| Max | 22.9 | 20.4 | 19.1 | 20.5 | 20.4 | 22.1 | 24.6 | 13.4 | 28.7 | 21.1 | 30.1 | |
| (B) Variation of the meristic characters of the fishes | ||||||||||||
| GR | Min | 14 | 14 | 14 | 14 | 14 | 15 | 15 | 15 | 15 | 14 | 14 |
| Mean | 15.9 ± 1.1 | 14.7 ± 0.7 | 14.4 ± 0.5 | 15.0 ± 0.7 | 14.5 ± 0.8 | 15.6 ± 0.5 | 15.7 ± 0.5 | 15.0 ± 0.0 | 15.7 ± 0.5 | 14.7 ± 0.5 | 16.0 ± 1.1 | |
| Max | 18 | 16 | 10 | 16 | 16 | 16 | 16 | 15 | 16 | 15 | 18 | |
| LL | Min | 26 | 27 | 26 | 27 | 27 | 26 | 27 | 26 | 25 | 28 | 26 |
| Mean | 29.1 ± 2.2 | 28.4 ± 0.6 | 28.3 ± 0.9 | 27.7 ± 0.4 | 28.0 ± 0.4 | 28.5 ± 0.9 | 27.9 ± 0.3 | 27.2 ± 1.2 | 25.7 ± 1.3 | 28.3 ± 0.5 | 27.4 ± 1.2 | |
| Max | 34 | 30 | 29 | 28 | 29 | 29 | 29 | 30 | 29 | 29 | 29 | |
| CPS | Min | 9 | 9 | 10 | 10 | 11 | 10 | 10 | 10 | 9 | 10 | 9 |
| Mean | 11.3 ± 1.4 | 10.9 ± 1.1 | 11.0 ± 0.4 | 10.6 ± 0.3 | 11.6 ± 0.5 | 11.0 ± 0.4 | 10.9 ± 0.5 | 10.9 ± 0.9 | 10.0 ± 0.7 | 11.3 ± 0.5 | 10.1 ± 0.7 | |
| Max | 14 | 13 | 12 | 12 | 12 | 12 | 12 | 12 | 11 | 12 | 12 | |
| PCFR | Min | 14 | 14 | 13 | 13 | 15 | 15 | 15 | 14 | 13 | 13 | 14 |
| Mean | 15.9 ± 0.9 | 14.8 ± 0.4 | 13.9 ± 1.0 | 14.2 ± 0.9 | 15.8 ± 0.6 | 15.3 ± 0.5 | 15.8 ± 0.5 | 14.7 ± 0.3 | 14.1 ± 0.7 | 14.0 ± 0.7 | 14.9 ± 0.7 | |
| Max | 17 | 15 | 16 | 15 | 17 | 16 | 17 | 16 | 15 | 15 | 16 | |
| PLFR | Min | 7 | 7 | 6 | 6 | 6 | 6 | 7 | 6 | 7 | 6 | 6 |
| Mean | 8.1 ± 0.5 | 7.0 ± 0.0 | 6.8 ± 0.6 | 6.2 ± 0.4 | 6.6 ± 0.5 | 6.7 ± 0.5 | 7.2 ± 0.4 | 6.7 ± 0.7 | 7.1 ± 0.3 | 6.3 ± 0.4 | 6.6 ± 0.5 | |
| Max | 9 | 7 | 6 | 7 | 7 | 7 | 8 | 8 | 8 | 7 | 7 | |
| DFR | Min | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 7 | 8 |
| Mean | 9.3 ± 0.5 | 8.6 ± 0.9 | 8.4 ± 0.9 | 8.6 ± 0.5 | 8.4 ± 0.5 | 8.7 ± 0.5 | 8.0 ± 0.0 | 8.3 ± 0.5 | 8.2 ± 0.4 | 7.9 ± 0.3 | 9.4 ± 0.6 | |
| Max | 10 | 10 | 10 | 9 | 9 | 9 | 8 | 9 | 9 | 8 | 11 | |
| AFR | Min | 8 | 8 | 8 | 9 | 9 | 9 | 8 | 8 | 8 | 9 | 9 |
| Mean | 9.8 ± 0.6 | 9.1 ± 0.5 | 8.9 ± 0.3 | 9.0 ± 0.0 | 9.0 ± 0.0 | 9.1 ± 0.3 | 8.9 ± 0.3 | 9.0 ± 0.9 | 8.5 ± 0.5 | 9.1 ± 0.3 | 9.6 ± 0.4 | |
| Max | 11 | 10 | 9 | 9 | 9 | 10 | 9 | 10 | 9 | 10 | 10 | |
| (C) Variation of the morphometric characters of the otoliths | ||||||||||||
| Excisura angle | Min | 100 | 92 | 109 | 105 | 112 | 93 | 91 | 86 | 84 | 67 | 93 |
| Mean | 117.0 ± 10.6 | 108.0 ± 8.2 | 119.0 ± 12.1 | 121.0 ± 17.1 | 120.0 ± 6.7 | 114.0 ± 19.1 | 99.0 ± 10.8 | 115.0 ± 14.4 | 101.0 ± 12.5 | 83.2 ± 9.6 | 120.0 ± 14.3 | |
| Max | 140 | 136 | 134 | 144 | 132 | 144 | 115 | 128 | 113 | 101 | 142 | |
| Posterior angle | Min | 63 | 80 | 60 | 85 | 76 | 60 | 69 | 71 | 98 | 68 | 64 |
| Mean | 85.7 ± 9.7 | 86.2 ± 6.6 | 78.0 ± 12.4 | 92.0 ± 7.7 | 88.0 ± 11.7 | 83.0 ± 13.1 | 81.0 ± 10.8 | 92.0 ± 13.8 | 102.0 ± 5.4 | 84.0 ± 10.1 | 92.0 ± 13.2 | |
| Max | 100 | 95 | 89 | 102 | 106 | 95 | 92 | 107 | 110 | 157 | 110 | |
| Posteroventral angle | Min | 128 | 140 | 130 | 126 | 137 | 133 | 130 | 130 | 127 | 124 | 125 |
| Mean | 144.0 ± 8.5 | 144.0 ± 3.3 | 144.0 ± 12.8 | 139.0 ± 8.9 | 144.0 ± 5.1 | 140.0 ± 6.3 | 141.0 ± 12.5 | 138.0 ± 6.1 | 141.0 ± 10.1 | 143.0 ± 9.1 | 140.0 ± 8.1 | |
| Max | 154 | 148 | 161 | 145 | 152 | 150 | 159 | 147 | 150 | 157 | 152 | |
| L/H index | Min | 1.2 | 0.99 | 0.99 | 0.99 | 1.0 | 0.99 | 0.99 | 1.1 | 0.99 | 1.2 | 1.0 |
| Mean | 1.3 ± 0.08 | 1.2 ± 0.07 | 1.2 ± 0.08 | 1.1 ± 0.06 | 1.1 ± 0.06 | 1.1 ± 0.06 | 1.1 ± 0.20 | 1.2 ± 0.06 | 1.0 ± 0.07 | 1.3 ± 0.07 | 1.1 ± 0.07 | |
| Max | 1.4 | 1.3 | 1.3 | 1.2 | 1.2 | 1.2 | 1.6 | 1.3 | 1.1 | 1.4 | 1.3 | |
| Rel. medial length | Min | 69.6 | 76.1 | 72.6 | 76.6 | 77.6 | 79.6 | 76.3 | 77.5 | 87.0 | 71.3 | 70.5 |
| Mean | 79.2 ± 4.2 | 80.1 ± 3.1 | 80.6 ± 3.1 | 81.2 ± 3.7 | 82.1 ± 3.5 | 84.2 ± 2.6 | 79.8 ± 1.2 | 79.3 ± 1.2 | 82.0 ± 3.5 | 72.0 ± 2.4 | 82.5 ± 2.3 | |
| Max | 89.4 | 84.0 | 87.0 | 85.6 | 87.7 | 95.1 | 86.7 | 80.7 | 90.0 | 79.7 | 88.7 | |
| Rel. rostrum length | Min | 11 | 17.1 | 12.3 | 13.5 | 12.6 | 5.8 | 11.2 | 12.4 | 14.2 | 22.8 | 7.0 |
| Mean | 17.1 ± 6.2 | 20.5 ± 2.3 | 18.1 ± 4.1 | 16.5 ± 3.4 | 16.8 ± 2.9 | 16.2 ± 2.4 | 18.3 ± 1.5 | 17.2 ± 2.5 | 16.6 ± 2.8 | 26.8 ± 2.7 | 17.7 ± 3.1 | |
| Max | 26.4 | 22.8 | 21.5 | 20.9 | 20.4 | 20.6 | 22.5 | 20.9 | 20.2 | 32.0 | 25.0 | |
| Rel. rostrum height | Min | 40.4 | 46.9 | 45.6 | 46.0 | 40.3 | 33.7 | 43.1 | 39.7 | 41.3 | 44.6 | 39.7 |
| Mean | 50.8 ± 3.1 | 49.3 ± 2.3 | 49.4 ± 3.1 | 46.8 ± 1.1 | 42.3 ± 1.8 | 47.5 ± 6.0 | 49.3 ± 1.3 | 43.8 ± 2.6 | 43.4 ± 2.1 | 51.1 ± 3.7 | 49.2 ± 4.8 | |
| Max | 58.7 | 52.2 | 53.2 | 48.3 | 46.4 | 53.2 | 52.4 | 46.9 | 46.1 | 55.3 | 55.0 | |
| Rel. antirostrum length | Min | 3.4 | 4.8 | 4.4 | 1.9 | 4.8 | 6.0 | 7.5 | 3.1 | 8.6 | 6.0 | 3.7 |
| Mean | 8.3 ± 3.1 | 10.4 ± 4.6 | 7.0 ± 2.9 | 6.8 ± 4.9 | 6.7 ± 1.5 | 10.1 ± 7.8 | 9.9 ± 2.1 | 7.6 ± 3.6 | 10.8 ± 2.3 | 16.5 ± 3.7 | 8.0 ± 3.2 | |
| Max | 13.4 | 15.2 | 11.0 | 11.6 | 9.3 | 14.1 | 12.4 | 14.7 | 17.3 | 21.2 | 14.3 | |
| Rel. antirostrum height | Min | 23.4 | 24.2 | 28.6 | 17.8 | 22.5 | 24.2 | 23.9 | 17.1 | 27.0 | 28.8 | 24.0 |
| Mean | 29.3 ± 2.7 | 32.5 ± 3.1 | 31.3 ± 6.9 | 23.1 ± 6.1 | 28.2 ± 3.8 | 29.2 ± 2.5 | 28.3 ± 6.2 | 28.0 ± 3.1 | 31.3 ± 2.5 | 36.7 ± 2.3 | 28.5 ± 3.7 | |
| Max | 38.9 | 39.0 | 34.0 | 28.9 | 35.8 | 35.3 | 31.4 | 39.1 | 39.2 | 44.6 | 34.7 | |
| Rel.dorsal length | Min | 62.0 | 79.0 | 71.0 | 77.0 | 74.0 | 83.0 | 79.0 | 75.0 | 87.0 | 77.0 | 75.0 |
| Mean | 73.6 ± 3.5 | 82.3 ± 3.7 | 79.6 ± 3.0 | 79.7 ± 2.3 | 80.2 ± 5.5 | 88.9 ± 3.7 | 85.0 ± 2.8 | 79.7 ± 3.2 | 87.8 ± 4.2 | 89.3 ± 5.1 | 83.3 ± 4.3 | |
| Max | 89.0 | 88.0 | 87.0 | 82.0 | 88.0 | 97.0 | 96.0 | 88.0 | 90.0 | 89.0 | 91.0 | |
- Abbreviations of morphometric characters according to Table 2. See also abbreviation of meristic characters in text. I = Makran Basin, II = Hormuzgan Basin, III = Helleh Basin. RU, HL, KH etc represent population codes (see Table 1), populations from hot springs are indicated by *. N = number of individuals.
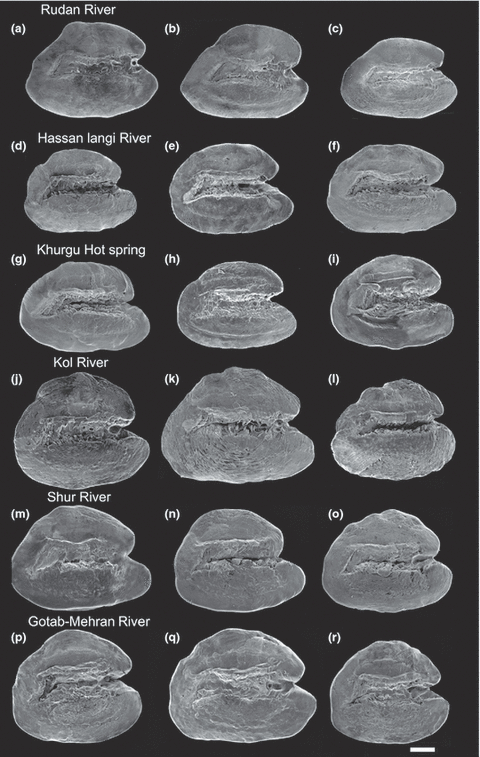
SEM micrographs of left otoliths (in medial view) of Aphanius dispar from populations in the Makran Basin (a–c) and Hormuzgan Basin (d–r). The otoliths include males (a, d, e, f, g, l, n and p) and females (b, c, h, i, j, k, m, o, q and r). Scale bar = 200 μm
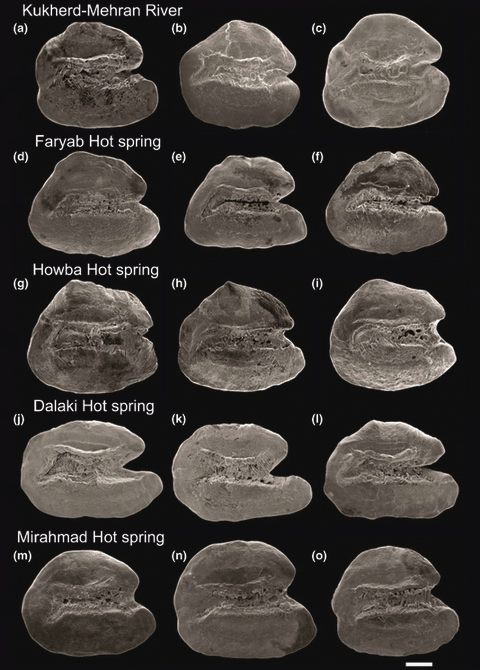
SEM micrographs of left otoliths (in medial view) of Aphanius dispar from populations in the Hormuzgan Basin (a–i) and Helleh Basin (j–o). The otoliths include males (c, d, g, h, l and m) and females (a, b, e, f, i, j, k, n and o). Scale bar = 200 μm
Makran Basin
The population from the Makran Basin is a river population (RU). The overall variability of the RU population is relatively low (Table 3). Five morphometric characters appear variable (Prdd.HL, Lcaup.Prad, HL.Lpcf, Ldf.Lpcf and Lplf.Lpcf). The two variable meristic characters are the number of lateral line series scales and the number of caudal peduncle scales. The otoliths are trapezoid to narrow triangular in shape; the rostrum is as long as or longer than the antirostrum and has a rounded tip (Fig. 3a–c). The two variable otolith characters concern the relative medial length and relative rostrum length (Table 3).
Hormuzgan Basin
The eight samples from this basin include five populations from river habitats and three from hot springs (Table 1). Considering the morphometric characters, within-population variability is visible in 21 (of 27) characters (Table 3). Characters with high variability include those that are related to the predorsal distance (Prdd), preanal distance (Prad), length of caudal peduncle (Lcaup), head length (HL), length of dorsal fin (Ldf), length of the pectoral fin (Lpcf) and the length of pelvic fin (Lplf) (Table 3). Two hot spring populations (KH and FA) showed the highest variability of morphometric characters, with nine characters being variable in each population (Table 3). Notably, the within-population variation in KH and FA is not affecting equal phenotypic characters, but the characters referring to the predorsal distance (Prdd), head length (HL), length of dorsal (Ldf) and pelvic (Lplf) fins are variable in both populations (Table 3). The lowest variability of morphometric characters was observed in the population from the river habitat KU.
With regard to the meristic characters, the eight populations show a relatively uniform pattern. However, distinctive variation was observed with regard to the lateral line series scales (variable in four populations), and number of gill rakers (variable in three populations) (Table 3). The two hot spring populations (FA, KH), which have already shown high variation of morphometric characters, but also the river population HL display a slightly higher variability of the meristic characters than the other populations, with four and three (in FA and KH) as well as three (in HL) meristic characters being variable (Table 3). As observed with regard to the morphometric characters, the within-population variation found in FA, KH and HL does not affect equal phenotypic characters, only the number of dorsal fin rays, the number of caudal peduncle scales and the lateral line series scales are variable in two of the three populations (Table 3). As observed for the morphometric characters, low variability of meristic characters was found in the population from the river habitat (KU) but also in another river (GO) and hot spring (HO) population.
The otoliths from the eight populations in the Hormuzgan Basin are rounded-trapezoid or rounded-triangular in shape, and the rostrum is generally longer than the antirostrum and displays a rounded, pointed or blunt tip (3, 4). The otolith characters show within-population variation in five of ten otolith variables. The relative antirostrum length is variable in four populations, whereas the relative rostrum length and height, the relative antirostrum height, and the relative dorsal length show variation in no more than two populations (Table 3). The river population GO and the hot spring populations KH and FA contain two variable otolith characters and thus have a slightly higher otolith variability than the remaining river (HL, KO, SH, KU) and hot spring (HO) populations.
In all, the five populations from river habitats have a low variation in their morphometric characters and, with the exception of the HL population, also a low variation in their meristic characters. Moreover, the otolith characters of the river populations also show a rather consistent morphology, a slightly increased variability is present in the GO population. The three populations from the hot spring habitats may have a distinct (FA and KH) or low (HO) variation regarding both the morphometric and meristic characters; their otolith variation is consistent with this pattern, that is, slightly increased in the KH and FA, but low in the HO population.
Helleh Basin
The two populations from this basin are from hot springs (DA and MI). The DA and MI specimens show within-population variation with regard to the predorsal distance (Prdd.HL), caudal peduncle (Lcaup.Prad), head length (HL.Lpcf) and the length of dorsal (Ldf.HL, Ldf.Lpcf) and pelvic (Lplf.Lpcf) fins (Table 3). In addition, the postdorsal distance (Podd.Prad) and head length (HL.SL) vary only in the DA specimens, while the total length (TL.SL) and preorbital distance (Prod.HL, Prod.Lpcf) display variation only in the MI specimens (Table 3).
The variability of the meristic characters is low in both populations and concerns the number of gill rakers and lateral line series scales (MI) and the number of pectoral fin rays (DA) (Table 3).
The otolith morphology of the DA and MI specimens is clearly different. The DA otoliths are elongate-rectangular in shape and possess a deeply incised V-shaped excisura, and a well-developed rostrum (Fig. 4j–l). The MI otoliths have a rounded-triangular shape and a moderately incised U-shaped excisura (Fig. 4m–o). Both populations yield otoliths characterized by a well-developed rostrum that is considerably longer than the antirostum and rounded at the end. The otoliths show within-population variability with regard to the relative length of the dorsal part (DA and MI) and the relative height of the rostrum (MI) (Table 3).
In conclusion, the DA and MI specimens depict a distinct within-population variation of their morphometric characters and also some level of variation in the otolith variables.
Variation between river and hot spring populations
To evaluate the influence of habitat differences on morphological differentiation, we used the populations from the Hormuzgan Basin, which have been collected from rivers (five populations) and hot springs (three populations) (Table 1). The statistical analyses do not indicate significant differences of the studied characters between the fish specimens from the two habitat systems (T-test, p < 0.05), which is also supported by the CDA. The CDA analysis for all eight populations indicates a strong overlap and low classification success (51.8%, Wilks lambda λ = 0.570).
Between-basin variation (analyses based on all populations)
Because of the sex dimorphism that occurs within the Aphanius dispar populations, the comparison of specimens between the three drainage basins I, II and III was conducted for males and females separately. anova with Duncan post hoc test (p < 0.05) was used for the comparisons based on univariate analyses, and the CDA was employed for the multivariate analyses.
Morphometric and meristic characters of the fish body
The univariate analyses show that three (of 27) morphometric characters (Prdd.HL, Prod.SL, HL.SL) differ significantly between both the males and females from the three basins. Moreover, HL.Prad is different between the males (p < 0.05). Considering the meristics, the pelvic and anal fin ray numbers are only different between the males of the three basins, while the pelvic fin rays numbers and gill rakers are only different between the females of the three basins (p < 0.05). The CDA is based on all morphometric (27) and meristic characters (seven). The results indicate a clear separation between the A. dispar populations from the three basins with a high classification success both for males (93.4%) and females (99.5%) (Table 4, Fig. 5).
| Basin | Predicted group membership | Total | ||
|---|---|---|---|---|
| I | II | III | ||
| (A) Morphometric and meristic characters | ||||
| Males | ||||
| I | 100.0 (22) | 0 | 0 | 22 |
| II | 2.55 (1) | 94.9 (38) | 2.55 (1) | 40 |
| III | 0 | 10.7(3) | 89.3 (17) | 20 |
| Females | ||||
| I | 100.0 (20) | 0 | 0 | 20 |
| II | 0 | 97.5 (39) | 2.5 (1) | 40 |
| III | 0 | 0 | 100.0 (21) | 21 |
| (B) Otolith characters | ||||
| Males | ||||
| I | 100.0 (13) | 0 | 0 | 13 |
| II | 0 | 82.4 (14) | 3 | 17 |
| III | 0 | 13.3 (2) | 86.7 (13) | 15 |
| Females | ||||
| I | 100.0 (13) | 0 | 0 | 13 |
| II | 0 | 93.3 (14) | 6.7 (1) | 15 |
| III | 0 | 7.7 (1) | 92.3 (12) | 13 |
- The percentages in rows represent the classification into the populations of each group given in columns (correct classifications are bold-typed). Corresponding number of individuals are given in brackets. (Wilks’λ = 0.09 in males and λ = 0.05 in females for morphometric and meristic characters analysis; Wilks’λ = 0.12 in males and λ = 0.08 in females for otolith characters analysis). I = Makran Basin, II = Hormuzgan Basin and III = Helleh Basin.
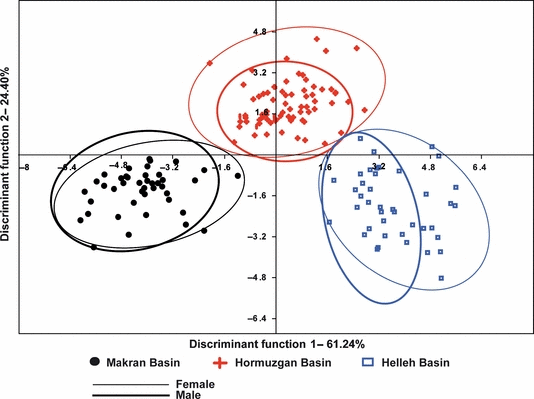
Discriminant function scores for the Aphanius dispar populations from the three studied basins based on morphometric and meristic characters for males and females
The mean discriminant coefficients were calculated to reveal the separation power of any given character. Accordingly, three morphometric characters (Prdd.SL, HL.SL, Lplf.SL) strongly contribute both in the separation of males and females; two of these characters relate to the same morphometric parameters as have been recognized by univariate analysis (i.e. Prdd and HL). Four morphometric characters (Prdd.Prad, Prad.SL, HL.Prad and Lplf.Prad) are important in the discrimination of the males, and four other morphometric characters (Prdd.HL, HL.Lpcf, Lplf.HL and Lplf.Lpcf) are important in the discrimination of the females (Table 5). The calculation of the mean discriminant coefficients of the meristic characters shows the same characters to be important in the separation between males and females as obtained through univariate analysis (see above and Table 5).
| (A) Morphometric characters | (B) Meristic characters | ||||
|---|---|---|---|---|---|
| Characters | ♂N = 82 | ♀N = 81 | Characters | ♂N = 82 | ♀N = 81 |
| TL.SL | 0.43 | 0.08 | Gill rakers | 0.42 | 0.98 |
| Prdd.SL | 3.07 | 2.25 | Lateral line series scales | 0.45 | 0.35 |
| Prdd.HL | — | 2.80 | Caudal peduncle scales | 0.03 | 0.05 |
| Prdd.Prad | 4.09 | — | Pectoral fin rays | 0.05 | 0.21 |
| Podd.SL | 0.22 | 0.45 | Pelvic fin rays | 0.56 | 0.60 |
| Podd.Prad | 0.98 | 0.35 | Dorsal fin rays | 0.30 | 0.25 |
| Prad.SL | 7.54 | 0.27 | Anal fin rays | 0.50 | 0.23 |
| Prod.SL | 0.37 | 0.41 | (C) Otolith characters | ||
| Prod.HL | 0.02 | 0.41 | Characters | N = 45 | N = 41 |
| Prod.Lpcf | — | 1.23 | Excisura angle | 0.62 | 1.02 |
| Lcaup.SL | 0.17 | 0.47 | Posterior angle | 0.42 | 0.24 |
| Lcaup.Prad | — | 0.56 | Posteroventral angle | 0.17 | 0.07 |
| Maxb.SL | 0.73 | 0.78 | Length-height | 0.84 | 1.00 |
| HL.SL | 8.08 | 4.36 | Relative length of medial part | 2.04 | 1.34 |
| HL.Prad | 6.78 | 0.42 | Relative length of rostrum | 1.00 | 1.58 |
| HL.Lpcf | — | 3.40 | Relative height of rostrum | 0.36 | 0.09 |
| Ldf.SL | 0.08 | 0.74 | Relative length of antirostrum | 2.13 | 0.30 |
| Ldf.HL | 0.17 | 0.05 | Relative height of antirostrum | 0.76 | 0.15 |
| Ldf.Lpcf | — | 0.80 | Relative length of dorsal part | 1.37 | 0.54 |
| Lpcf.SL | 1.04 | 1.42 | |||
| Lpcf.HL | 0.37 | 0.48 | |||
| Lpcf.Prad | 1.20 | — | |||
| Lplf.SL | 2.30 | 3.38 | |||
| Lplf.HL | — | 6.80 | |||
| Lplf.Prad | 3.11 | — | |||
| Lplf.Lpcf | — | 2.30 | |||
| Ddf.SL | 0.21 | 0.26 | |||
- Bold faced indicates the characters with high values, that is, which contributed most in the separation. Characters with no value in separation are shown by —. N = number of the males and females. See Table 2 for abbreviation of morphometric characters
Otolith morphology
The otoliths from the Makran Basin are trapezoid to triangular in shape and characterized by the anteriorly pronounced dorsal rim (Fig. 3a–c), while those from the Hormuzgan Basin are rounded-trapezoid or rounded-triangular (3, 4) and those from the Helleh Basin are elongate-rectangular (DA) or rounded-triangular in shape (MI) (Fig. 4j–o). The excisura is moderately incised in the Makran and Hormuzgan otoliths, but deeply incised in the Helleh otoliths; it is mostly V-shaped in specimens from Makran and Hormuzgan Basins and V- or U-shaped in the Helleh specimens. The rostrum of the Makran otoliths is shorter than that seen in the otoliths from Hormuzgan, and the rostrum of the latter is shorter than in the Helleh specimens (see 3, 4). The antirostrum of the Makran otoliths is mostly blunt, while it is rounded in the Hormuzgan and Helleh otoliths. In addition, the otoliths show differences with regard to their margins. The posterior rim of the otoliths from the Makran Basin is sloping, the ventral rim is straight or slightly curved and the dorsal rim is slightly curved. The posterior rim of the otoliths from Hormuzgan is rounded (in some specimens also sloping), the ventral rim is generally straight or very slightly curved and the dorsal rim is completely curved (3, 4); a few specimens possess a small dorsal tip in the posterior part of the dorsal rim (Fig. 4a–c,g–i). In the otoliths from the Helleh Basin, the posterior rim is rounded, the ventral rim straight and the dorsal rim only slightly curved (Fig. 4j–o).
Otolith morphometry
The univariate analysis revealed that four (of 10) otolith variables differ significantly between both males and females of Aphanius dispar from the three drainage basins (i.e., length/height, relative medial length, relative rostrum length and relative dorsal length, p < 0.05). The CDA was conducted based on all ten otolith variables and reveals a high classification success for both males (89.3%) and females (95.4%) (Table 4). Also, the scatter plots of the discriminant function scores support well the clear separation of A. dispar from the three drainage basins based on the otolith variables (plots not shown).
The calculation of the mean discriminant coefficients of the otolith variables indicates that the relative medial length and relative rostrum length are important otolith characters for the separation of males and females from the three basins (Table 5); both characters have also been indicated by univariate comparison. In addition, calculation of the mean discriminant coefficients supports the significance of the relative antirostrum length and relative dorsal length as discriminants between males, while the excisura angle and the length/height index are important features in the separation of females (Table 5).
Phenotypic relations of Aphanius dispar between the basins
The average linkage dendrogram based on the Euclidean distance was calculated for all morphometric and meristic variables (Fig. 6a), as well as for all otolith variables (Fig. 6b). Both dendrograms categorize the Aphanius dispar populations in three groups. Group I contains the population of the Makran Basin, group II consists of the populations of the Hormuzgan Basin and group III includes the populations of the Helleh Basin. The only exception is the inconsistent position of the MI population (see Fig. 6a versus Fig. 6b), which is because of the distinct differences between the otoliths from the two available Helleh populations. Moreover, both dendrograms (Fig. 6a,b) indicate a separate clade for the HO population within group II, which is probably related to the geographically isolated position of this population (see Discussion).
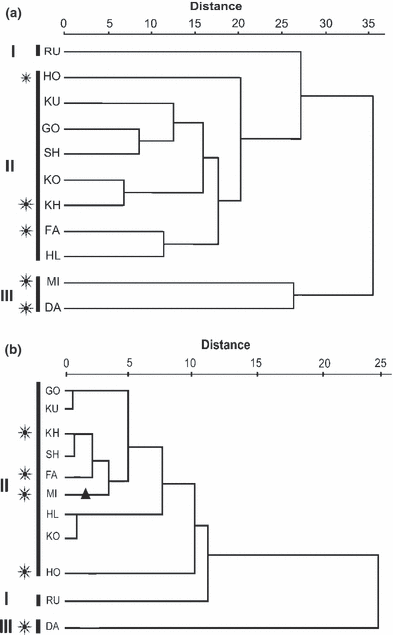
Average linkage dendrogram (based on Euclidean distance) showing the phenotypic relations between Aphanius dispar from the Makran Basin (I), Hormuzgan Basin (II) and Helleh Basin (III). Dendrograms are based on all morphometric and meristic characters (A) and on the otolith characters (B). Code for populations, see Table 1, hot spring habitats are indicated by (*). The MI population (indicated by) belongs to the Helleh Basin
Distinctness of Aphanius dispar versus Aphanius ginaonis
Here, we compare the newly obtained data on Aphanius dispar with the morphometric and meristic characters of six males and four females of Aphanius ginaonis (unpublished data, males and females were merged in the analyses). The morphometric characters related to the dorsal fin (Ddf.SL, Ldf.SL, Ldf.HL. Ldf.Lpcf), head length (HL.SL, HL.Prad) and predorsal distance (Prdd.Prad), as well as four meristic characters (number of pectoral, dorsal, anal fin rays; number of lateral line series scales) were significant for the separation of these A. ginaonis specimens from all studied A. dispar populations (anova, with Duncan’s post hoc test, p < 0.05). In addition, A. ginaonis was found to be most different from the Makran specimens of A. dispar, whereas lesser differences were present with regard to the Hormuzgan and Helleh specimens.
Discussion
Distinctness of Aphanius dispar phenotypes from southern Iran
All analyses have revealed a high phenotypic differentiation of Aphanius dispar in the three isolated drainage basins in southern Iran. However, because we lack morphometric and meristic data for A. dispar from areas outside Iran, a comparison of our data with other populations is only possible with regard to the otolith characters. The comparison of the studied otoliths with the otoliths of A. dispar populations from the United Arab Emirates (UAE, see Reichenbacher et al. 2009a) reveals that the otoliths considered in this study are different from the UAE specimens with regard to overall shape, rims and angles. In addition, otoliths from certain coastal populations (e.g. Khor Hulaylah location) from the United Arab Emirates possess a distinct dorsal tip, while in otoliths of the Iranian populations, this tip is mostly absent. CDA supports this difference when our data set is combined with that obtained for the UAE population from Khor Hulaylah (data from Reichenbacher et al. 2009a). The classification success for the groups was 100% for the Makran Basin, 72.4% for the Hormuzgan 75% for the Helleh Basin and 90.5 for the otoliths from the UAE (overall classification success was 76.7%).
Evaluation of phenotypic characters
Here, we discuss only those characters that have the same implication in both males and females (according to Table 5).
Between-basin variation analysis revealed that six phenotypic characters could be used to discriminate between Aphanius dispar populations in both sexes (Table 5). These six characters include the measurements related to the predorsal distance (Prdd.SL), head length (HL.SL), and pelvic fin length (Lplf.SL), the number of pelvic fin rays, and in otoliths, the relative medial length and relative rostrum length. All six characters have demonstrated low within-population variation, that is, the mentioned morphometric and meristic characters are variable in one of the 11 studied populations, the otolith character relative medial length is not variable in any one of the populations, and the otolith character relative rostrum length displays within-population variation in two populations (Table 3). As a result, the six characters have consistent appearances within an individual population and are significant within a group of populations, each of which perhaps representing a separate taxonomic unit.
Furthermore, certain phenotypic characters are insignificant for a taxonomic separation between Aphanius dispar regardless of their low within-population variation. These characters include four morphometric characters, that is, the postdorsal distance (Podd), preorbital distance (Prod), as well as the dimensions of the dorsal (Ldf, Ddf) and pectoral fins (Lpcf) (Tables 3 and 5). However, the characters related to the dimensions of the dorsal fin were found to be suitable for the separation between A. dispar and A. ginanonis. With regard to the otoliths, the posteroventral angle is not variable within populations and has no separation significance. Several other characters are also insignificant for a taxonomic separation, but show high within-population variation (i.e. variable in 3–5 populations). These characters include one morphometric character (length of caudal peduncle, Lcaup), which is variable in up to five populations, and one meristic character (number of the lateral line series scales), which is variable in six populations (Table 3). All these characters do not seem to be appropriate to separate A. dispar and, more generally, may be not appropriate to identify individual species among Aphanius (see also Hrbek et al. 2006 for the poor taxonomic significance of these characters).
Taxonomic evaluation of Aphanius dispar groups
The differences described above indicate that the three groups of Aphanius dispar may represent distinct taxonomic units such as subspecies or even species. This is strongly supported by the taxonomic meaning of the character HL.SL for the separation between A. ginaonis and A. dispar (this study) and for the discrimination of four closely related Aphanius species from central Iran (Hrbek et al. 2006). These authors recognize the HL.SL and also the head depth (related to SL) as the most important characters to separate between the four species (males and females) in the multivariate space, while they did not find any of the univariate morphometric or meristic characters to be different (otoliths were not studied). It can therefore be assumed that the dimensions of the head represent a significant taxonomic character to differentiate between closely related Aphanius species.
Moreover, also the otolith characters strongly support the hypothesis that each individual studied basin probably contains its distinct subspecies or even species. The relative rostrum length and the relative medial length (significantly different between the three A. dispar groups) is also significantly different in the otoliths of closely related Aphanius species (Reichenbacher et al. 2007, 2009b) and can therefore be considered as important taxonomic characters on the species level.
Possible causes of morphogeographical differentiation
Changes in morphology may result from certain environmental conditions (ecoplasticity), represent an expression of genetic differences and/or result from gene pool pauperization. In most cases, changes in morphology are the result of an interplay between environmental factors and genetic plasticity (Scheiner 1993; Smith 1993; Svanbäck and Eklöv 2006).
The results from our study raise the question as to whether the observed differentiation between A. dispar groups results from geographical isolation and allopatric divergence or from environmental or physiological adaptation. The results from the Hormuzgan Basin, however, clearly demonstrate that the differences in the phenotypic characters among the populations in the Hormuzgan Basin are not related to differences in habitat conditions. It can be concluded that this is also true of the phenotypic differences observed in A. dispar from the Makran and Helleh Basins.
Furthermore, the active geology of Iran, resulting from collisions of the Arabian with the Iranian plate during the late Miocene (5–10 million years), Pliocene (1.8–5 million years) and Pleistocene (1.8 million years –10.000 y.) (Dercourt et al. 1986; Allen et al. 2004) has led to rapid isolation of multiple areas owing to physical features such as migration barriers and changing hydrological networks (Sborschchikov et al. 1981; Dercourt et al. 1986; Hatzfeld et al. 2010). This provided excellent conditions for the speciation of freshwater fishes in southern and western Iran (Zagros mountains) (Hrbek et al. 2006), similar to the recorded speciation of reptiles (Rastegar-Pouyani and Nilson 2002). A similar scenario can be assumed for the study area, which is part of the Central Zagros Mountains (Fig. 7). In the following paragraphs, we provide a summary of the geological history of the study area and evaluate whether geological processes could have led to geographical isolation and thus could be used to explain the observed geographical differentiation of A. dispar.
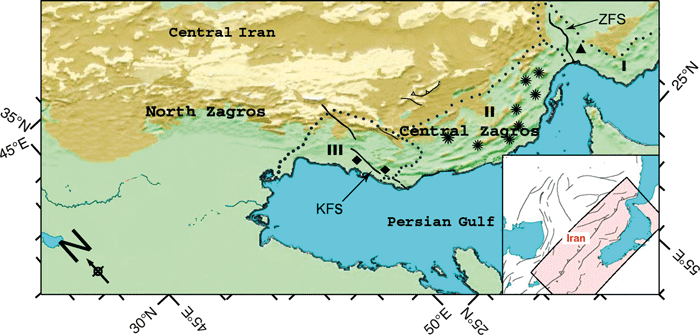
Map showing the main geographical and tectonic features of the Zagros (Iran) modified after Hatzfeld et al. (2010). KFS, Kazerun Fault System, which separates the North Zagros from the Central Zagros, and ZFS, Zendan Fault System, which separates the Makran Basin from the central Zagros. I = Makran Basin, II = Hormuzgan Basin and III = Helleh Basin
Makran Basin
The Makran Basin represents the coastal region of south-eastern Iran between the Straits of Hormuz and the Pakistani border (1, 7). It is separated from the easternmost part of the Central Zagros Mountains and Hormuzgan Basin, respectively, by the Zendan Fault system, which was active during the late Pliocene and lower Pleistocene; the last connection of the Makran Basin with the Hormuzgan Basin probably dates back to the Pleistocene (1.8 million years before present) (Regard et al. 2004). Therefore, the Aphanius dispar population in this basin (Rudan River) may have become isolated at least several hundred thousand years ago.
Hormuzgan Basin
This basin in the Zagros Coastal Plain or Central Zagros is delimited to the west by the North Zagros and to the east by the Makran Basin (Regard et al. 2004; Hatzfeld et al. 2010). The Hormuzgan Basin probably is separated from the other units of the Zagros Mountains since the late Pliocene and Pleistocene (Hatzfeld et al. 2010). The geologic events within the Hormuzgan Basin are responsible for the presence of many unusual and isolated habitats (e.g. hot springs). This habitat fragmentation probably increased the species diversity among fishes (see Esmaeili et al. 2010) and also among the populations of Aphanius dispar owing to genetic drift, founder effects and local adaptation to specific environmental conditions (see Carson 1971; Barton and Charlesworth 1984; Plath et al. 2010). An example for high phenotypic (and perhaps genetic) differentiation is the population from the hot spring Howba (HO), which has been shown in this study to occupy an isolated position (Fig. 6a,b). An example for another Aphanius species that probably evolved locally in the Hormuzgan Basin because of the above-mentioned factors is A. ginaonis Holly, 1929, which is represented by a single isolated population in the Genow hot spring within the A. dispar distribution region (Abdoli 2000; Hrbek and Meyer 2003; Teimori 2006; Reichenbacher et al. 2009b; Coad 2011; this study).
Helleh Basin
The Helleh Basin belongs to the North Zagros Mountains (1, 7) and represents a separate drainage area since the Pliocene (Hatzfeld et al. 2010). During the late Pliocene, the NW-SE extending Kazeroun Fault System (KFS in Fig. 7) subdivided the Helleh Basins into a western and eastern part (Kompani-Zare and Moore 2001; Hatzfeld et al. 2010). It is likely that this fault has caused the isolation of the Aphanius dispar populations east and west of it, that is, the isolation of the Dalaki (west of KFS) and Mirahmad hot spring populations (east of KFS) (Fig. 7). Accordingly, the distinct differences in otolith morphology between Dalaki and Mirahmad (Fig. 4j–o), as well as the similarity of the latter with the otoliths from the Hormuzgan Basin populations (Fig. 6b), could be easily explained by the geographical isolation and allopatric divergence of the DA and MI populations since at least 2 million years.
Summary
The results presented in this study suggest a morphological differentiation of Aphanius dispar in southern Iran into three phenotypes (groups). The differentiation is supported by all data sets, that is, morphometric characters, meristic data, otolith morphology and otolith characters. The characters related to the predorsal distance (Prdd.SL), head length (HL.SL), pelvic fin length (Lplf.SL), number of pelvic fin rays, and the otolith characters relative medial length and rostrum length play the most important role in the distinction. Major driving forces of the differentiation probably include geographical isolation and habitat fragmentation resulting from the geologic history and also local adaptation to unusual habitats (hot springs). The three groups of A. dispar recognized here probably represent separate subspecies or even species. However, the differentiation is slightly lesser pronounced than seen between A. dispar and A. ginaonis.
Acknowledgements
Financial support has been provided by the Iranian Ministry of Sciences, Research and Technology. The authors would like to thank the Editor, Stefan T. Hertwig and the potential reviewers, W. Schwarzhans and Gloria Arratia for their valuable comments and recommendation on this manuscript. We would like to thank A. Gholami, R. Khaefi and H. Dehdar (Shiraz) for assistance in the collection of fish material, R. Melzer for providing access to the SEM at the Bavarian State Collection of Zoology (ZSM, Munich) and M. Krings (Paleontology and Geobiology, Munich) for improving the English of our manuscript. In addition, we acknowledge Mr. Kamran for logistic support in the field.
Kurzfassung
Vom arabischen Cyprinodontiden Aphanius dispar (Rüppell, 1829) ist eine beachtliche morphologische Variabilität bekannt. Es ist jedoch weitgehend unbekannt, ob diese Variabilität auf ökologische Unterschiede oder geographische Isolation und allopatrische Divergenz zurück zu führen ist. In der vorliegenden Studie wurden 11 Populationen von A. dispar aus drei geographisch isolierten Becken im Südiran untersucht, nämlich aus dem Makran Becken (I, ein Flusssystem), dem Hormuzgan Becken (II, fünf Flüsse und drei heiße Quellen) und dem Helleh Becken (III, zwei heiße Quellen). Statistische Untersuchungen zeigen keinen signifikanten Unterschied zwischen den beiden Habitatsystemen (T-test, p < 0,05), was auch durch die CDA unterstützt wird. Morphometrische und meristische Merkmale der Fische zeigen jedoch ebenso wie die Otolithenmorphologie und –morphometrie, dass signifikante phänotypische Unterschiede zwischen den Populationen aus den drei Becken bestehen. Sechs phänotypische Merkmale, nämlich (1) prädorsale Distanz (Prdd.SL), (2) Kopflänge (HL.SL), (3) Bauchflossenlänge (Lplf.SL), (4) Anzahl der Flossenstrahlen der Bauchflossen so wie die relative Länge des (5) medialen Anteils der Otolithen und (6) des Rostrums der Otolithen grenzen die Populationen von A. dispar aus den drei Becken gut gegeneinander ab. Die oben genannten Merkmale weisen darüber hinaus ein konsistentes Muster der Variabilität auf, was die Annahme stützt, dass die phänotypische Variabilität zwischen den Populationen von A. dispar eher auf geographische Isolation bedingt durch die geologische Geschichte der Entwässerungssysteme als auf ökologische Unterschiede zurück zu führen ist. Die Ereignisse, die zur Isolation geführt haben, datieren möglicherweise bis in das Pleistozän zurück. Die phänotypischen Unterschiede könnten darauf hindeuten, dass die Populationen von A. dispar aus den drei untersuchten Becken separate Unterarten oder sogar Arten darstellen.




