Phylogenetic relationships of the paraphyletic ‘caprimulgiform’ birds (nightjars and allies)
Verwandtschaftsbeziehungen der paraphyletischen “Caprimulgiformes” (Schwalmvögel und Verwandte)
Abstract
enIn the past years, paraphyly of the traditional ‘Caprimulgiformes’ (nightjars and allies) with respect to Apodiformes (swifts and hummingbirds) has been well established, but the relationships between the five ‘caprimulgiform’ family-level taxa remain controversial. These crepuscular or nocturnal birds differ in numerous anatomical features, and here an analysis of 69 morphological characters is performed. Except for the position of the Nyctibiidae (potoos), the topology of the single most-parsimonious tree agrees with the results of a recently published large-scale ‘phylogenomic’ study. Whereas molecular data support a clade including Nyctibiidae and the Steatornithidae (oilbird), potoos were shown to be the sister taxon of Caprimulgidae (nightjars) in the present analysis. A sister group relationship between Nyctibiidae and Caprimulgidae is strongly supported, both in terms of bootstrap robustness and the number of synapomorphies, and it is detailed that the morphological data are more likely to reflect the true relationships of these birds. A classification is proposed, and the term Strisores is introduced for a clade including all ‘Caprimulgiformes’ and Apodiformes. It is most parsimonious to assume a single origin of dark activity in the stem lineage of Strisores and a reversal to diurnal activity in Apodiformes. However, a fourfold origin of dark-activity in the stem lineages of Steatornithidae, Podargidae, Aegothelidae and the Caprimulgidae/Nyctibiidae cannot be conclusively excluded with the data at hand.
Zusammenfassung
deWährend Paraphylie der traditionellen ‘Caprimulgiformes” (Ziegenmelker und Verwandte) in Bezug auf die Apodiformes (Segler und Kolibris) in den vergangenen Jahren überzeugend begründet wurde, bleiben die Verwandtschaftsbeziehungen zwischen den fünf ‘caprimulgiformes’ Taxa umstritten. Diese dämmerungs- oder nachtaktiven Vögel unterscheiden sich in zahlreichen anatomischen Eigenschaften, und hier wird eine Analyse durchgeführt, welche 69 morphologische Merkmale umfasst. Mit Ausnahme der Stellung der Nyctibiidae (Tagschläfer) stimmt die Topologie des sparsamsten Stammbaumes mit den Resultaten einer vor kurzem erschienenen umfangreichen ‘phylogenomischen’ Studie überein. Während molekulare Daten ein Monophylum stützen, welches Nyctibiidae und Steatornithidae (Fettschwalme) beinhaltet, resultierten Tagschläfer in der vorliegenden Analyse als Schwestertaxon der Caprimulgidae (Nachtschwalben). Das Schwestergruppenverhältnis zwischen Nyctibiidae und Caprimulgidae wird durch eine hohe Boostrap-Robustheit und eine große Zahl von Synapomorphien gestützt, und es wird dargelegt, dass die morphologischen Daten die tatsächlichen Verwandtschaftsverhältnisse zwischen diesen Vögel besser wiederspiegeln. Eine Klassifikation wird vorgeschlagen und der Ausdruck Strisores für ein Monophylum eingeführt, welches alle ‘Caprimulgiformes” und Apodiformes beinhaltet. Es ist am sparsamsten, einen einzelnen Ursprung der Nachtaktivität in der Ahnenlinie der Strisores und eine Rückkehr zur Tagaktivität in der Ahnenlinie der Apodiformes anzunehmen. Ein vierfacher Ursprung von Nachtaktivität in den Ahnenlinien der Steatornithidae, Podargidae, Aegothelidae und Caprimulgidae/Nyctibiidae kann allerdings anhand der vorliegende Daten nicht ausgeschlossen werden.
Introduction
Phylogenetic analyses of both morphological and molecular data have congruently shown that the traditional avian taxon ‘Caprimulgiformes’ (nightjars and allies) is not monophyletic. A sister group relationship between the ‘caprimulgiform’ Aegothelidae (owlet-nightjars) and Apodiformes (swifts and hummingbirds) is well-established by multiple lines of independent evidence. This hypothesis was first proposed from an analysis of 25 morphological characters (Mayr 2002), and is supported by all subsequent molecular studies in which these taxa were included (Mayr et al. 2003; Cracraft et al. 2004; Barrowclough et al. 2006; Ericson et al. 2006; Hackett et al. 2008; Pratt et al. 2009).
In addition to the Aegothelidae, the ‘Caprimulgiformes’ include four other taxa of crepuscular or nocturnal birds, i.e. the Steatornithidae (oilbird), Podargidae (frogmouths), Caprimulgidae (nightjars) and Nyctibiidae (potoos). Mayr (2002) established a clade including Caprimulgidae, Nyctibiidae, Aegothelidae and apodiform birds, and considered the relationships of Podargidae and Steatornithidae uncertain. Subsequent analyses of morphological data supported a sister group relationship between Steatornithidae and the clade including Caprimulgidae, Nyctibiidae, Aegothelidae and apodiform birds, but did not resolve the position of the Podargidae (Mayr 2005a,b). All ‘caprimulgiform’ and apodiform birds share an elongated crus longum of the os carpi ulnare (Fig. 1), but this feature is also present in some other avian taxa (e.g. trogons, Trogonidae), and no unambiguous morphological apomorphies of a clade including these birds have as yet been identified. Although a clade including all ‘caprimulgiform’ and apodiform taxa was obtained in an analysis of 2954 morphological characters (Livezey and Zusi 2007), none of the three putative apomorphies (Livezey and Zusi 2007: tab. 2) is present in all of its representatives.1
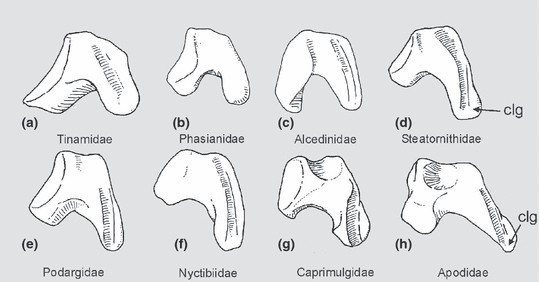
Os carpi ulnare in comparison, to illustrate the unusually long crus longum of ‘caprimulgiform’ and apodiform birds. (a) Thicket Tinamou, Crypturellus cinnamomeus (Tinamidae). (b) Golden Pheasant, Chrysolophus pictus (Phasianidae). (c) Collared Kingfisher, Todiramphus chloris (Alcedinidae). (d) Oilbird, Steatornis caripensis (Steatornithidae). (e) Tawny Frogmouth, Podargus strigoides (Podargidae). (f) Common Potoo, Nyctibius griseus (Nyctibiidae). (g) White-throated Nightjar, Eurostopodus mystacalis (Caprimulgidae). (h) Great Dusky Swift, Cypseloides senex (Apodidae). clg, crus longum. Not to scale
Meanwhile, however, there exists congruent molecular support for a clade including Apodiformes and all taxa of the paraphyletic ‘Caprimulgiformes’, which resulted from analyses of multiple individual gene loci (Ericson et al. 2006; Mayr 2008; Hackett et al. 2008; Fig. 2a,b), and received high bootstrap support in Hackett et al.'s (2008) comprehensive ‘phylogenomic’ study (Fig. 2c). Equal molecular support does not exist for alternative phylogenies, and the fact that this clade was obtained in independent analyses of different kind of molecular data constitutes strong evidence that the included taxa indeed constitute a monophyletic group (see also Mayr 2008).
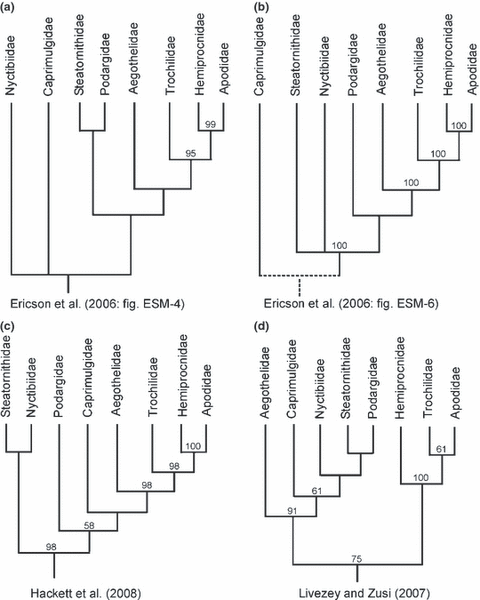
Recent phylogenies in comparison. (a) Bayesian analysis of the ß-fibrinogen intron 7 sequence (Ericson et al. 2006: fig. ESM-4). (b) Bayesian analysis of sequences of the c-myc exon 3, RAG-1 exon, myoglobin intron 2 and ornithine decarboxylase introns 6 and 7 with intercepting exon 7 (Ericson et al. 2006: fig. ESM-6; note that this analysis did not support position of the Caprimulgidae within the ‘Caprimulgiformes’/Apodiformes clade). (c) Maximum likelihood analysis of 19 gene loci (Hackett et al. 2008). (d) Analysis of 2954 morphological characters (Livezey and Zusi 2007). Posterior probabilities (a, b) and bootstrap support values (c, d) from the original publications are listed above the nodes
The relationships between Steatornithidae, Podargidae, Caprimulgidae and Nyctibiidae, however, remain controversial, and the results of the above studies and other molecular analyses (Mariaux and Braun 1996; Cracraft et al. 2004; Barrowclough et al. 2006; Harshman 2007; Larsen et al. 2007; Brown et al. 2008) do not agree concerning their position. Whereas, for example, Cracraft et al. (2004) assumed that Steatornithidae, Nyctibiidae and Caprimulgidae form a clade, Livezey and Zusi (2007) hypothesized the traditional ‘Caprimulgiformes’ to be monophyletic, and the analysis of Hackett et al. (2008) resulted in a sister group relationship between Steatornithidae and Nyctibiidae, with Podargidae and Caprimulgidae being successive sister taxa of the Aegothelidae/Apodiformes clade (Fig. 2).
Despite a superficially similar external appearance, ‘caprimulgiform’ birds distinctly differ in anatomical features, and the aim of the present study is to show that morphological data contribute to a resolution of the relationships between these taxa. Sixty-nine morphological characters are included in the analysis, and the implications of the resulting phylogeny for the evolution of dark-activity are discussed.
Material and Methods
Skeletons of the following species were examined (all in the collection of Forschungsinstitut Senckenberg): Steatornithidae: Steatornis caripensis; Podargidae: Batrachostomus septimus, Podargus strigoides; Aegothelidae: Aegotheles cristatus; Nyctibiidae: Nyctibius griseus; Caprimulgidae: Caprimulgus europaeus, Caprimulgus pectoralis, Chordeiles minor, Eurostopodus mystacalis, Hydropsalis torquata (‘brasiliana’), Lurocalis semitorquatus, Macrodipteryx longipennis (skull), Nyctidromus albicollis, Phalaenoptilus nuttalii; Apodidae: Apus apus, Apus melba, Chaetura vauxi, Collocalia salangana, Collocalia vanikorensis, Cypseloides senex, Streptoprocne zonaris; Hemiprocnidae: Hemiprocne comata; Trochilidae: Amazilia versicolor, Archilochus colubris, Chrysolampis mosqitus, Glaucis hirsuta, Melanotrochilus fuscus, Phaethornis pretrei, Thalurania furcata, Trochilus polytmus. Osteological terminology follows Baumel and Witmer (1993), if not indicated otherwise.
The phylogenetic analysis was performed with the heuristic search modus of nona 2.0 (Goloboff 1993) through the winclada 1.00.08 interface (Nixon 2002), using the commands hold 10 000, hold/10, mult*1000 and max*. Sixty-nine characters were scored for eight ingroup taxa, based on the revised and emended matrix of Mayr (2005a) (see Appendix 2). Outgroup comparisons were made with Tinamidae (tinamous) and Anseriformes (waterfowl). The consistency index (CI) and retention index (RI) were calculated. Bootstrap support values were calculated with 1000 replicates, three searches holding one tree per replicate, and TBR branch swapping without max*.
Results of phylogenetic analysis
Analysis of the character matrix in Appendix 2 resulted in a single most parsimonious tree, which is depicted in Fig. 3. The following characters were optimized as apomorphies of a clade including Podargidae, Caprimulgidae, Nyctibiidae, Aegothelidae and apodiform birds to the exclusion of the Steatornithidae:
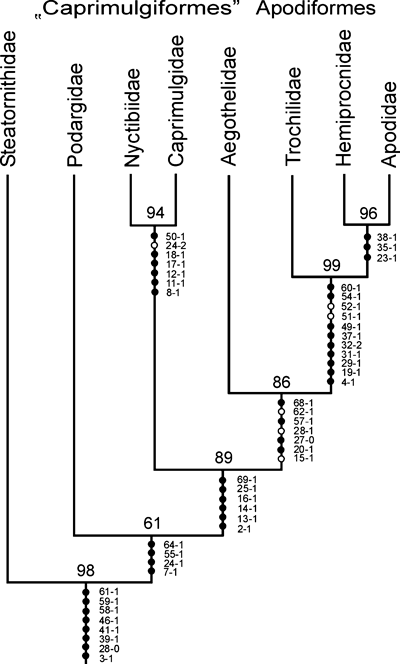
Most parsimonious tree resulting from analysis of the character matrix in Appendix 2 with nona 2.0 (Length = 118, CI = 0.66, RI = 0.71). Bootstrap support values are listed above the nodes, characters and characters states next to the internodes. Filled circles represent strict apomorphies, open circles homoplastic ones
- 1
Palatinum with strongly protruding angulus caudolateralis. This character was listed as an apomorphy of a clade including Aegothelidae and Apodiformes by Mayr (2002), but is also present in the Caprimulgidae and Nyctibiidae, whose palatinum exhibits an apomorphic morphology (character 7; Fig. 4). The angulus caudolateralis is secondarily reduced in Cypseloides (Apodidae) and Trochilidae.
- 2
Seventeen or 18 free praesacral vertebrae (character 24); Steatornithidae have 19 free praesacral vertebrae, which by outgroup comparison with other neornithine birds is the plesiomorphic number (e.g. Mayr and Clarke 2003).
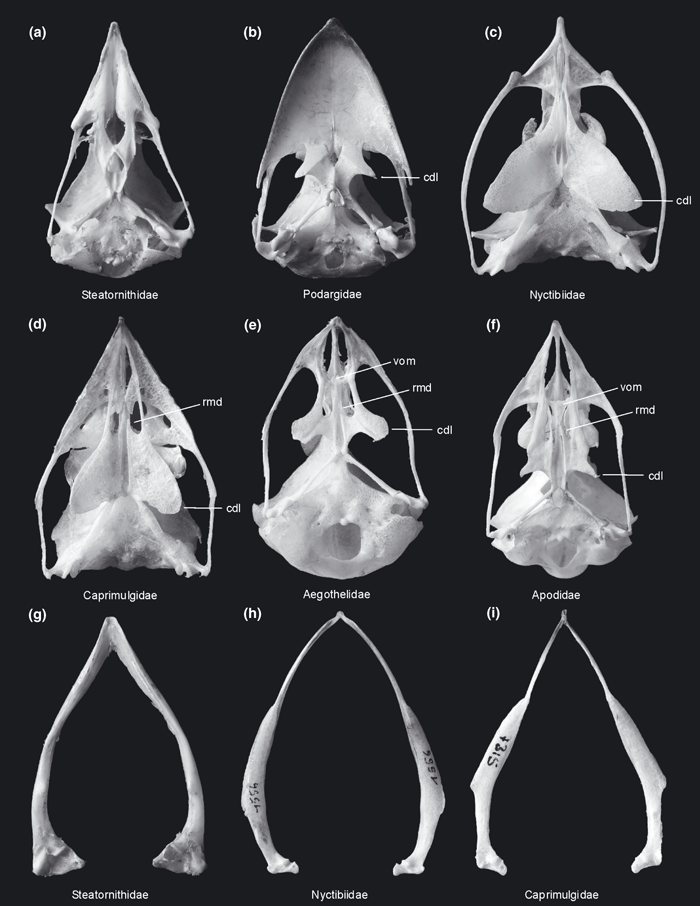
(a)-(f) Skull (ventral view) and (g)-(i) mandible (dorsal view) of ‘caprimulgiform’ and apodiform birds in comparison. (a), (g) Oilbird, Steatornis caripensis (Steatornithidae; right quadratum detached). (b) Tawny Frogmouth, Podargus strigoides (Podargidae). (c), (h) Common Potoo, Nyctibius griseus (Nyctibiidae). (d), (i) White-throated Nightjar, Eurostopodus mystacalis (Caprimulgidae). (e) Australian Owlet-nightjar, Aegotheles cristatus (Aegothelidae). (f) Alpine Swift, Apus melba (Apodidae). cdl, angulus caudolateralis; rmd, processus rostromedialis; vom, vomer. Not to scale
Two homoplastic characters were further shown to be apomorphies of this clade. One of these (tarsometatarsus without canalis interosseus distalis; character 55) occurs in many neornithine birds and is absent in the Nyctibiidae. The other (vinculum between tendons of musculus flexor perforans et perforatus digiti III and m. perforatus digiti III absent; character 64) is variable in the Caprimulgidae (see Appendix 1).
A clade including Caprimulgidae, Nyctibiidae, Aegothelidae and apodiform birds is supported by six apomorphies:
- 1
Short beak that is very wide at its base and with narial openings extending into its tip (character 2).
- 2
Processus orbitalis of quadratum greatly reduced (character 13; Fig. 5).
- 3
Condylus caudalis of quadratum reduced, condylus lateralis separated from elongated condylus medialis by a narrow furrow (character 14; Fig. 5).
- 4
Distal part of rami mandibulae very narrow, pars symphysialis very short (Fig. 4; character 16).
- 5
Furcula with distinct facies articularis acrocoracoidea for articulation with processus acrocoracoideus of coracoid (Fig. 5; character 25).
- 6
Cerebellum with reduced anterior lobe, particularly small folia II and III, and a relatively large posterior lobe (Iwaniuk et al. 2006: 53; character 69). Iwaniuk et al. (2006) hypothesized that the small anterior lobe is due to the weakly developed hindlimb musculature.
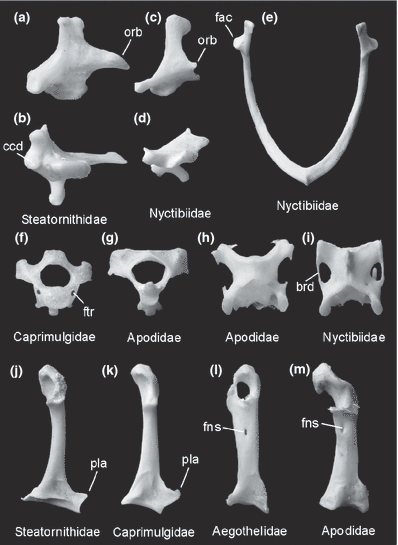
(a)-(d) Right quadratum in lateral (a, c) and caudal (b, d) view, (e) furcula, (f), (g) axis, (h), (i) third cervical vertebra, and (j)-(m) right coracoid of ‘caprimulgiform’ and apodiform birds in comparison. (a), (b), (j) Oilbird, Steatornis caripensis (Steatornithidae). (c), (d), (e), (i) Common Potoo, Nyctibius griseus (Nyctibiidae). (f) Common Nighthawk, Chordeiles minor (Caprimulgidae). (g), (h), (m) Alpine Swift, Apus melba (Apodidae). (k) Scissor-tailed Nightjar, Hydropsalis torquata (Caprimulgidae). (l) Australian Owlet-nightjar, Aegotheles cristatus (Aegothelidae). brd, bridge from processus transversus to processus articularis caudalis; ccd, condylus caudalis; fac, facies articularis acrocoracoidea; fns, foramen nervi supracoracoidei; ftr, foramen transversarium; orb, processus orbitalis; pla, processus lateralis. Not to scale
Characters (1)–(3) were already mentioned as apomorphies of this clade by Mayr (2002), who listed two further apomorphies. Both were included in the present analysis, but were not unambiguously optimized as apomorphies of any of the nodes (palatinum with well-developed processus rostromedialis; character 6 [Fig. 4], and fossa dorsalis of phalanx proximalis digiti majoris divided into two depressions by distinctly raised oblique bulge; character 43 [Fig. 6]). Characters (5) and (6) are newly identified in the present study.
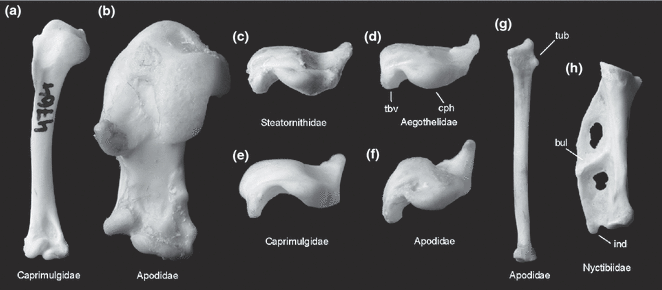
Right humerus in (a), (b) cranial and (c)-(f) proximal view, (g) right radius, (h) left phalanx proximalis digiti majoris of ‘caprimulgiform’ and apodiform birds in comparison. (a), (e) Scissor-tailed Nightjar, Hydropsalis torquata. (b), (f), (g) Alpine Swift, Apus melba (Apodidae). (c) Oilbird, Steatornis caripensis (Steatornithidae). (d) Australian Owlet-nightjar, Aegotheles cristatus (Aegothelidae). (h) Common Potoo, Nyctibius griseus (Nyctibiidae). bul, oblique bulge dividing phalanx proximalis digiti majoris; cph, caput humeri; ind, processus internus indicis; tbv, tuberculum ventrale; tub, tubercle on distal radius of swifts. Not to scale
A sister group relationship between Caprimulgidae and Nyctibiidae is supported by seven synapomorphies:
- 1
Os palatinum, pars lateralis extremely widened (Fig. 4; character 8). This character is a unique synapomorphy of Caprimulgidae and Nyctibiidae, and may constitute a safety device to protect the large eyes of these birds against damage by prey insects.
- 2
Processus paroccipitales widely separated and strongly ventrally protruding; basis cranii concave (character 11).
- 3
Presence of cone-like bony protrusion at caudal margin of foramen nervi optici (Fig. 7; character 12). The process was considered the attachment site of the musculi bulbi oculi by Livezey and Zusi (2006: fig. 2d), and is a unique synapomorphy of Caprimulgidae and Nyctibiidae.
- 4
Mandible with intraramal joint and caudal half of rami mandibulae greatly widened and flattened (Fig. 4; character 17). This character is a unique synapomorphy of Caprimulgidae and Nyctibiidae and is related to the fact that these birds use their beak in a brailer-like fashion when hunting insects (Bühler 1970).
- 5
Proximal end of mandible unusually small (Fig. 4; character 18); this character is a unique synapomorphy of Caprimulgidae and Nyctibiidae.
- 6
Only 17 free praesacral vertebrae (character 25).
- 7
Presence of os sesamoideum intertarsale (Fig. 8; character 51). This character occurs in only few other birds (e.g. Pteroclidae [sandgrouse]), and was not included in the analysis of Livezey and Zusi (2007) (see Livezey and Zusi 2006: 340).
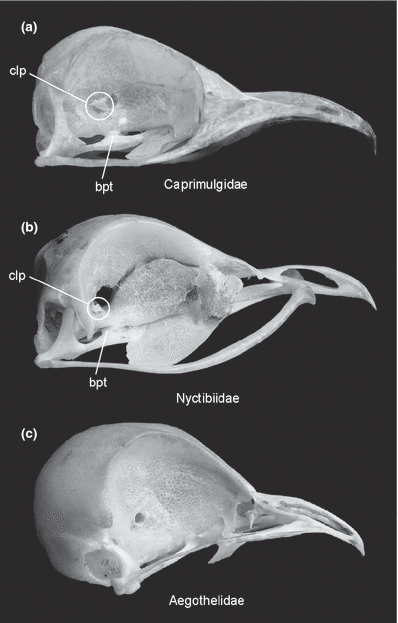
Skull in lateral view. (a) White-throated Nightjar, Eurostopodus mystacalis (Caprimulgidae). (b) Common Potoo, Nyctibius griseus (Nyctibiidae). (c) Australian Owlet-nightjar, Aegotheles cristatus (Aegothelidae). bpt, processus basipterygoideus; clp, cone-like protrusion. Not to scale
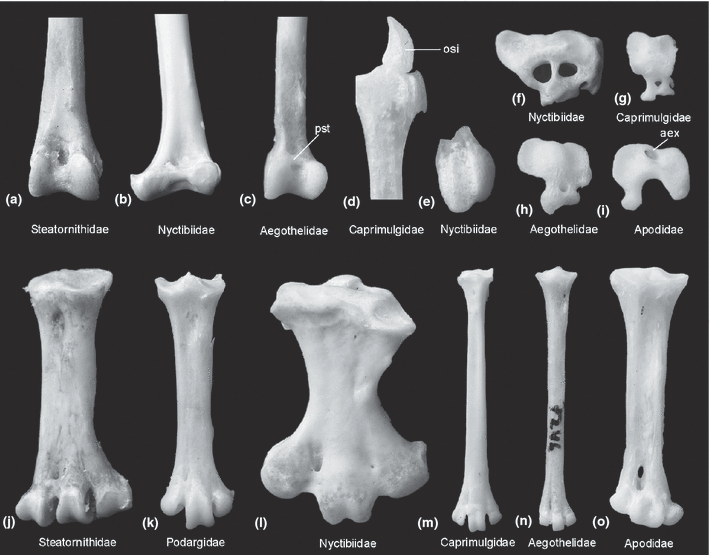
(a)-(c) Distal end of right tibiotarsus, (d), (e) os sesamoideum intertarsale, (f)-(i) proximal view of right hypotarsus, and (j)-(o) right tarsometatarsus of ‘caprimulgiform’ and apodiform birds in comparison. (a), (j) Oilbird, Steatornis caripensis (Steatornithidae). (b), (e), (f), (l) Common Potoo, Nyctibius griseus (Nyctibiidae). (c), (h), (n) Australian Owlet-nightjar, Aegotheles cristatus (Aegothelidae). (d) Common Nighthawk, Chordeiles minor (Caprimulgidae). (g), (m) Scissor-tailed Nightjar, Hydropsalis torquata. (k) Tawny Frogmouth, Podargus strigoides (Podargidae). (i), (o) Alpine Swift, Apus melba (Apodidae). aex, arcus extensorius; osi, os sesamoideum intertarsale; pst, pons supratendineus. Not to scale
Characters (1)–(5) were discussed by Mayr (2002), characters (6) and (7) are newly identified as apomorphies of this clade in the present study.
The sister group relationship between Aegothelidae and Apodiformes is supported by seven synapomorphies:
- 1
Quadratum, processus oticus, dorsal margin of caudal surface with many small pneumatic foramina (character 15).
- 2
Axis without foramina transversaria (Fig. 5; character 20).
- 3
Coracoid with foramen nervi supracoracoidei (Fig. 5; character 27).
- 4
Coracoid with very short processus lateralis (Fig. 5; character 289).
- 5
Musculus splenius capitis with cruciform origin (character 57). This feature was first noted by Burton (1971) and its exaggerated condition, which is accompanied by a derived morphology of the processus spinosus of the axis, is unique to Apodiformes and Aegothelidae.
- 6
Musculus fibularis longus absent (character 62). This muscle is absent in only few other avian taxa, including Strigidae (true owls), Upupidae (hoopoes), and Bucerotidae (hornbills) (George and Berger 1966; McKitrick 1991).
- 7
Caeca absent (character 68). Because Aegothelidae, Hemiprocnidae, and Apodidae have a similar insectivorous diet like Caprimulgidae and Nyctibiidae, caeca development does not seem to be correlated with the composition of the diet of these birds.
Characters (1), (4), (5) and (7) were identified as apomorphies of the Aegothelidae/Apodiformes clade by Mayr (2002), who discussed two further apomorphies of this clade. One of these (os palatinum with strongly protruding angulus caudolateralis; character 7 in Appendix 2) is optimized as an apomorphy of a more inclusive clade in the present study (see beginning of this chapter). The polarity of the other character (absence of processus basipterygoidei; character 10 in Appendix 2) is uncertain. Steatornithidae, Caprimulgidae and Nyctibiidae exhibit well-developed processus basipterygoidei, whereas these processes are absent in Podargidae and the Aegothelidae/Apodiformes clade. It is equally parsimonious to assume that they were reduced in the latter taxa, or that they were regained in Steatornithidae and the clade including Caprimulgidae and Nyctibiidae. Processus basipterygoidei are found in palaeognathous birds and Galloanseres, i.e. all non-neoavian Neornithes, and are part of the groundplan of Neornithes, to which ‘Caprimulgiformes’ and Apodiformes belong. In order to show whether they also belong to the groundplan of the ‘Caprimulgiformes’/Apodiformes clade, it would be necessary to either identify the sister taxon of this clade, or to examine whether or not these processes occur in the early ontogeny of those taxa, in which they are absent in the adult representatives.
Classification
Based on the results of the phylogenetic analysis, the following classification is proposed:
Strisores Baird, 1858.
- 1
Steatornithiformes, new taxon
- 1.1
Steatornithidae Bonaparte, 1842
- 1.1
- 2
Podargocypseli, new taxon
- 2.1
Podargiformes Mathews, 1918
- 2.1.1
Podargidae Bonaparte, 1838
- 2.1.1
- 2.2
Cypselomorphae Huxley, 1867
- 2.2.1
Caprimulgi Ridgway, 1881
- 2.2.1.1
Caprimulgidae Vigors, 1825
- 2.2.1.2
Nyctibiidae Chenu and des Murs, 1851
- 2.2.1.1
- 2.2.2
Apodimorphae Sibley et al., 1988
- 2.2.2.1
Aegotheliformes Worthy et al., 2007
- 2.2.2.1.1
Aegothelidae Bonaparte, 1853
- 2.2.2.1.1
- 2.2.2.2
Apodiformes Peters, 1940
- 2.2.2.2.1
Apodi Peters, 1940
- 2.2.2.2.1.1
Apodidae Olphe-Galliard, 1887
- 2.2.2.2.1.2
Hemiprocnidae Oberholser, 1906
- 2.2.2.2.1.1
- 2.2.2.2.2
Trochilidae Vigors, 1825
- 2.2.2.2.1
- 2.2.2.1
- 2.2.1
- 2.1
Sangster (2005) introduced the term ‘Daedalornithes’ for the clade including Aegothelidae and Apodiformes, considering the name Apodimorphae inappropriate, because Sibley et al. (1988) restricted it to apodiform birds. However, taxon names above the ‘family-level’ are not regulated by the International Code of Zoological Nomenclature, and I prefer the latter term, which more appropriately reflects the actual content of this clade.
Worthy et al. (2007: 25) stated that Aegothelidae were first elevated into ‘ordinal’ rank by Simonetta (1967). However, this assumption is erroneous, as Simonetta (1967) classified owlet-nightjars within Caprimulgiformes and just assigned them to a new ‘suborder’ Aegothelae, which was listed in his linear sequence between Podargi (including Podargidae) and Caprimulgi (including Caprimulgidae and Nyctibiidae). The term Aegotheliformes was thus actually introduced by Worthy et al. (2007).
Discussion
Except for the position of the Nyctibiidae, the results of the present analysis are in concordance with the phylogeny of Hackett et al. (2008). Potoos resulted as the sister taxon of the Steatornithidae in the Hackett et al. (2008) analysis, but neither the Steatornithidae/Nyctibiidae clade nor the clade including Caprimulgidae, Aegothelidae and apodiform birds received significant bootstrap support (Fig. 2c). By contrast, a sister group relationship between Nyctibiidae and Caprimulgidae is strongly supported in the present study, both in terms of bootstrap robustness and the number of shared apomorphies. Likewise well-corroborated is a clade including Nyctibiidae, Caprimulgidae, Aegothelidae and apodiform birds, and a sister group relationship between Steatornithidae and Nyctibiidae would imply homoplasy of at least 13 morphological features, some of which are unparalleled within Aves (see chapter ‘Results of phylogenetic analysis’).
A sister group relationship between Steatornithidae and Nyctibiidae is not recovered in other analyses of sequence data (Mariaux and Braun 1996; Barrowclough et al. 2006; Ericson et al. 2006; Brown et al. 2008). Although a Steatornithidae/Nyctibiidae clade resulted from the DNA-DNA hybridization studies of Sibley and Ahlquist (1990), these have been criticized for methodological reasons (Houde 1987; Lanyon 1992; Harshman 1994), and Nyctibiidae were not included in the analyses of ‘caprimulgiform’ birds using the Fitch algorithm (Sibley and Ahlquist 1990: fig. 334), which according to Harshman (1994) should be preferred to the trees based on the UPGMA algorithm.
Except for the greatly abbreviated tarsometatarsus and the possibly functionally correlated absence of an ossified pons supratendineus on the tibiotarsus (Fig. 8), Nyctibiidae and Steatornithidae do not share derived morphological features that indicate a sister group relationship, and a clade consisting of Nyctibiidae and Caprimulgidae is certainly much better supported by morphological data. Because molecular evidence for a Steatornithidae/Nyctibiidae clade is weak, a sister group relationship between Nyctibiidae and Caprimulgidae is more likely to reflect the true phylogenetic affinities of these birds. The present study may thus serve as an example where morphological features contribute to phylogenetic issues that are not conclusively resolved by molecular data.
In contrast to all other recent studies, Livezey and Zusi’s (2007) analysis resulted in monophyly of the traditional ‘Caprimulgiformes’, with Aegothelidae as the sister group of the other taxa, and Nyctibiidae as sister taxon of a clade including Steatornithidae and Podargidae. Apomorphies that support ‘caprimulgiform’ relationships were not listed, and as I have detailed elsewhere (Mayr 2008), the results of the analysis are called into question by disputable character scorings, which in several instances are based on unverified assumptions.
Steatornithidae, Caprimulgidae, Nyctibiidae, Podargidae and Aegothelidae are crepuscular or nocturnal birds, whereas all Apodiformes are diurnal like most other birds. Irrespective of the exact affinities of the Nyctibiidae, the most parsimonious interpretation of the distribution of dark-activity in Strisores is a single origin in the stem lineage of these birds, and a reversal to a diurnal way of living in the stem lineage of the Apodiformes (Fig. 9a). Such an evolutionary scenario was first proposed by Mayr (2002), and has also been assumed by Cracraft et al. (2004) and Hackett et al. (2008). So far, however, no anatomical or physiological features were identified that indicate a nocturnal stem species of apodiform birds. Steatornithidae and Caprimulgidae further show distinct differences in retina morphology, with oilbirds having much more rods and lacking a tapetum lucidum (Rojas et al. 2004; to the best of my knowledge, the retina of other ‘caprimulgiform’ birds has not been studied so far). Fidler et al. (2004) also reported differences between Aegothelidae, Podargidae and Caprimulgi in the nucleotide sequences of the arylalkylamine N-acetyltransferase gene, which regulates melatonin biosynthesis and is assumed to play a role in nocturnal activity (Steatornithidae and Apodiformes were not included in this study). Although these differences per se do not prove an independent origin of dark-activity in ‘caprimulgiform’ birds, it is not straightforward to explain their existence under the assumption that the stem species of the Strisores was crepuscular or nocturnal. None of the extant ‘caprimulgiform’ taxa includes secondarily diurnal representatives, and Holyoak (2001: 12) hypothesized that the ‘successful return to diurnal foraging may be prevented by combinations of factors, such as loss of colour vision, susceptibility to hawks and other diurnal predators, and potential competitive interactions with the many diurnal insectivorous bird species’. According to Holyoak (2001: 10) there are further ‘huge evolutionary incentives for insectivorous birds to become nocturnal’, such as the great abundance of insects and the fewer number of predators. Despite being a less parsimonious scenario, a fourfold origin of dark-activity in the stem lineages of the Steatornithidae, Podargidae, Caprimulgi and Aegothelidae (Fig. 9b) is thus not implausible, and remains an alternative to be seriously considered in future studies. Possible reasons for a multiple independent origin of dark-activity may have been competition with diurnal insectivorous birds, or access to a new ecological zone due to an early Paleogene radiation of nocturnal insects (see Mayr 2005c, 2009).
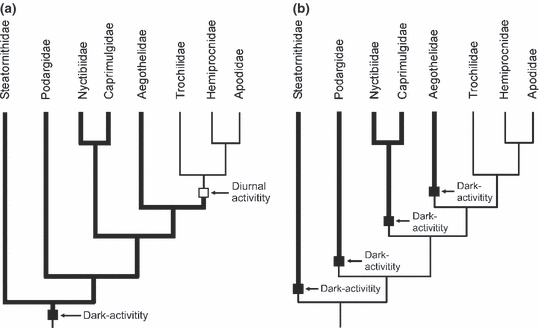
Two hypotheses on the origin of dark-activity (thick lines) in Strisores. (a) Single origin in the stem lineage of the taxon and reversal into diurnal activity in the stem lineage of the Apodiformes. (b) Fourfold origin in the stem lineages of Steatornithidae, Podargidae, Caprimulgi and Aegothelidae
Many ‘caprimulgiform’ and apodiform birds are capable of torpor, i.e. facultative hypothermic responses to environmental conditions, which has been reported for Podargidae, Caprimulgidae, Aegothelidae, Apodidae and Trochilidae (Bartholomew 1985; Brigham et al. 2001; Körtner et al. 2001; McKechnie and Lovegrove 2002; Lane et al. 2004). The distribution of this trait in the extant taxa indicates that it was already present in the stem species of the Podargocypseli. Kitto and Wilson (1966) hinted at the possibility that occurrence of torpor in ‘caprimulgiform’ and apodiform birds may be related to an unusually slow motility of the enzyme S-malate dehydrogenase in the studied representatives of these taxa. By reducing oxaloacetate to malate, this enzyme indeed plays a role in gluconeogenesis after the hibernation of mammals (Epperson et al. 2004). A correlation between torpor and the properties of the S-malate dehydrogenase may also be indicated by the fact that its motility is particularly slow (only 27% of that of the chicken enzyme) in the Common Poorwill, Phalaenoptilus nuttalii, which is the only bird known to be capable of extended periods of torpor over several weeks (e.g. Bartholomew 1985). However, Kitto and Wilson (1966), showed that the S-malate dehydrogenase is also unusually slow in Charadriiformes, which are not known to be capable of torpor, and torpor regulation is likely to be a multifactorial process that is dependent on more than a single enzyme. P. nuttalii was the only representative of the ‘Caprimulgiformes’ included in the study of Kitto and Wilson (1966), and studies of the motility of the S-malate dehydrogenase in other ‘caprimulgiform’ birds are warranted.
Footnotes
Acknowledgements
I thank S. Tränkner for taking the photographs and A. Manegold for comments on an earlier version of the manuscript. The latter was further improved by reviews from D. Ksepka and an anonymous referee.
Appendices
Appendix 1. Character descriptions (see Mayr 2005a for further comments).
- 1
Skull, largely/completely ossified septum nasale: absent (0), present (1).
- 2
Beak short and very wide at its base, with narial openings large and reaching far into its tip: no (0), yes (1).
- 3
Os lacrimale vestigial or completely reduced: no (0), yes (1).
- 4
Os ectethmoidale, greatly expanded and plate-like, with dorsal margin largely fused with ossa frontalia: no (0), yes (1).
- 5
Vomer: not as follows (0), with truncate rostral and bifurcate caudal end (typical of the ‘aegithognathous’ palate) (1). This character is coded as unknown in taxa in which the vomer is vestigial or reduced.
- 6
Os palatinum, well-developed processus rostromedialis (Fig. 4; terminology after Zusi and Livezey 2006): absent (0), present (1).
- 7
Os palatinum with strongly protruding angulus caudolateralis (Fig. 4): no (0), yes (1). In the examined Apodidae, this character is absent in Cypseloides.
- 8
Os palatinum, pars lateralis extremely widened (Fig. 4): no (0), yes (1).
- 9
Os palatinum and os pterygoideum fused: yes (0), no (1).
- 10
Well developed processus basipterygoidei that articulate with the ossa pterygoidea (Fig. 7): yes (0), no (1).
- 11
Processus paroccipitales widely separated and strongly ventrally protruding; basis cranii concave: no (0), yes (1).
- 12
Cone-like bony protrusion at caudal margin of foramen nervi optici (Fig. 7; processus musculi bulbi oculi of Livezey and Zusi 2006: fig. 2D): absent (0), present (1).
- 13
Quadratum, processus orbitalis greatly reduced (Fig. 5): no (0), yes (1).
- 14
Quadratum, condylus caudalis completely reduced, condylus lateralis separated from elongate condylus medialis by a deep but narrow furrow (Fig. 5): no (0), yes (1).
- 15
Quadratum, processus oticus, dorsal margin of caudal surface with many small pneumatic foramina: no (0), yes (1).
- 16
Mandible, distal part of rami mandibulae very narrow, pars symphysialis very short (Fig. 4): no (0), yes (1).
- 17
Mandible with intraramal joint and caudal half of rami mandibulae greatly widened and dorso-ventrally flattened (Fig. 4): no (0), yes (1).
- 18
Mandible, proximal end unusually small, with very short cotyla lateralis and stout processus medialis (Fig. 4): no (0), yes (1).
- 19
Atlas, incisura fossae: open (0), closed (1).
- 20
Axis, foramina transversaria (Fig. 5): present (0), absent (1).
- 21
Third cervical vertebra, osseous bridge from processus transversus to processus articularis caudalis (Fig. 5): absent (0), present (1).
- 22
5th cervical vertebra, osseous bridge from processus costalis to midsection of corpus vertebrae: no (0), yes (1).
- 23
Tip of processus ventralis of 14th and 15th praesacral vertebrae forming large horizontal plate: no (0), yes (1).
- 24
Number of free praesacral vertebrae: 19 or more (0), 18 (1), 17 (2).
- 25
Furcula, extremitas omalis with distinct, laterally protruding facies articularis acrocoracoidea (Fig. 5): no (0), yes (1). Within Caprimulgidae, this character is present in, e.g., Eurostopodus, but only weakly developed in, e.g., Caprimulgus.
- 26
Coracoid, facies articularis scapularis excavated and cup-like: yes (0), no (1).
- 27
Coracoid, foramen nervi supracoracoidei (Fig. 5): present (0), absent (1).
- 28
Coracoid, processus lateralis (Fig. 5): short (0), long (1).
- 29
Sternum, spina externa forming a ridge that extends dorsoventrally over entire rostrum sterni: no (0), yes (1).
- 30
Sulci coracoidei cross in midline of bone: no (0), yes (1).
- 31
Sternum, facies articularis coracoideus weakly saddle-shaped or convex: no (0), yes (1).
- 32
Caudal margin of sternum: with four notches/fenestrae (0), with two notches/fenestrae (1), or without notches/fenestrae (2).
- 33
Humerus, proximal end, sulcus transversus very deep, long, and rectangular-shaped: no (0), yes (1).
- 34
Humerus, proximal end, caudal prominence of tuberculum ventrale distinctly greater than that of caput humeri (Fig. 6): no (0), yes (1). This character corresponds to character 1365b of Livezey and Zusi (2006), which was listed as an apomorphy of a clade including all ‘caprimulgiform’ and apodiform birds by Livezey and Zusi (2007).
- 35
Humerus, distal end, tuberculum supracondylare ventrale elongated and narrow: no (0), yes (1).
- 36
Humerus, distal end, fossa musculi brachialis deep and sharply delimited: no (0), yes (1).
- 37
Humerus, greatly abbreviated and stocky, ratio length of bone: width of shaft in midsection less than 7.0 (Fig. 6): no (0), yes (1).
- 38
Radius, distal end with marked tubercle on ventral side of shaft, opposite to tuberculum carpale of ulna (Fig. 6): no (0), yes (1).
- 39
Ulna distinctly exceeding humerus in length: no (0), yes (1).
- 40
Carpometacarpus, os metacarpale minus distinctly exceeding os metacarpale majus in length: no (0), yes (1).
- 41
Os carpi ulnare, crus longum much longer than crus breve (Fig. 1): no (0), yes (1).
- 42
Phalanx digiti alulae, claw: present (0), absent or rudimentary in adulthood (1); after Stephan (1992).
- 43
Fossa dorsalis of phalanx proximalis digiti majoris divided into two depressions by a distinctly raised oblique bulge (Fig. 6): no (0), yes (1).
- 44
Phalanx proximalis digiti majoris, well developed processus internus indicis (Fig. 6): absent (0), present (1).
- 45
Pelvis, foramen ilioischiadicum caudally closed: no (0), yes (1).
- 46
Pelvis wide in mediolateral direction, width across antitrochanters as much as or more than length of synsacrum: no (0), yes (1). In Podargidae, this character is present in Batrachostomus but absent in Podargus.
- 47
Pelvis, well developed tubercula praeacetabularia: present (0), absent (1).
- 48
Tibiotarsus, distal end, pons supratendineus (Fig. 8): ossified (0), tendinous (1).
- 49
Tibiotarsus, distal end, trochlea cartilaginis tibialis with very deep sulcus: no (0), yes (1).
- 50
Os sesamoideum intertarsale (Fig. 8): absent (0), present (1).
- 51
Tarsometatarsus greatly abbreviated and stout, measuring less than half of carpometacarpus length (Fig. 8): no (0); yes (1).
- 52
Tarsometatarsus, tuberositas musculi tibialis cranialis very prominent: no (0), yes (1).
- 53
Tarsometatarsus, hypotarsus, tendons of musculus flexor digitorum longus and m. flex. hallucis longus enclosed in bony canals (Fig. 8): no (0), yes (1).
- 54
Tarsometatarsus, arcus extensorius (Fig. 8; ossified retinaculum extensorium tarsometatarsi): absent (0), present (1).
- 55
Tarsometatarsus, canalis interosseus distalis: present (0), absent (1).
- 56
Second and third phalanx of fourth toe greatly abbreviated, measuring less than half the length of the fourth phalanx: no (0), yes (1).
- 57
Musculus splenius capitis with cruciform origin: no (0), yes (1).
- 58
Musculus ambiens: present (0), absent (1); after McKitrick (1991).
- 59
Musculus iliofemoralis externus: present (0), absent (1); after McKitrick (1991).
- 60
Musculus flexor cruris lateralis, pars accessoria: present (0), absent (1); after McKitrick (1991).
- 61
Musculus caudofemoralis, pars pelvica: present (0), absent (1); after McKitrick (1991).
- 62
Musculus fibularis longus: present (0), absent (1); after McKitrick (1991).
- 63
Musculus popliteus: present (0), absent (1); after McKitrick (1991).
- 64
Vinculum between tendons of musculus flexor perforans et perforatus digiti III and m. perforatus digiti III: present (0), absent (1). Occurrence of this character is variable in the Caprimulgidae, being absent in Chordeiles but present in Nyctidromus, Phalaenoptilus, and Caprimulgus (Hoff 1966: 120).
- 65
Musculus flexor hallucis longus and musculus flexor digitorum longus, type of arrangement. See George and Berger (1966: 447) for description of types; Trochilidae were coded 0, following McKitrick (1991).
- 66
Wing: diastataxic (0), eutaxic (1). Within Apodidae, the Cypseloidinae are diastataxic, whereas the Apodinae are eutaxic (Stephan 1970). Of the 104 genera of Trochilidae studied by Stephan (1970), 41 are eutaxic and 63 diastataxic.
- 67
Rictal bristles: absent (0), present (1). Within Caprimulgidae, rictal bristles are absent in Chordeiles, Eurostopodus, and Veles (Holyoak 2001).
- 68
Caeca: present (0), absent (1).
- 69
Cerebellum with reduced anterior lobe, particularly small folia II and III, and relatively large posterior lobe: no (0), yes (1); after Iwaniuk (2006).
Appendix 2. Character matrix of 69 morphological characters for the ten taxa included in the analysis (see Appendix 1 for character definitions). Polymorphic characters are coded as such, unknown character states are indicated by ‘?’. Tinamidae and Anseriformes were used for outgroup comparison.
| Taxa | Characters and character states | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | |
| Tinamidae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anseriformes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 01 | 0 | 0 | 0 |
| Steatornithidae | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Podargidae | 1 | 0 | ? | 0 | ? | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Caprimulgidae | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 01 |
| Nyctibiidae | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 1 |
| Aegothelidae | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| Apodidae | 0 | 1 | 1 | 1 | 1 | 1 | 01 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Hemiprocnidae | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Trochilidae | 0 | 0 | ? | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 1 |
| Taxa | Characters and character states | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | |
| Tinamidae | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anseriformes | 0 | 01 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 01 | 0 | 0 | 0 |
| Steatornithidae | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| Podargidae | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 01 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 01 | 1 | 0 | 0 | 0 |
| Caprimulgidae | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| Nyctibiidae | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | ? | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Aegothelidae | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Apodidae | 0 | 0 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 01 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Hemiprocnidae | 0 | 0 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Trochilidae | 0 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Taxa | Characters and character states | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | |
| Tinamidae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 1 | 0 | 0 | 0 |
| Anseriformes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 0 | 0 | 0 | 0 |
| Steatornithidae | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | ? | 0 | 1 | 0 | 0 |
| Podargidae | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 01 | 1 | ? | 0 | 1 | 0 | 0 |
| Caprimulgidae | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 01 | 5 | 0 | 01 | 0 | 1 |
| Nyctibiidae | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | ? | 0 | 0 | 0 | 1 |
| Aegothelidae | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | ? | 0 | 1 | 1 | 1 |
| Apodidae | 1 | 1 | 0 | 1 | 1 | 01 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 5 | 01 | 0 | 1 | 1 |
| Hemiprocnidae | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | ? | ? | ? | ? | ? | ? | 0 | 0 | 1 | ? |
| Trochilidae | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 01 | 0 | 1 | 1 |




